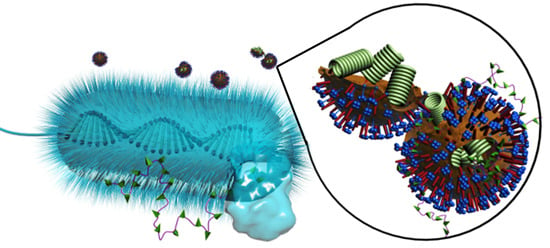
Antibacterial/Antiviral Property and Mechanism of Dual-Functional Quaternized Pyridinium-type Copolymer
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Test Microorganisms
2.3. Characterizations
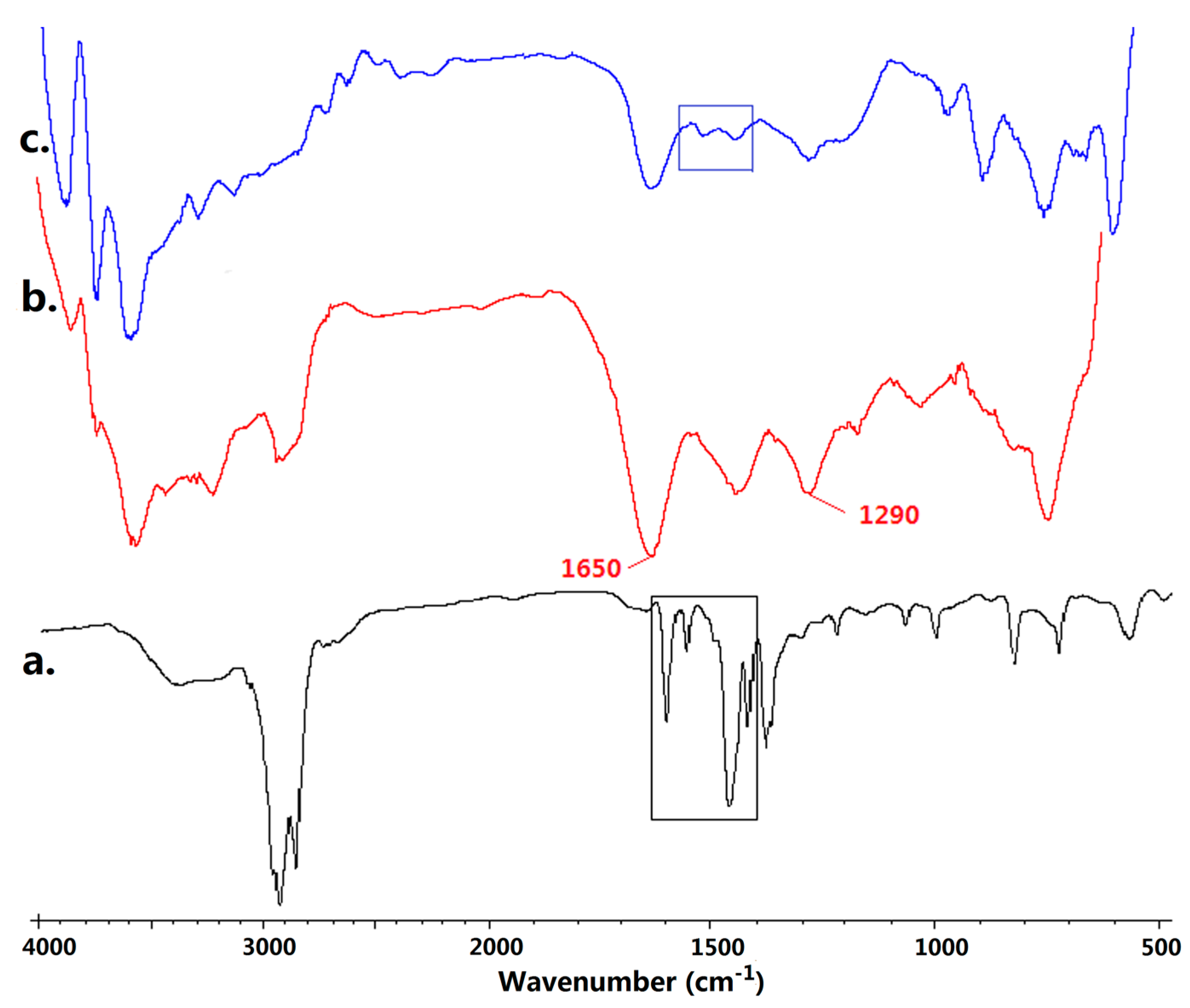
2.3.1. Fourier Transform Infrared (FTIR) Spectroscopy
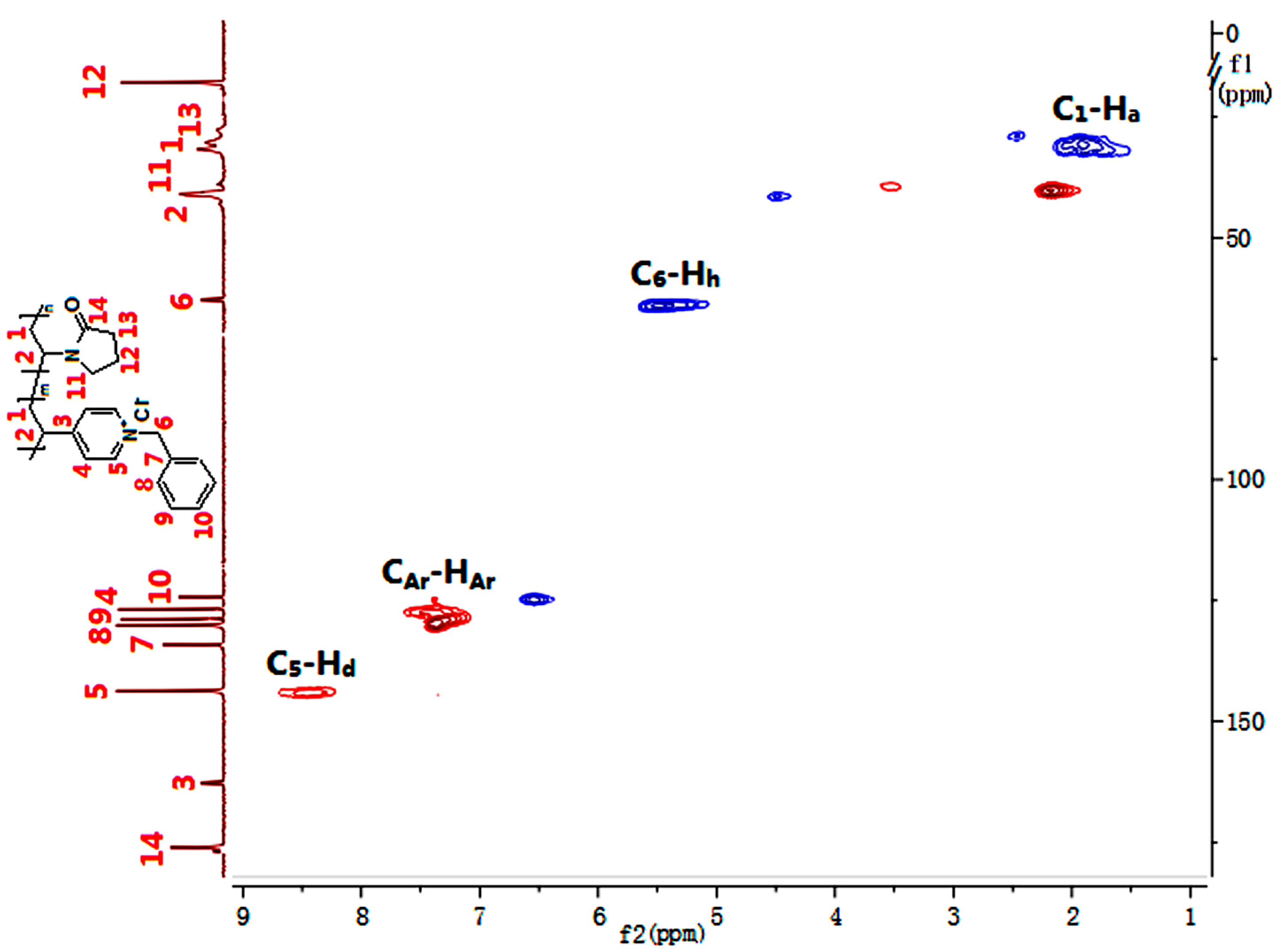
2.3.2. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.3.3. Static Light Scattering (SLS)
2.3.4. Apparent Charge Density
2.3.5. UV Spectrophotometry
2.3.6. Atomic Force Microscopy (AFM) Analysis
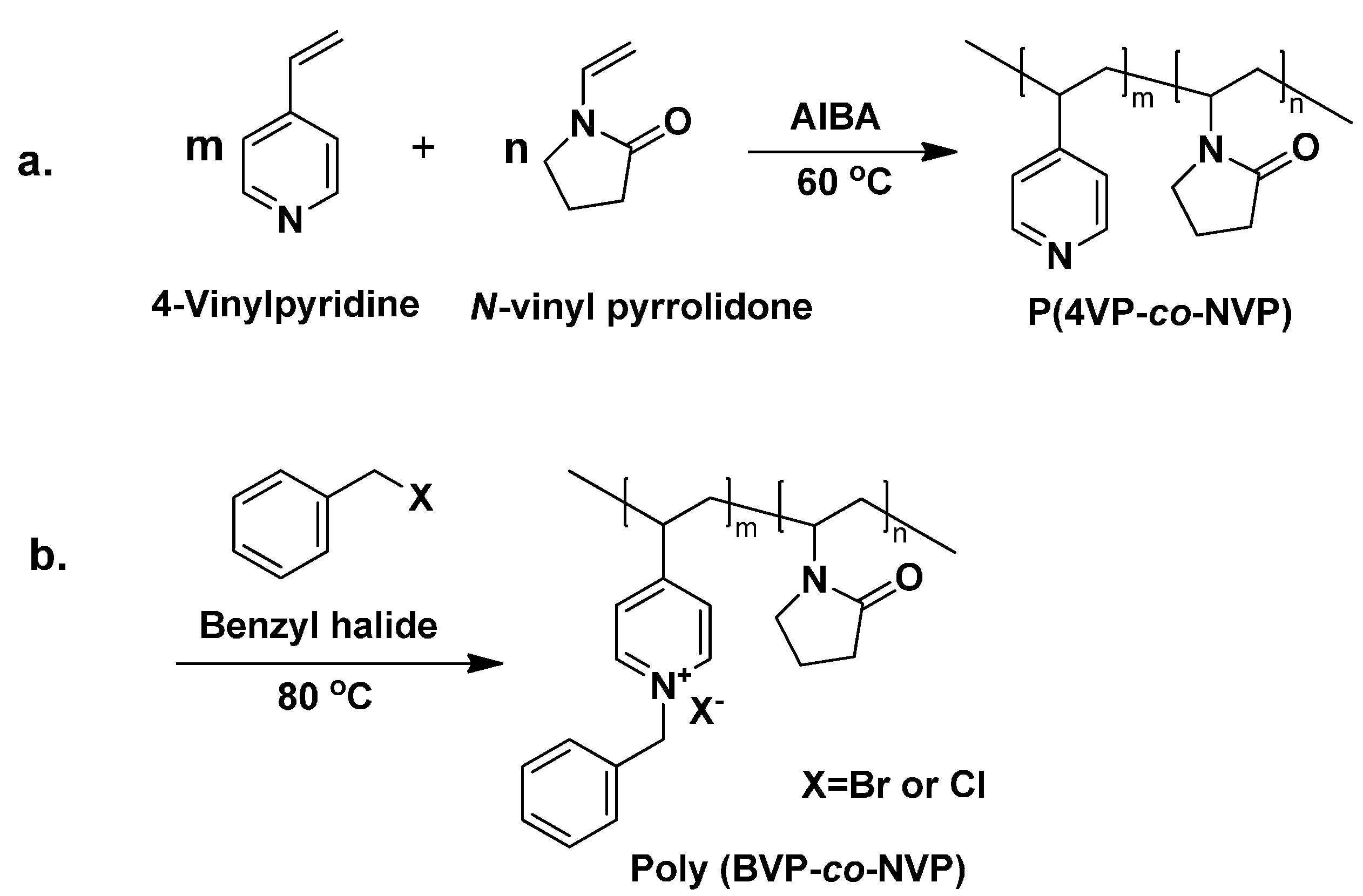
2.4. Copolymerization of 4VP and NVP
2.5. Quaternization of Poly(4VP-co-NVP) via N-Alkylation
2.6. Antibacterial Assessment
2.7. Antiviral Assessment
3. Results and Discussion
3.1. Copolymerization of 4VP and NVP
| Sample | Feed molar ratio of 4VP (mol%) | 4VP content a (mol%) | 4VP content b (mol%) |
|---|---|---|---|
| P4VP | 100 | 100 | 100 |
| Poly(4VP-co-NVP)1 | 50 | 52.8 | 55.9 |
| Poly(4VP-co-NVP)2 | 30 | 34.5 | 35.3 |
| Poly(4VP-co-NVP)3 | 10 | 11.6 | 11.2 |

3.2. Quternization of Poly(4VP-co-NVP)
| Sample | Charge density a (meq/g) | Quaternization efficiency (%) | Mw (kDa) |
|---|---|---|---|
| PBVPC | 3.16 | 55.4 | 94.5 |
| Poly(BVPC-co-NVP)1 | 2.98 | 92.3 | 92.8 |
| Poly(BVPC-co-NVP)2 | 2.24 | 97.4 | 96.9 |
| Poly(BVPC-co-NVP)3 | 0.82 | 90.1 | 93.8 |
| PBVPB | 2.83 | 52.8 | 93.4 |
| Poly(BVPB-co-NVP)1 | 2.60 | 90.4 | 98.5 |
| Poly(BVPB-co-NVP)2 | 1.97 | 94.9 | 95.5 |
| Poly(BVPB-co-NVP)3 | 0.75 | 83.1 | 94.4 |
3.3. Evaluation of Antimicrobial Activity
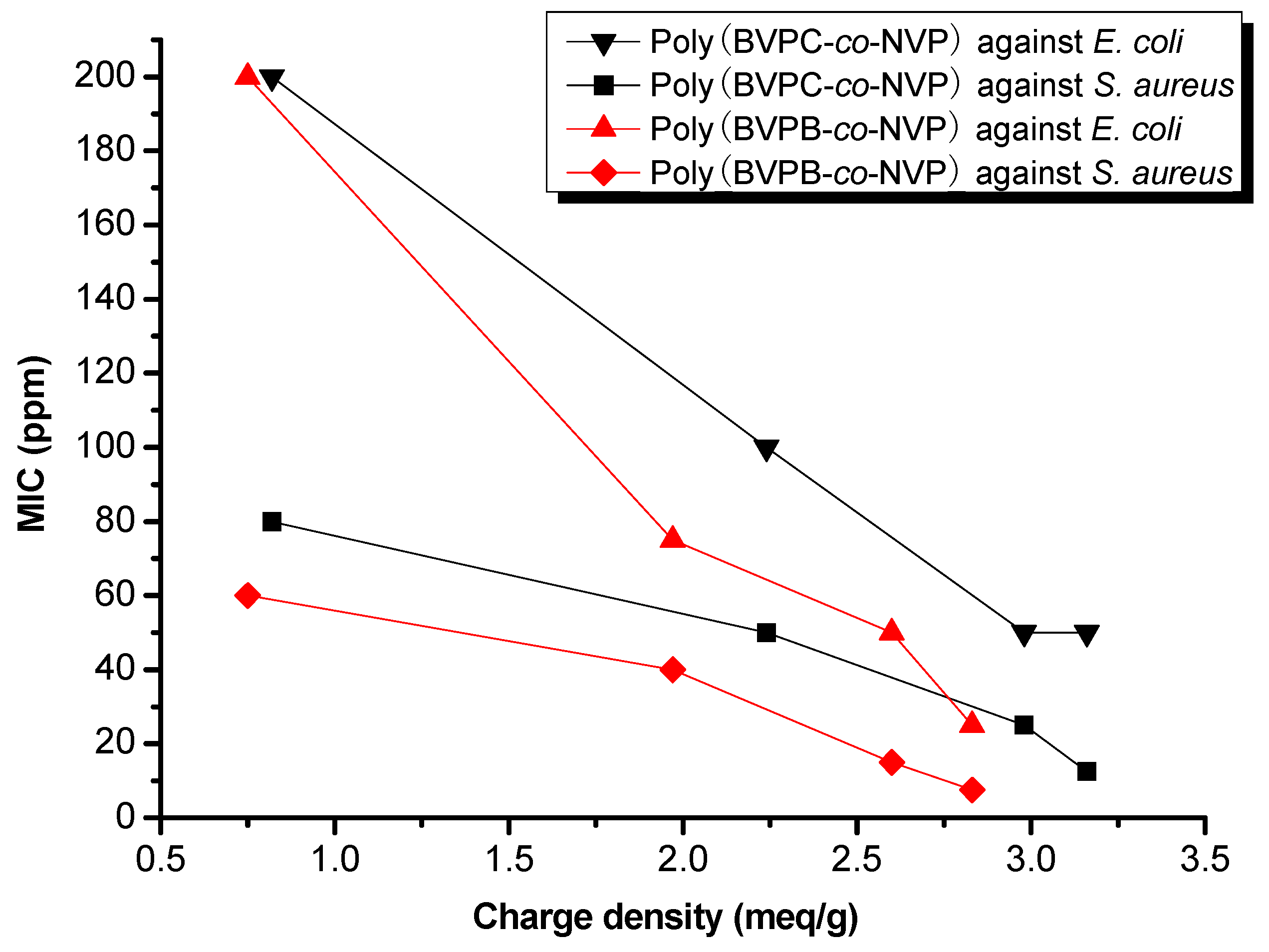
3.4. AFM Analysis
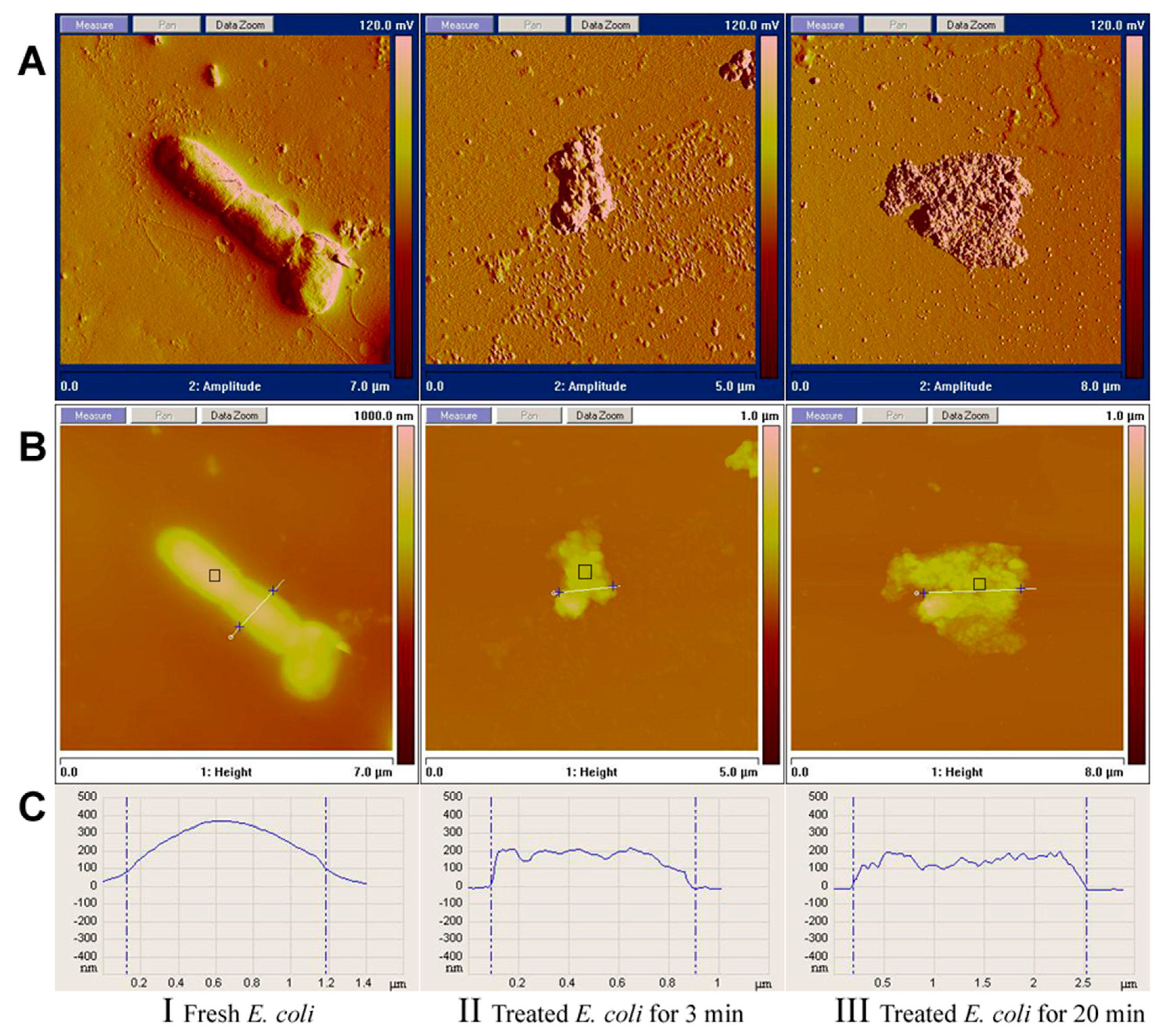
3.5. Virucidal Assessment

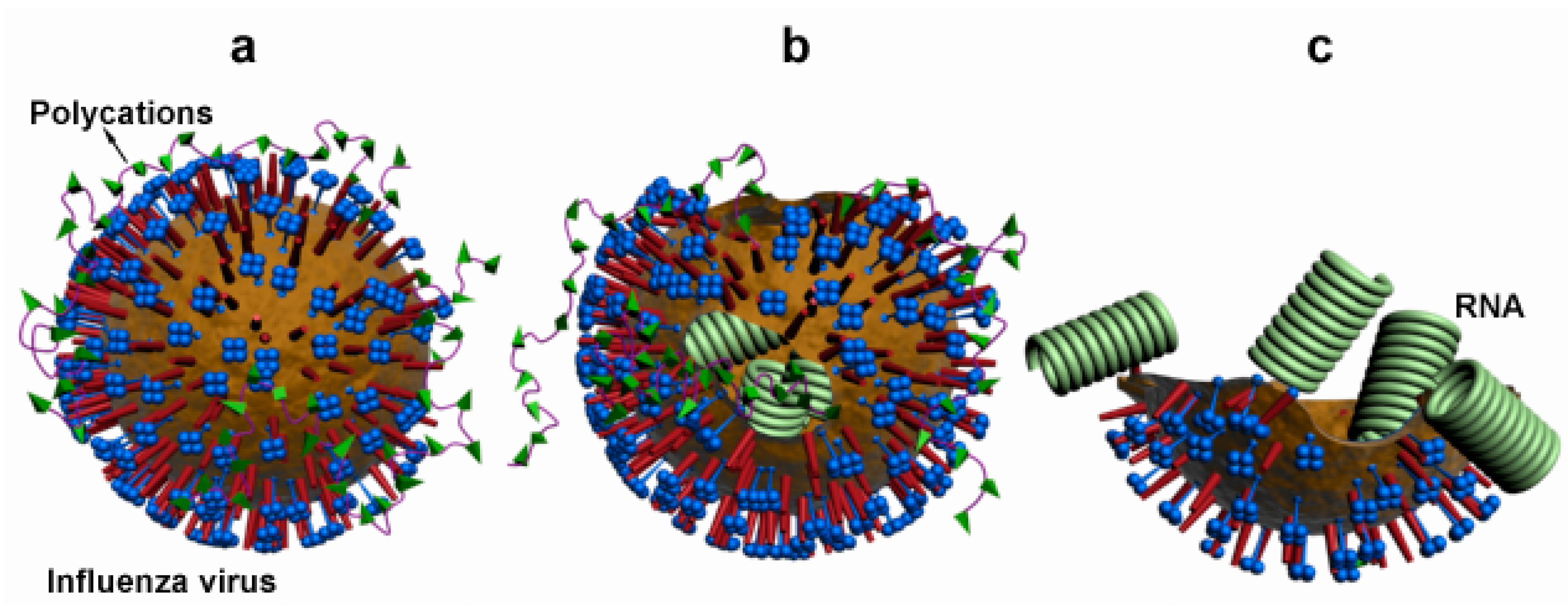
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kenawy, E.; Worley, S.D.; Broughton, R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef] [PubMed]
- Siedenbiedel, F.; Tiller, J.C. Antimicrobial polymers in solution and on surfaces: Overview and functional principles. Polymers 2012, 4, 46–71. [Google Scholar] [CrossRef]
- Malmsten, M. Antimicrobial and antiviral hydrogel. Soft Matter 2011, 7, 8725–8736. [Google Scholar] [CrossRef]
- Huang, J.; Murata, H.; Koepsel, R.R.; Russell, A.J.; Matyjaszewski, K. Antibacterial polypropylene via surface-initiated atom transfer radical polymerization. Biomacromolecules 2007, 8, 1396–1399. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Pan, Y.; Xiao, H.; Zhao, Y. Novel quaternary phosphonium-type cationic polyacrylamide and elucidation of dual-functional antibacterial/antiviral activity. RSC Adv. 2014, 4, 46887–46895. [Google Scholar] [CrossRef]
- Gong, S.; Epasinghe, D.J.; Zhou, B.; Niu, L.; Kimmerling, K.A.; Rueggeberg, F.A.; Yiu, C.K.Y.; Mao, J.; Pashley, D.H.; Tay, F.R. Effect of water-aging on the antimicrobial activities of an ormosil-containing orthodontic acrylic resin. Acta Biomater. 2013, 9, 6964–6973. [Google Scholar] [PubMed]
- Muňoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Chen, Y.; Wilbon, P.A.; Chen, Y.P.; Zhou, J.; Nagarkatti, M.; Wang, C.; Chu, F.; Decho, A.W.; Tang, C. Amphipathic antibacterial agents using cationic methacrylic polymers with natural rosin as pendant group. RSC Adv. 2012, 2, 10275–10282. [Google Scholar] [CrossRef]
- Haldar, J.; An, D.; de Cienfuegos, L.Á.; Chen, J.; Klibanov, A.M. Polymeric coatings that inactivate both influenza virus and pathogenic bacteria. Proc. Natl. Acad. Sci. USA 2006, 103, 17667–17671. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, N.; Ujino, I. Removal of virus from air by filtration using a composite microporous membrane made of crosslinked poly(N-benzyl-4-vinylpyridinium chloride). React. Funct. Polym. 1998, 37, 213–218. [Google Scholar] [CrossRef]
- Kawabata, N.; Yamazaki, K.; Otake, T.; Oishi, I.; Minekawa, Y. Removal of pathogenic human viruses by insoluble pyridinium-type resin. Epidemiol. Infect. 1990, 105, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, N. Syntheses of biodegradable vinyl polymers by insertion of N-benzyl-4-vinylpyridinium chloride into the main chain. React. Funct. Polym. 2007, 67, 1292–1300. [Google Scholar] [CrossRef]
- Cen, L.; Neoh, K.G.; Kang, E.T. Surface functionalization technique for conferring antibacterial properties to polymeric and cellulosic surfaces. Langmuir 2003, 19, 10295–10303. [Google Scholar] [CrossRef]
- Yuan, S.J.; Pehkonen, S.O.; Ting, Y.P.; Neoh, K.G.; Kang, E.T. Inorganic–organic hybrid coatings on stainless steel by layer-by-layer deposition and surface-initiated atom-transfer-radical polymerization for combating biocorrosion. ACS Appl. Mater. Interfaces 2009, 1, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Ward, R.J.; Hexemer, A.; Sohn, K.E.; Lee, K.L.; Angert, E.R.; Fischer, D.A.; Kramer, E.J.; Ober, C.K. Surfaces of fluorinated pyridinium block copolymers with enhanced antibacterial activity. Langmuir 2006, 22, 11255–11266. [Google Scholar] [CrossRef] [PubMed]
- Tiller, J.C.; Liao, C.-J.; Lewis, K.; Klibanov, A.M. Designing surfaces that kill bacteria on contact. Proc. Natl. Acad. Sci. USA 2001, 98, 5981–5985. [Google Scholar] [CrossRef] [PubMed]
- Dizman, B.; Elasri, M.O.; Mathias, L.J. Synthesis and characterization of antibacterial and temperature responsive methacrylamide polymers. Macromolecules 2006, 39, 5738–5746. [Google Scholar] [CrossRef]
- Eren, T.; Som, A.; Rennie, J.R.; Nelson, C.F.; Urgina, Y.; Nüsslein, K.; Coughlin, E.B.; Tew, G.N. Antibacterial and hemolytic activities of quaternary pyridinium functionalized polynorbornenes. Nat. Rev. Microbiol. 2008, 209, 516–524. [Google Scholar] [CrossRef]
- Sambhy, V.; Peterson, B.R.; Sen, A. Antibacterial and hemolytic activities of pyridinium polymers as a function of the spatial relationship between the positive charge and the pendant alkyl tail. Angew. Chem. Int. Ed. 2008, 47, 1250–1254. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.G.; Wang, X.; Li, X.Y.; Chian, W.; Li, Y.; Liao, Y.Z. Electrospun biphasic drug release polyvinylpyrrolidone/ethyl cellulose core/sheath nanofibers. Acta Biomater. 2013, 9, 5665–5672. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhu, L.; Zhu, L.; Zhang, H.; Zhu, B.; Xu, Y. Antifouling and antimicrobial polymer membranes based on bioinspired polydopamine and strong hydrogen-bonded poly(N-vinyl pyrrolidone). ACS Appl. Mater. Interfaces 2013, 5, 12895–12904. [Google Scholar] [CrossRef] [PubMed]
- Gizdavic-Nikolaidis, M.R.; Bennett, J.R.; Swift, S.; Easteal, A.J.; Ambrose, M. Broad spectrum antimicrobial activity of functionalized polyanilines. Acta Biomater. 2011, 7, 4204–4209. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xi, X.; Shi, J.; Cao, S. A homogeneous catalyst made of poly(4-vinylpyridine-co-N-vinylpyrrolidone)-Pd(0) complex for hydrogenation of aromatic nitro compounds. J. Mol. Catal. A Chem. 2000, 160, 287–292. [Google Scholar] [CrossRef]
- Gatica, N.; Gargallo, L.; Radić, D. 2-Vinylpyridine-co-N-vinyl-2-pyrrolidone copolymers: Synthesis and reactivity ratios. Polym. Int. 1998, 45, 285–290. [Google Scholar] [CrossRef]
- Cheng, L.; Weir, M.D.; Xu, H.H.K.; Antonucci, J.M.; Kraigsley, A.M.; Lin, N.J.; Lin-Gibson, S.; Zhou, X. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent. Mater. 2012, 28, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Majumdar, P.; Chisholm, B.; Stafslien, S.; Chen, Z. Antifouling and antimicrobial mechanism of tethered quaternary ammonium salts in a cross-linked poly(dimethylsiloxane) matrix studied using sum frequency generation vibrational spectroscopy. Langmuir 2010, 26, 16455–16462. [Google Scholar] [CrossRef] [PubMed]
- Shalev, T.; Gopin, A.; Bauer, M.; Stark, R.W.; Rahimipour, S. Non-leaching antimicrobial surfaces through polydopamine bio-inspired coating of quaternary ammonium salts or an ultrashort antimicrobial lipopeptide. J. Mater. Chem. 2012, 22, 2026–2032. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhou, Z.; Zhu, J.; Tang, Y.; Canady, T.D.; Chi, E.Y.; Schanze, K.S.; Whitten, D.G. Dark antimicrobial mechanisms of cationic phenylene ethynylene polymers and oligomers against Escherichia coli. Polymers 2011, 3, 1199–1214. [Google Scholar] [CrossRef]
- Xue, Y.; Guan, Y.; Zheng, A.; Wang, H.; Xiao, H. Synthesis and characterization of ciprofloxacin pendant antibacterial cationic polymers. J. Biomat. Sci. Polym. Ed. 2012, 23, 1115–1128. [Google Scholar] [CrossRef] [PubMed]
- Luczak, J.; Jungnickel, C.; Lacka, I.; Stolte, S.; Hupka, J. Antimicrobial and surface activity of 1-alkyl-3-methylimidazolium derivatives. Green Chem. 2010, 12, 593–601. [Google Scholar] [CrossRef]
- Yudovin-Farber, I.; Beyth, N.; Weiss, E.I.; Domb, A.J. Antibacterial effect of composite resins containing quaternary ammonium polyethyleneimine nanoparticles. J. Nanopart. Res. 2010, 12, 591–603. [Google Scholar] [CrossRef]
- Chen, C.Z.; Beck-Tan, N.C.; Dhurjati, P.; van Dyk, T.K.; LaRossa, R.A.; Cooper, S.L. Quaternary ammonium functionalized poly(propylene imine) dendrimers as effective antimicrobials: Structure-activity studies. Biomacromolecules 2000, 1, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Weng, Y.; Guo, X.; Zhao, J.; Gregory, R.L.; Zheng, C. Preparation and evaluation of a novel glass-ionomer cement with antibacterial functions. Dent. Mater. 2011, 27, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, N.; Kahne, D.; Silhavy, T.J. Advances in understanding bacterial outer-membrane biogenesis. Nat. Rev. Microbiol. 2006, 4, 57–66. [Google Scholar] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Koepsel, R.R.; Matyjaszewski, K.; Russell, A.J. Permanent, non-leaching antibacterial surface 2: How high density cationic surfaces kill bacterial cells. Biomaterials 2007, 28, 4870–4879. [Google Scholar] [CrossRef] [PubMed]
- Asri, L.A.T.W.; Crismaru, M.; Roest, S.; Chen, Y.; Ivashenko, O.; Rudolf, P.; Tiller, J.C.; van der Mei, H.C.; Loontjens, T.J.A.; Busscher, H.J. A shape-adaptive, antibacterial-coating of immobilized quaternary-ammonium compounds tethered on hyperbranched polyurea and its mechanism of action. Adv. Funct. Mater. 2014, 24, 346–355. [Google Scholar] [CrossRef]
- Harrison, S.C. Viral membrane fusion. Nat. Struct. Mol. Biol. 2008, 15, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.E.; Helenius, A. How viruses enter animal cells. Science 2004, 304, 237–242. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Xiao, H. Antibacterial/Antiviral Property and Mechanism of Dual-Functional Quaternized Pyridinium-type Copolymer. Polymers 2015, 7, 2290-2303. https://doi.org/10.3390/polym7111514
Xue Y, Xiao H. Antibacterial/Antiviral Property and Mechanism of Dual-Functional Quaternized Pyridinium-type Copolymer. Polymers. 2015; 7(11):2290-2303. https://doi.org/10.3390/polym7111514
Chicago/Turabian StyleXue, Yan, and Huining Xiao. 2015. "Antibacterial/Antiviral Property and Mechanism of Dual-Functional Quaternized Pyridinium-type Copolymer" Polymers 7, no. 11: 2290-2303. https://doi.org/10.3390/polym7111514