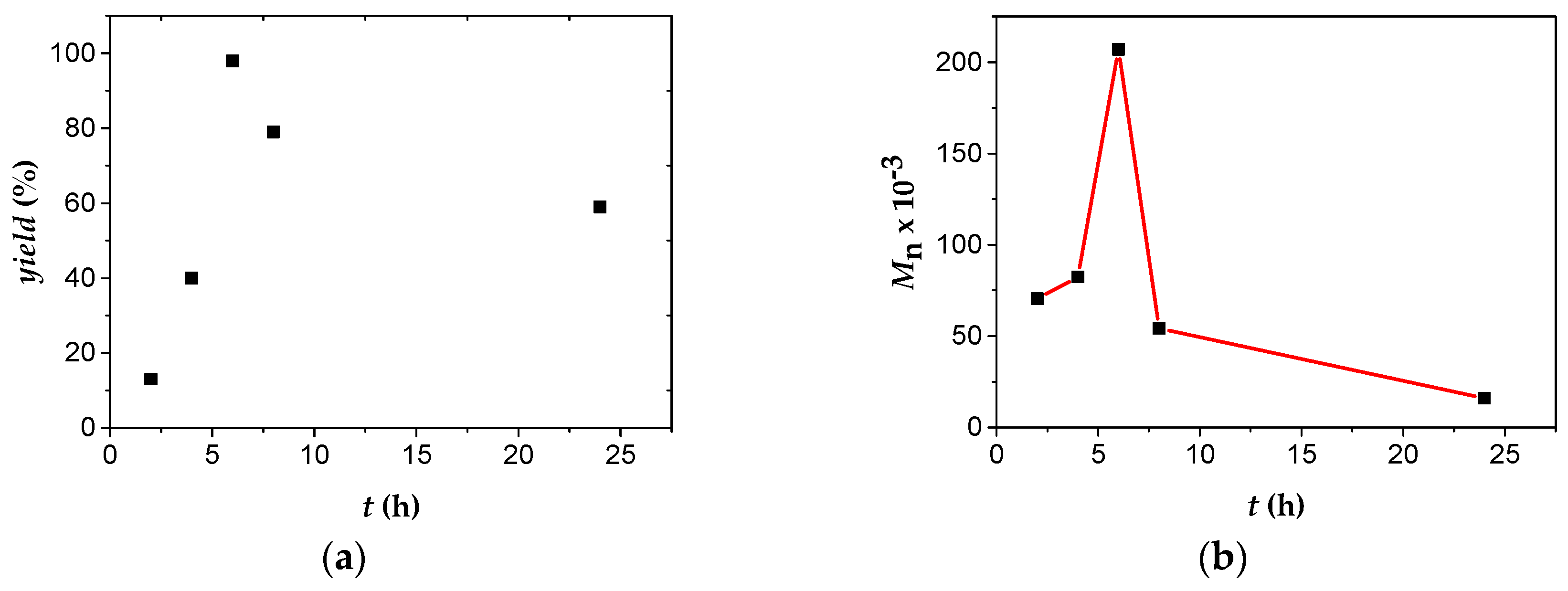3.1. Catalyst Synthesis and Characterization
Compound
1 was synthesized from the reaction of (Ph
4P)[W(CO)
5Br] and 1,2-dibromoethane in refluxing chlorobenzene, according to the literature procedure for the synthesis of the analogous
nPr
4N
+ salt [
22], which features face-sharing bioctahedral (FSBO) geometry, with three terminal bromide ligands on each tungsten atom and three bridging ones (
Scheme 2), and contains a metal-metal bond of order 2.5. Crystals of
1 suitable for X-ray analysis could not be obtained, but the complex was characterized by UV-Vis spectroscopy in CH
3CN (
Figure S1) and MALDI-TOF mass spectrometry (
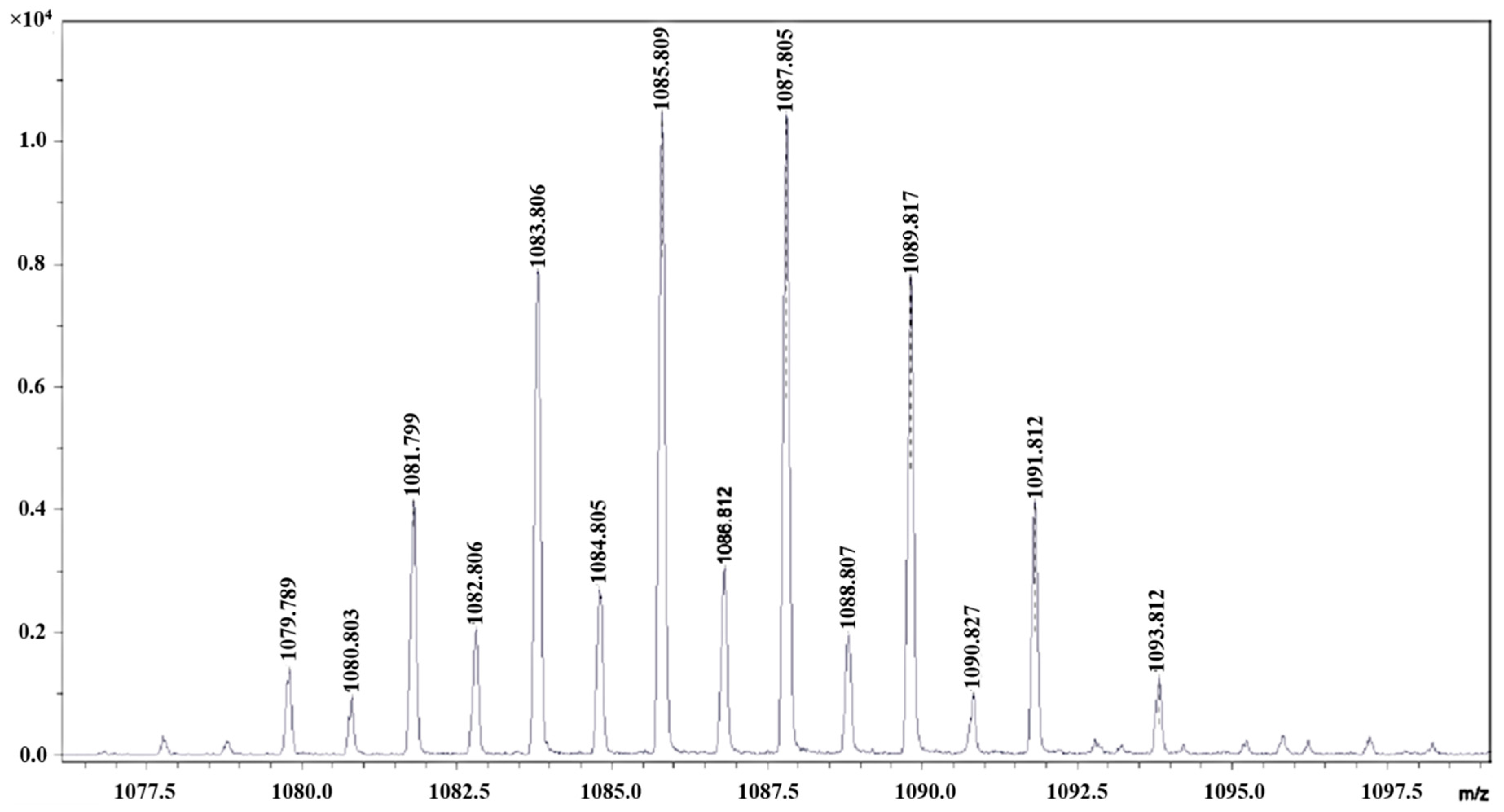
Figure 1 and
Figures S2–S6). UV-Vis spectra of both compounds (
1 and the
nPr
4N
+ salt) featured four peaks at the same wavelengths and with very similar molar absorption coefficients (
Table S1). MALDI-TOF mass spectra in the positive ion region revealed fragments attributed to Ph
4P
+ and [(Ph
4P)
3W
2Br
9]
+, while in the negative ion region mononuclear and dinuclear fragments were observed (
Table 1).
Scheme 2.
Schematic representation of the dianion of complex 1, [W2(μ-Br)3Br6]2−.
Scheme 2.
Schematic representation of the dianion of complex 1, [W2(μ-Br)3Br6]2−.
Figure 1.
MALDI-TOF mass spectrum of 1 in trans-2-[3-(4-tert-butylphenyl)-2-methyl-2-propenylidene]malononitrile (DCTB) (fragment [W2Br9]−).
Figure 1.
MALDI-TOF mass spectrum of 1 in trans-2-[3-(4-tert-butylphenyl)-2-methyl-2-propenylidene]malononitrile (DCTB) (fragment [W2Br9]−).
Table 1.
Fragments (m/z) of 1.
Table 1.
Fragments (m/z) of 1.
| Fragment | m/z (Theoretical) | m/z (Experimental) |
|---|
| Ph4P+ | 339 | 339.213 |
| [(Ph4P)3W2Br9]+ | 2104 | 2106.932 |
| [W2Br9]− | 1086 | 1085.809 |
| [Ph4PW2Br9]− | 1427 | 1427.563 |
| [WBr5]− | 583 | 582.207 |
| [W2Br7]− | 926 | 925.977 |
1 is moderately air-sensitive (oxygen, moisture); in the solid state, it is stable in air at room temperature for a few hours. It is soluble in CH2Cl2 and CH3CN, less soluble in tetrahydrofuran (THF) and CHCl3, and insoluble in toluene and Et2O. It was repeatedly recrystallized and checked carefully for purity (UV-Vis) before use.
3.2. Polymerization Reactions
Polymerization reactions were carried out at room temperature, for a given time, as shown in
Table 2,
Table 3,
Table 4,
Table 5 and
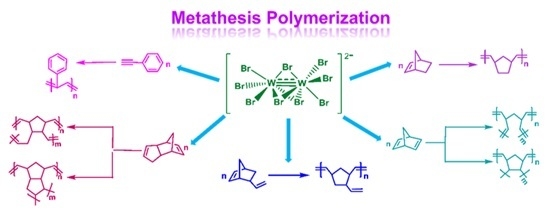
Table 6. Monomers studied include phenylacetylene (PA), norbornene (NBE), 5-vinyl-2-norbornene (VNBE), norbornadiene (NBD) and dicyclopentadiene (DCPD). All possible products are shown in
Scheme 3. Soluble polymers were characterized using
1H NMR spectroscopy (
Figures S7–S9) and insoluble polymers using
13C Cross Polarization Magic Angle Spinning (CPMAS) spectroscopy. Addition of AgBF
4 was required, otherwise
1 was inactive towards polymerization reactions. The role of AgBF
4 was to remove one or more bromide ligands, thus generating vacant coordination sites at the bimetallic core, available for monomer coordination.
Table 2.
Polymerization of phenylacetylene (PA) with the catalytic system 1/AgBF4.
Table 2.
Polymerization of phenylacetylene (PA) with the catalytic system 1/AgBF4.
| Entry | Solvent | 1/AgBF4/PA Molar ratio | t (h) | Yield (%) | Mn × 10−3 e | Mw/Mn e | cis (%) f |
|---|
| 1 | CH2Cl2 | 1/2/350 a | 24 | 9 | 5.2 | 1.45 | 0 |
| 2 | CH2Cl2 | 1/3/350 b | 24 | 12 | 4.6 | 1.37 | 0 |
| 3 | CH2Cl2 | 1/4/350 c | 24 | 20 | 2.8 | 1.89 | 0 |
| 4 | THF | 1/2/350 a | 8 | 4 | 41.8 | 1.86 | 75 |
| 5 | THF | 1/2/350 a | 16 | 88 | 67.8 | 1.60 | 77 |
| 6 | THF | 1/2/350 a | 24 | 95 | 59.5 | 1.58 | 88 |
| 7 | THF | 1/3/350 b | 2 | 13 | 70.5 | 1.72 | 85 |
| 8 | THF | 1/3/350 b | 6 | 98 | 207 | 1.16 | 87 |
| 9 | THF | 1/3/350 b | 8 | 79 | 54.2 | 1.61 | 85 |
| 10 | THF | 1/3/350 b | 24 | 59 | 16.0 | 2.07 | 85 |
| 11 | Toluene | 1/3/350 b | 24 | - d | - d | - d | - d |
| 12 | CH3CN | 1/3/350 b | 24 | - d | - d | - d | - d |
Table 3.
Polymerization of norbornene (NBE) with the catalytic system 1/AgBF4.
Table 3.
Polymerization of norbornene (NBE) with the catalytic system 1/AgBF4.
| Entry | Solvent | 1/AgBF4/NBE Molar ratio | [NBE] (M) | t (h) | Yield (%) | Mn × 10−3 g | Mw/Mn g | cis (%) h |
|---|
| 1 | CH2Cl2 | 1/2/350 a | 0.6 | 24 | 6 | 103.8 | 1.60 | 65 |
| 2 | CH2Cl2 | 1/3/350 b | 0.6 | 24 | 8 | 82.3 | 1.80 | 68 |
| 3 | CH2Cl2 | 1/4/350 c | 0.6 | 24 | 22 | 186.7 | 1.25 | 62 |
| 4 | CH2Cl2 | 1/4/500 d | 0.8 | 1 | 15 | 810.5 | 1.34 | 42 |
| 5 | CH2Cl2 | 1/4/500 d | 0.8 | 2 | 94 | 547.1 | 1.28 | 48 |
| 6 | CH2Cl2 | 1/4/500 e | 1.7 | 0.4 | 98 | - i | - i | - i |
| 7 | THF | 1/4/500 e | 1.7 | 24 | - f | - f | - f | - f |
| 8 | CH3CN | 1/4/500 e | 1.7 | 24 | - f | - f | - f | - f |
Table 4.
Polymerization of 5-vinyl-2-norbornene (VNBE) with the catalytic system 1/AgBF4.
Table 4.
Polymerization of 5-vinyl-2-norbornene (VNBE) with the catalytic system 1/AgBF4.
| Entry | Solvent | 1/AgBF4/VNBE Molar ratio | [VNBE] (M) | t (h) | Yield (%) | Mn × 10−3 f | Mw/Mn f |
|---|
| 1 | CH2Cl2 | 1/4/500 a | 0.8 | 22 | 10 | - | - |
| 2 | CH2Cl2 | 1/4/500 b | 2.7 | 22 | 20 | - | - |
| 3 | CH2Cl2 | 1/4/500 c | 4.9 | 22 | 17 | - | - |
| 4 | CH2Cl2 | 1/4/1000 d | 2.3 | 26 | Traces | - | - |
| 5 | CH2Cl2 | 1/4/2000 e | 4.9 | 7 | 87 | 8.0 | 1.27 |
| 6 | - | 1/4/2000 e | - | 23 | 39 | 16.7 | 1.16 |
Table 5.
Polymerization of norbornadiene (NBD) with the catalytic system 1/AgBF4.
Table 5.
Polymerization of norbornadiene (NBD) with the catalytic system 1/AgBF4.
| Entry | Solvent | 1/AgBF4/NBD Molar ratio | [NBD] (M) | t (h) | Yield (%) |
|---|
| 1 | CH2Cl2 | 1/2/500 a | 1.7 | 0.75 | 72 |
| 2 | CH2Cl2 | 1/4/500 b | 1.7 | 1.5 | 92 |
| 3 | CH2Cl2 | 1/4/500 c | 3.4 | 21 | 98 |
| 4 | CH2Cl2 | 1/8/500 d | 1.7 | 4.5 | 95 |
| 5 | THF | 1/4/500 b | 1.7 | 22 | - h |
| 6 | THF | 1/4/500 c | 3.4 | 22 | 42 |
| 7 | Toluene | 1/4/500 b | 1.7 | 23 | traces |
| 8 | Toluene | 1/4/1000 e | 3.4 | 23 | traces |
| 9 | Toluene | 1/-/1000 g | 3.4 | 4.5 | 80 |
| 10 | Toluene | 1/-/1000 f | 3.4 | 4.5 | traces |
| 11 | - | 1/4/500 b | - | 22 | traces |
| 12 | - | 1/4/1000 e | - | 3 | traces |
| 13 | - | 1/-/1000 g | - | 22 | traces |
Table 6.
Polymerization of dicyclopentadiene (DCPD) with the catalytic system 1/AgBF4.
Table 6.
Polymerization of dicyclopentadiene (DCPD) with the catalytic system 1/AgBF4.
| Entry | Solvent | 1/AgBF4/DCPD Molar ratio | [DCPD] (M) | t (h) | Yield (%) |
|---|
| 1 | CH2Cl2 | 1/4/250 a | 0.8 | 16 | 95 |
| 2 | CH2Cl2 | 1/4/500 b | 1.4 | 4 | 43 |
| 3 | CH2Cl2 | 1/4/750 c | 1.4 | 22 | 66 |
| 4 | CH2Cl2 | 1/4/750 d | 2.0 | 4 | 9 |
| 5 | CH2Cl2 | 1/4/1000 e | 2.6 | 22 | 15 |
| 6 | Toluene | 1/4/250 a | 0.8 | 22 | Traces |
| 7 | Toluene | 1/4/500 b | 1.4 | 22 | 53 |
| 8 | Toluene | 1/4/750 d | 2.0 | 22 | 52 |
| 9 | Toluene | 1/4/1000 e | 2.6 | 22 | 30 |
| 10 | - | 1/4/250 a | - | 22 | 37 |
| 11 | - | 1/4/500 b | - | 22 | 10 |
| 12 | - | 1/4/750 d | - | 21 | 17 |
| 13 | - | 1/4/1000 e | - | 21 | 47 |
| 14 | Toluene | 1/-/750 f | 2.1 | 24 | 10 |
| 15 | Toluene | 1/-/1250 f | 3.0 | 24 | 10 |
Scheme 3.
Metathesis polymerization of PA and ROMP of NBE, VNBE, NBD and DCPD.
Scheme 3.
Metathesis polymerization of PA and ROMP of NBE, VNBE, NBD and DCPD.
Polymerization of PA by
1/AgBF
4 was studied in toluene, CH
3CN, CH
2Cl
2, and THF (
Table 2).
1/AgBF
4 was inactive in toluene and CH
3CN (entries 11 and 12). Toluene is a very poor solvent for both
1 and AgBF
4. CH
3CN is a strongly coordinating solvent and most likely prevented coordination of substrate to the bimetallic core. In CH
2Cl
2 (entries 1–3) similar results were obtained under all reaction conditions. The color of the reaction mixture gradually changed from green to the characteristic deep red color of polyphenylacetylene (PPA), but very low molecular weight polymers were isolated in low yields. Polymerization proceeded smoothly in THF when two equivalents of AgBF
4 were added, providing PPA in high yield after 24 h (entries 4–6). When the AgBF
4/
1 molar ratio was raised to 3/1, the polymerization proceeded faster and maximum yield was obtained after 6 h (entry 8). The molecular weight of PPA was higher than that of PPA obtained in CH
2Cl
2, whereas the molecular weight distribution was not much altered. At longer reaction times (entries 9 and 10), lower yields, lower molecular weights, and broader molecular weight distributions were observed. That could be attributed to depolymerization of PPA due to secondary metathesis and uncontrolled chain-transfer reactions, as was also observed with the catalytic system Na[W
2(μ-Cl)
3Cl
4(THF)
2]·(THF)
3 (
2) [
18]. Interestingly, the stereochemistry of polymers obtained changed from
all-trans in CH
2Cl
2 to
high-cis (77%–87%) in THF (
Figure S7). Tuning the stereoselectivity of a catalytic system simply by changing the solvent is not frequently encountered.
2 exhibited similar behavior and provided mixtures of
cis and
trans PPA in CH
2Cl
2 and
high-cis (90%) polymers in THF [
18]. The molecular weights of PPA formed by both catalytic systems in THF were very similar, but the molecular weight distribution of PPA obtained in this this study was narrower. In CH
2Cl
2 the two catalytic systems differ significantly; as with
1, reaction rate and molecular weights obtained were significantly lower.
ROMP of NBE was studied in CH
3CN, THF and CH
2Cl
2 (
Table 3). The reaction did not proceed in THF and CH
3CN (entries 7 and 8). That could be attributed to: (a) the low solubility of
1; and (b) the high coordinating ability of those solvents. In CH
2Cl
2 maximum yield was obtained for molar ratio
1/AgBF
4/NBE equal to 1/4/500 (entries 5 and 6) with the reaction being faster at higher NBE concentrations (entry 6). The reaction was complete within minutes and provided polynorbornene (PNBE) that was insoluble in common organic solvents, probably because of very high molecular weight. In more dilute solutions and with various AgBF
4/
1 molar ratios, polymerization was slower and yields were low to moderate (entries 1–4). The configuration of soluble polymers was determined by
1H NMR spectroscopy (
Figure S8) [
19]. The fraction of
cis double bonds (σ
c = 0.62) was estimated by integration of the signals at δ
H 2.79 (HC
1,4 cis-PNBE) and 2.43 ppm (HC
1,4 trans-PNBE). By comparison to
2 [
19] or
2/PA [
20], the present catalytic system was less stereoselective, but provided quantitatively very high molecular weight polymers.
VNBE polymerization was studied only in CH
2Cl
2 (
Table 4), because in that solvent the solubility of
1 was high and the polymerization of NBE was more efficient. The reaction rendered almost quantitative within a few hours (entry 5), when high molar ratio of VNBE/
1 and high [VNBE] were employed, but the molecular weight of poly(5-vinyl-2-norbornene) (PVNBE) obtained was low. At lower concentrations yields remained low, even after long reaction times (entries 1–4). In bulk, PVNBE with low molecular weight and very narrow molecular weight distribution was obtained in moderate yield (entry 6). The
1H NMR spectrum of PVNBE (
Figure S9) could not provide information about the configuration of the polymer, because signals of olefinic protons of the polymeric chain overlap with the vinylic ones, but indicated that the ring-strained C=C bond was cleaved, while the vinylic one was left intact and available for functionalization [
23]. The same reactivity was observed with
2 [
19] and
2/PA [
20] and is rather unusual. The pendant vinyl group is usually involved in metathesis reactions, leading to cross-linked products; therefore, that monomer is used for the synthesis of self-healing polymers [
24]. Other than that, the reactivity of the catalytic systems was different, as very high (974,000) and high (97,000) molecular weight polymers were obtained with
2 and
2/PA, respectively, although with much broader molecular weight distributions (2.6).
CH
2Cl
2 was found to be the optimum solvent for the ROMP of NBD (
Table 5) as well. The reaction provided high yield of polynorbornadiene (PNBD) with molar ratio
1/AgBF
4 equal to 1/2 (entry 1) and was quantitative, or almost quantitative, when molar ratios
1/AgBF
4 equal to 1/4 or higher were employed (entries 2–4), but the rate was maximum when NBD concentration was equal to 1.7 M (entry 2),
i.e., under conditions identical to the polymerization of NBE. In THF, no reaction took place under the same conditions (entry 5), and when NBD concentration was higher (3.4 M), moderate yields were obtained (entry 6). In toluene, traces of polymer were obtained even after 23 h (entries 7 and 8). Interestingly, with
1/NBD molar ratio equal to 1/1000, without adding AgBF
4, and under reflux, PNBD was obtained in high yield (80%; entry 9). Under similar conditions, but at room temperature, the reaction provided traces of PNBD (entry 10). Such reactivity resembles that of latent catalysts [
25]. In bulk, traces of polymers were obtained in the presence or absence of AgBF
4, and even under reflux (entries 11–13). Molecular weights of polymers obtained could not be determined with size exclusion chromatography (SEC), because the polymers were insoluble. Differential thermogravimetry showed a bimodal decomposition peak at high temperatures (452 and 462 °C, respectively), indicating a high degree of crosslinking and a complex mechanism of thermal decomposition.
DCPD polymerization was studied in CH
2Cl
2, toluene, and in bulk (
Table 6). Quantitative yield was obtained in CH
2Cl
2, with molar ratio
1/DCPD equal to 1/250 (entry 1). When molar ratio and DCPD concentration were increased, yields were lowered (entries 2–5) and reaction times increased (entries 3 and 5). In toluene, after 22 h, yields were at best moderate. For molar ratios
1/DCPD 1/500 and 1/750 (entries 7 and 8), polymer in almost 50% yield was obtained, while increasing or decreasing the ratio gave lower yields (entries 6 and 9). In contrast, polymers in 40%–50% yield were obtained in bulk, with ratios
1/DCPD 1/250 and 1/1000 (entries 10 and 13). With molar ratios
1/DCPD 1/500 and 1/750 the yields were much lower (entries 11 and 12). Two reactions in the absence of co-catalyst and with heating were made under conditions similar to those in the polymerization of NBD, but the yields were very low in both cases, despite the long reaction time (entries 14 and 15). Molecular weights of polydicyclopentadiene (PDCPD) obtained could not be determined with SEC, because polymers were insoluble. Differential thermogravimetry showed a bimodal decomposition peak at high temperatures (462 and 470 °C respectively), indicating high degree of crosslinking and a complex mechanism of thermal decomposition, as in the case of PNBD polymers.
Regarding the ROMP of NBD, catalytic system
1/AgBF
4 was almost equally reactive to
2, which also provided quantitative yields within short reaction times [
19,
20]. In addition, it showed similar reactivity with
2/PA towards the ROMP of DCPD [
20], which seems to be the least reactive of the monomers studied, as long reaction times were required with either system.
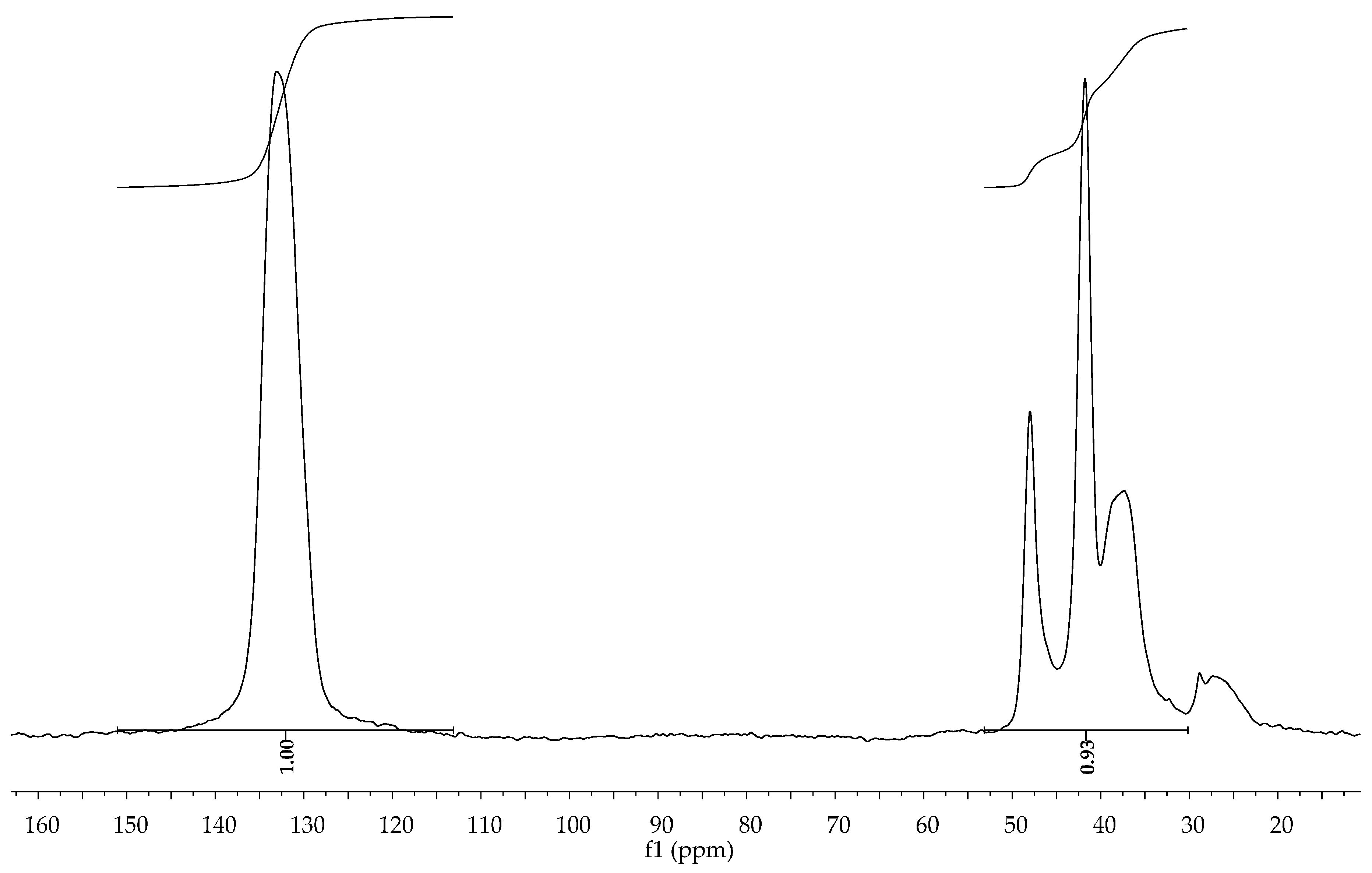
Polymer characterization for insoluble polymers PNBD and PDCPD was done using
13C CPMAS spectroscopy (
Figure 2 and
Figure 3). Peaks at 133 (PNBD) and 129 (PDCPD) ppm are due to olefinic carbons, and peaks in the regions 30–53 (PNBD) and 26–60 ppm (PDCPD) are due to aliphatic carbons.
Scheme 3 shows all possible products of NBD and PDCPD polymerization via ROMP. Polymers obtained may be linear or cross-linked. Cross-linked polymers are formed by reactions taking place on the double bond of the cyclopentene ring. Those can be either metathetic or olefin coupling reactions and have been well-studied for PDCPD [
26]. The ratio of olefinic/aliphatic carbons depends on the mechanism of cross-linking and is equal to 4/3 (metathetic) and 2/5 (olefin coupling) for PNBD and 2/3 (metathetic) and 1/4 (olefin coupling) for PDCPD. Therefore, the olefin coupling contribution can be calculated by integration of the corresponding
13C CPMAS peaks, and by using the following equations for PNBD (Equation (1)) and PDCPD (Equation (2)) [
27]. In those equations C
olefinic refers to carbons of double bonds, C
aliphatic to carbons of single bonds, and
x is the fraction of polymer double bonds that participate in cross-linking via olefin coupling. Integration of the PNBD spectrum provided a ratio of 1/0.93 and integration of the PDCPD spectrum provided a ratio of 1/1.81. By replacing the experimental values in Equations (1) and (2);
x = 0.19 for PNBD and
x = 0.22 for PDCPD,
i.e., 19% of NBD and 22% of DCPD double bonds participated in crosslinking via olefin coupling.
Figure 2.
13C Cross Polarization Magic Angle Spinning (CPMAS) spectrum of PNBD obtained from the reaction of
1/AgBF
4/NBD (
Table 5, entry 4) in CH
2Cl
2.
Figure 2.
13C Cross Polarization Magic Angle Spinning (CPMAS) spectrum of PNBD obtained from the reaction of
1/AgBF
4/NBD (
Table 5, entry 4) in CH
2Cl
2.
Figure 3.
13C CPMAS spectra of PDCPD obtained from the reaction of
1/AgBF
4/DCPD (
Table 6, entry 1) in CH
2Cl
2.
Figure 3.
13C CPMAS spectra of PDCPD obtained from the reaction of
1/AgBF
4/DCPD (
Table 6, entry 1) in CH
2Cl
2.
3.3. Kinetic Studies
The progress of reaction of each monomer, with the exception of NBE polymerization, which was very rapid, was monitored by measuring the polymerization yield gravimetrically, and the molecular characteristics of the polymers formed (number average molecular weight and molecular weight distribution) by SEC analysis. Results for PA polymerization in THF using a molar ratio
1/AgBF
4/PA equal to 1/3/350 are displayed in
Table S2 and
Figure 4. It is obvious that the yield increased more or less linearly with time up to quantitative conversion in 6 h. In the same time the molecular weight increased to high values, whereas the molecular weight distribution diminished rapidly. Longer polymerization times, up to 24 h, lead to scission of the produced polymeric chains and therefore to lower yields of polymerization along with lower molecular weights and broader distributions. This behavior is similar to that observed during the polymerization of PA with the triply bonded complex
2 in THF solutions [
18]. In the present study, the maximum yield and molecular weight were observed in ~6 h of polymerization, whereas in the previous study in ~2 h. Therefore,
1 polymerized PA with a lower rate, but the reaction was more controlled, leading to products of higher molecular weight and considerably smaller molecular weight distributions.
Figure 4.
Polymerization of PA (653 μL, 608 mg, 6.0 mmol) with 1 (30.0 mg, 0.017 mmol), AgBF4 (9.9 mg, 0.051 mmol) and 10 mL THF (25 °C); (a) % yield vs. time plot of PPA; (b) Mn × 10−3 vs. time plot.
Figure 4.
Polymerization of PA (653 μL, 608 mg, 6.0 mmol) with 1 (30.0 mg, 0.017 mmol), AgBF4 (9.9 mg, 0.051 mmol) and 10 mL THF (25 °C); (a) % yield vs. time plot of PPA; (b) Mn × 10−3 vs. time plot.
Variation of polymerization yield with time for NBD is given in
Figure 5, whereas data are displayed in
Table S3. Apparently, the system was characterized by an induction period, which was equal to a few minutes. This period was devoted to the complexation of the monomer to the catalyst and the initiation step of the polymerization process. After this period, the yield increased linearly with time. Nearly quantitative yields were obtained after 90 min of reaction. This result indicates that both the polymerization reaction through the opening of the first double bond and the cross-linking reaction occuring at the second double bond proceeded smoothly with time and simultaneously in the same manner leading to a controlled synthesis of cross-linked PNBD.
Figure 5.
Polymerization of NBD (0.8 mL, 725.0 mg, 8.5 mmol) with 1 (30 mg, 0.017 mmol), AgBF4 (13.3 mg, 0.069 mmol) and 5 mL CH2Cl2 (25 °C); % yield vs. time plot of PNBD.
Figure 5.
Polymerization of NBD (0.8 mL, 725.0 mg, 8.5 mmol) with 1 (30 mg, 0.017 mmol), AgBF4 (13.3 mg, 0.069 mmol) and 5 mL CH2Cl2 (25 °C); % yield vs. time plot of PNBD.
The kinetics of polymerization of VNBE was studied in CH
2Cl
2 solutions, as shown in
Table S4 and
Figure 6. As in the case of NBD polymerization, an induction period was also observed for VNBE polymerization, indicating that the same mechanism took place in both cases. Compared to NBD, the initiation, as well as the propagation reaction, proceeded in a slower manner, probably due to the increased steric hindrance of VNBE. However, the yield scaled linearly with time indicating that the polymerization reaction proceeded in a well-controlled way.
Figure 6.
Polymerization of VNBE (4.9 mL, 4.1 g, 34.0 mmol) with 1 (30 mg, 0.017 mmol), AgBF4 (13.2 mg, 0.068 mmol) and 2 mL CH2Cl2; % yield vs. time plot of PVNBE.
Figure 6.
Polymerization of VNBE (4.9 mL, 4.1 g, 34.0 mmol) with 1 (30 mg, 0.017 mmol), AgBF4 (13.2 mg, 0.068 mmol) and 2 mL CH2Cl2; % yield vs. time plot of PVNBE.
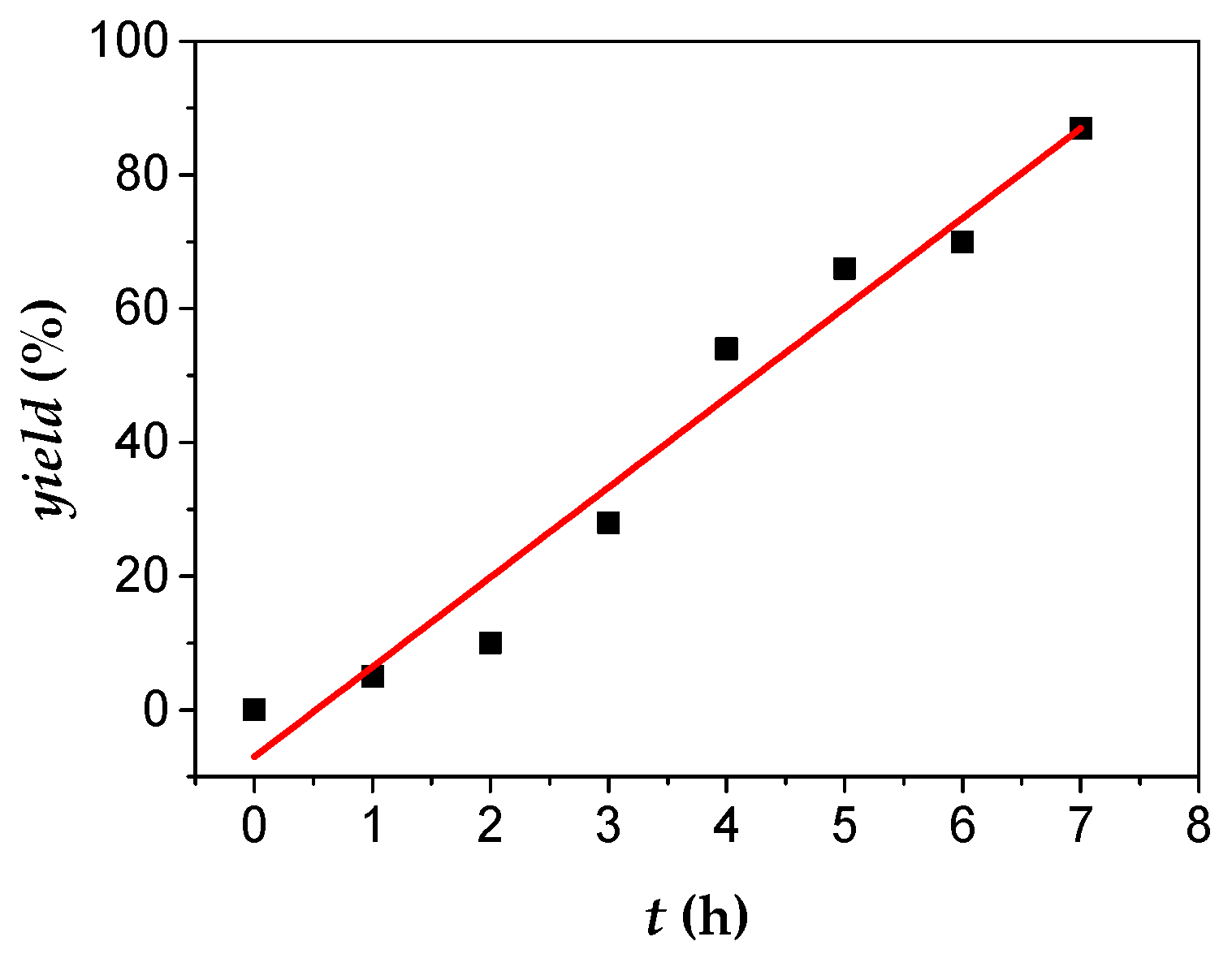
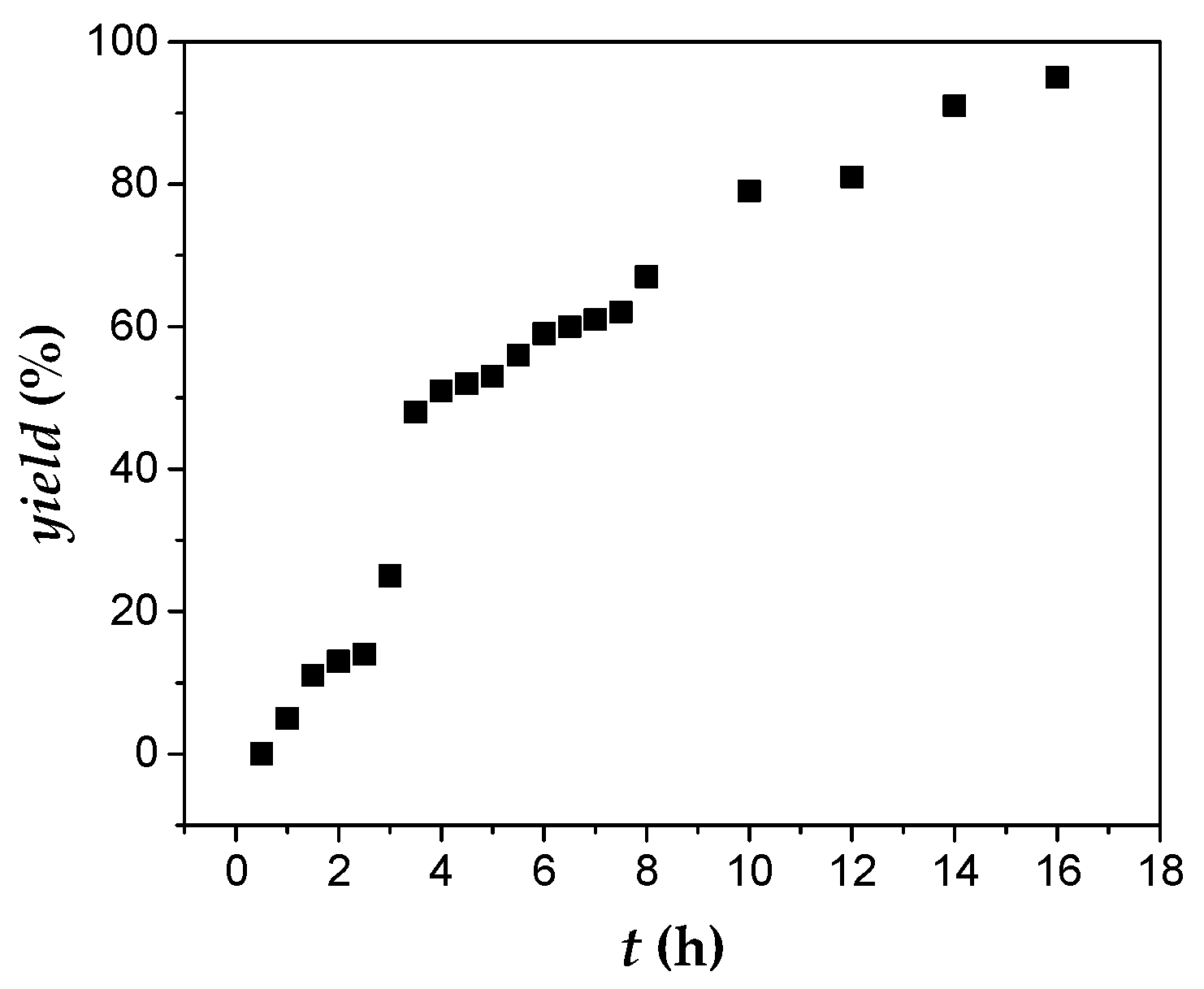
The initiation reaction for DCPD was very fast, since the plot of yield
vs. time passed through the origin, as shown in
Figure 7 and
Table S5. The polymerization rate was initially very fast without the presence of an appreciable induction period. However, upon progressing time, the rate of polymerization was substantially lowered. Compared to other monomers examined in this work, DCPD was the less reactive, probably due to the increased steric hindrance of this monomer. Retardation of the polymerization may be attributed to the increased time needed for the activation of the second double bond of the monomer leading to cross-linked products.
Figure 7.
Polymerization of DCPD (0.6 mL, 552.0 mg, 4.2 mmol) with 1 (30.0 mg, 0.017 mmol), AgBF4 (13.2 mg, 0.068 mmol) and 5.0 mL CH2Cl2; % yield vs. time plot of PDCPD.
Figure 7.
Polymerization of DCPD (0.6 mL, 552.0 mg, 4.2 mmol) with 1 (30.0 mg, 0.017 mmol), AgBF4 (13.2 mg, 0.068 mmol) and 5.0 mL CH2Cl2; % yield vs. time plot of PDCPD.