1. Introduction
Poly(vinyl chloride) (PVC) is one of the largest commodity thermoplastics for various commercial and domestic applications, with 39.3 million metric tons consumed in 2013 [
1]. PVC by itself has limited use because it is too rigid, with inadequate flexibility, posing difficulties in melt processing, in addition to having low thermal stability [
2,
3,
4]. To overcome these limitations, plasticizer is needed in the fabrication of the various PVC products. Adding a plasticizer modifies the mechanical and thermal properties of PVC by intercalating the PVC polymeric chain and thereby reduces the cohesive interaction forces between the polymeric chains. Thus, a plasticizer increases the mobility of each PVC chain, thereby making the polymer softer and easier to process. The utilization of PVC greatly depends on the formulation of additives, plasticizers, and fillers that are incorporated to produce the end product [
5,
6,
7].
Phthalate esters are the conventional petrochemical-based plasticizers for PVC. The esters, di-octyl phthalate (DOP) and di-isononyl phthalate (DiNP) are the most commonly and widely used plasticizers for PVC. Plasticized PVC is extensively used for packaging, toys, household products, and medical products such as blood bags, tubing, and catheters [
6,
7]. However, phthalate esters are not chemically bound to PVC. Consequently, phthalate esters can migrate to the surface and leach out from the PVC matrix to accumulate in the environment or in organisms via direct contact with skin and tissue. Hence, the effects and consequences of the use of phthalates have been of great concern in recent years.
Phthalate esters were reported to cause adverse effects to human health, including changes in hormone levels, causing respiratory difficulties, reproductive malformations, chronic toxicities, and hepatic tumorigenesis [
8,
9,
10,
11,
12,
13,
14,
15]. For these reasons, the use of several phthalate esters is restricted in the European Union, particularly in their application in PVC toys for young children [
8]. Given the increasing concerns of the adverse effects of phthalate esters, increased interest has been expressed and extensive research has been carried out in the development of safer plasticizers with lower toxicity and migration rates. These plasticizers could be more economically and technically viable as substitutes of petrochemical-based phthalate plasticizers for medical and other commodity PVC products [
16]. Renewable resources, such as cardanol acetate [
17], epoxidized vegetable oil [
18,
19] (including epoxidized soya bean oil and epoxidized sunflower oil), glucose ester [
20], and furandicarboxylate ester [
21] have been extensively investigated as alternative plasticizers for PVC.
Recently, a palm oil-based alkyd synthesized from palm stearin has been developed as a bio-based plasticizer of PVC [
22]. Palm stearin was selected for the production of the alkyd for economic reasons, as stearin is a by-product in the process of isolating palm olein from the oil palm fruit. The alkyd possesses biodegradable, biocompatible, and non-toxic properties. It is a polar compound with several functional groups, such as –COOH, –C=O, and –OH, to provide polar interaction with the –CH–Cl group in PVC. The palm oil-based alkyd is a relatively large molecule and has better migration resistance compared with the conventional phthalate-based plasticizers. The latter are small molecules not chemically bonded to PVC. The palm oil-based alkyd, with its higher molecular weight and longer polymeric side chains, can entangle with the PVC molecules, which can prevent its migration out of the PVC matrix.
This work investigates the application of the palm oil-based alkyd as a co-plasticizer for PVC by partial substitution of DOP or DiNP. The plasticized PVC was investigated for the film morphology, thermal and mechanical properties.
2. Experimental Section
2.1. Materials
The pure PVC resin powders were PVC (TL-700) and PVC (TL-1000) with K values of 57–58.8 and 65–67, respectively (K value is an indicator of molecular weight and degree of polymerization). Both powders were supplied by Tianjin LG Dagu Chemical Co., Ltd. (LG-DAGU, Tianjin, China). The industrial grade DOP and DiNP and the heat stabilizer, barium zinc (LX-BZ828), were purchased from LuxChem Trading Sdn Bhd, Selangor, Malaysia. For the alkyd synthesis, the palm stearin and glycerol (99.5%) were obtained from Emery Oleochemical Malaysia Sdn Bhd, Banting, Malaysia. Phthalic anhydride was obtained from Merk (Hohenbrunn, Germany), Ca(OH)2 from HmbG Chemicals (Hamburg, Germany). All the materials involved in this study were used as received without any further purification.
2.2. Preparation of the Alkyd
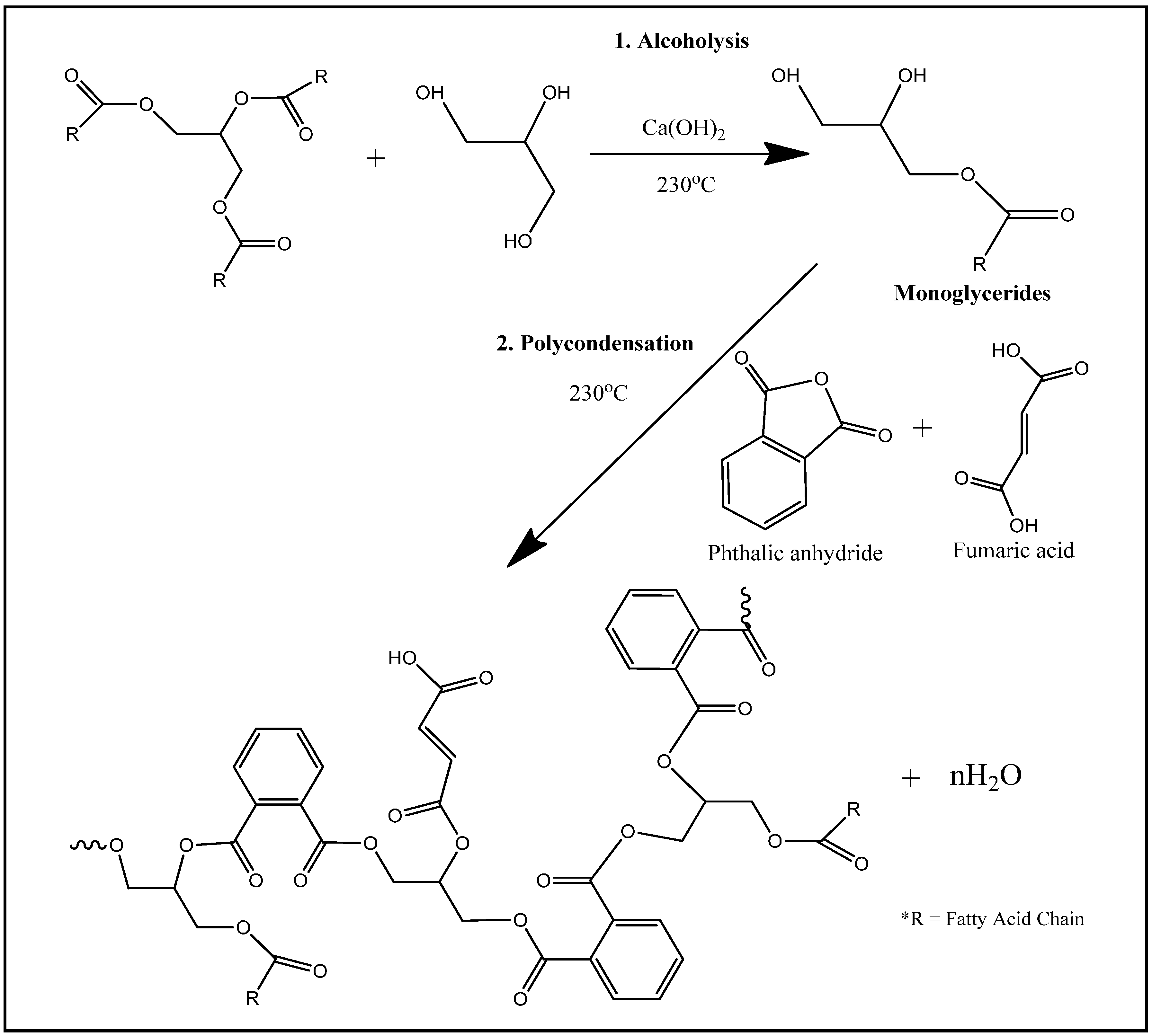
The alkyd used in this study was synthesized from palm stearin, glycerol, phthalic anhydride and fumaric acid. The synthesis began with alcoholysis of 304.4 g of palm stearin (0.34 mol) by 167.1 g of glycerol (1.8 mol) in the presence of 0.2 g of Ca(OH)
2 as catalyst to form monoglycerides. Phthalic anhydride and fumaric acid were then added to initiate the polycondensation reaction at high temperature (230–240 °C) until the acid number of the mixture dropped below 20 mg KOH/g resin (<10% of the initial acid number). Throughout the synthesis, water produced from the polycondensation was trapped in a Dean-Stark apparatus attached as a side-arm to the reaction flask. The plausible reactions involved can be summarized in
Figure 1. Molecular weight of the alkyd was recorded from Gel Permeation Chromatography (GPC) analysis at 3615 g·mol
−1, the transition temperature (
Tg) of the alkyd from Differential scanning calorimetry (DSC) was reported at −12 °C and the maximum decomposition peak of the alkyd from thermogravimetric analysis (TGA) was at 380 °C. Details of the synthesis and characterization of the alkyd were described elsewhere [
22].
Figure 1.
A plausible scheme of reactions in the synthesis of palm oil-based alkyd.
Figure 1.
A plausible scheme of reactions in the synthesis of palm oil-based alkyd.
2.3. Preparation of Plasticized PVC
Two different series of plasticized PVC were prepared from PVC (TL-700) and PVC (TL-1000) with the palm oil-based alkyd as a co-plasticizer to DOP and DiNP respectively. Each series of six samples was prepared with varying proportions of the palm oil-based alkyd to DOP or DiNP, the total plasticizer to PVC composition was maintained constant at 70:100. The only difference is the relative content of the palm oil-based alkyd to DOP or DiNP. The barium zinc stabilizer was fixed at 2 phr (parts per hundred resin) in all the plasticized PVC blends. PVC-L refers to PVC TL-700, with
K-value of 57–58.8, while PVC-H refers to PVC TL-1000, with
K-value of 65–67. ALK refers to the palm oil-based alkyd. The composition of the studied samples is reported in
Table 1. PVC-L/DOP7, PVC-L/DiNP7, PVC-H/DOP7 and PVC-H/DiNP7 with a relative content of 70 phr DOP or DiNP served as the control in this study.
The plasticized PVC was fabricated by mixing the required components of the formulation (as shown in
Table 1) with a Plastograph EC plus mixer (Brabender, Duisburg, Germany) at 120 °C for 50 min to obtain a uniform blend. Subsequently, PVC sheets were prepared by compression molding the plasticized PVC into 1 mm thickness with a hot press (GT-7014-A30C, GOTECH, Taiwan) at 120 °C for 14 min. The PVC was then cooled under the cooling plates to room temperature for 7 min, both under a pressure of 30 metric tons for heating and cooling. The samples were stored in sealed plastic bags before further characterization studies.
Table 1.
Formulation of palm oil-based alkyd with (a) PVC-L/DOP and PVC-L/DiNP blends and (b) PVC-H/DOP and PVC-H/DiNP blends. Poly(vinyl chloride) TL-700 (PVC-L). Di-octyl phthalate (DOP). Poly(vinyl chloride) TL-1000 (PVC-H). Di-isononyl phthalate (DiNP). Palm oil-based alkyd (ALK).
Table 1.
Formulation of palm oil-based alkyd with (a) PVC-L/DOP and PVC-L/DiNP blends and (b) PVC-H/DOP and PVC-H/DiNP blends. Poly(vinyl chloride) TL-700 (PVC-L). Di-octyl phthalate (DOP). Poly(vinyl chloride) TL-1000 (PVC-H). Di-isononyl phthalate (DiNP). Palm oil-based alkyd (ALK).
| Blends | Composition (phr) |
|---|
| DOP | DiNP | Palm Oil-Based ALK |
|---|
| (a) | PVC-L/DOP7 | 70 | - | 0 |
| PVC-L/DOP6/ALK1 | 60 | - | 10 |
| PVC-L/DOP5/ALK2 | 50 | - | 20 |
| PVC-L/DiNP7 | - | 70 | 0 |
| PVC-L/DiNP6/ALK1 | - | 60 | 10 |
| PVC-L/DiNP5/ALK2 | - | 50 | 20 |
| (b) | PVC-H/DOP7 | 70 | - | 0 |
| PVC-H/DOP6/ALK1 | 60 | - | 10 |
| PVC-H/DOP5/ALK2 | 50 | - | 20 |
| PVC-H/DiNP7 | - | 70 | 0 |
| PVC-H/DiNP6/ALK1 | - | 60 | 10 |
| PVC-H/DiNP5/ALK2 | - | 50 | 20 |
2.4. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
The analysis was performed with a Perkin Elmer Spectrum 400 ATR-FTIR spectrometer (Perkin Elmer, Waltham, MA, USA). The spectra of the unplasticized PVC, palm oil-based alkyd, and the different compositions of plasticized PVC blends were scanned in the range of 4000–400 cm−1 at a resolution of 4 cm−1 and recorded after 32 scans.
2.5. Mechanical Testing
The tensile test was conducted according to ASTM D-638 [
23] with a Shimadzu universal testing machine AGX-S (Shimadzu, Kyoto, Japan) fitted with a 500 N load cell, which was equipped with pneumatic grips. The mechanical properties of the plasticized PVC were examined in the tensile mode with a crosshead speed of 10 mm/min to determine the tensile strength, elastic modulus, and the elongation at break of each sample. The PVC sheets were cut into dumbbell shapes with a dumbbell shape cutter, which was prepared according to ASTM D-638, and the thickness was recorded with a digital thickness gauge. Five specimens were tested for each batch. The samples were pre-conditioned for 48 h prior to analysis, according to the ASTM D-638 standard specifications.
2.6. Field Emission Scanning Electron Microscopy (FESEM) Analysis
Morphology studies of the plasticized PVC were performed by field emission scanning electron microscopy with a JEOL JSM-7600F (JEOL, Peabody, MA, USA) using a low angle backscatter electron detector at an operating voltage of 10 kV. These morphology studies aim to obtain a backscatter image to investigate the contrast difference between phases of different compositions. The samples were sputter coated with a Pt layer under vacuum conditions to avoid sample charging under the electron beam before each analysis.
2.7. Thermogravimetric Analysis
The thermal degradations of plasticized PVC samples were analyzed with a Perkin Elmer TGA 6 (Perkin Elmer, Waltham, MA, USA). A sample of approximately 10 mg was loaded into the sample holder, and the TGA scans were conducted from 35 to 600 °C at a heating rate of 10 °C/min under 20 mL/min of nitrogen flow.
3. Results and Discussion
3.1. FTIR Analysis of PVC/Plasticizer Blends
ATR-FTIR analysis was conducted to investigate the specific interaction between PVC and the palm oil-based alkyd and to determine the vibrational shifting of functional groups of the peaks in the spectrum. The ATR-FTIR spectra within the range of 4000 cm
−1 to 400 cm
−1 of the alkyd, unplasticized PVC and PVC plasticized with varying proportions of alkyd and DOP or DiNP are shown in
Figure 2. The palm oil-based alkyd as a co-plasticizer exhibited strong absorbance peaks at 3466 cm
−1, which corresponded to –OH stretching, 1728 cm
−1 from the –C=O stretching, and 742 cm
−1 that was attributed to the out of plane angular bending of the orthosubstituted –C–H aromatic ring in the alkyd chain [
22,
24]. These three characteristic bands were also found in the plasticized PVCs that were composed with the alkyd. The PVC-L and PVC-H plasticized with 70 phr of DOP (PVC-L/DOP7 and PVC-H/DOP7), which served as the control in this study, were an exception because the –OH stretching band from the alkyd did not appear.
Figure 2.
Effect of palm oil-based alkyd on ATR-FTIR spectra of (a) plasticized PVC-L blends; and (b) plasticized PVC-H blends.
Figure 2.
Effect of palm oil-based alkyd on ATR-FTIR spectra of (a) plasticized PVC-L blends; and (b) plasticized PVC-H blends.
The unplasticized PVC had a characteristic band at 2974 cm
−1 from the stretching and deformation of the –C–H of –CHCl [
22]. After blending with the alkyd, it has shifted to a lower frequency and became a broader peak. In this study, the peaks for PVC-L at 2968 and 2912 cm
−1, as shown in
Figure 2, have shifted to 2928 cm
−1 after blending with plasticizers for all plasticized PVC blends. The same shifts were observed for the PVC-H samples. In particular, the PVC blends containing the alkyd had a lower shift compared with the control samples, which consisted of DOP only. On the other hand, the –C–Cl stretching of PVC at 608 cm
−1 has shifted to a higher frequency at around 612 cm
−1, which was observed after DOP or DiNP was partially replaced with the alkyd.
As shown in
Figure 2a,b, the alkyd exhibited an absorbance peak at 3466 cm
−1, which has become a broader band at 3444 to 3450 cm
−1 after mixing with the PVC. In addition, the carbonyl peak at 1728 cm
−1 has shifted to 1722 cm
−1 after mixing with PVC. Shifting of these peaks is due to intermolecular interaction between the plasticizers and PVC. The relative peak ratio
A1723/
A1426 between the carbonyl and –C–H absorbance peaks was taken to reflect the extent of interaction between plasticizer and PVC [
25]. The ratio has increased with the application of the alkyd. The PVC-H/DOP5/ALK2 sample showed the highest ratio, 5.94, signifying that the alkyd has increased the level of intermolecular interaction with the PVC.
3.2. Mechanical Properties of PVC/Plasticizer Blends
The mechanical properties of the plasticized PVC sheets with different proportions of the alkyd are presented in
Figure 3,
Figure 4 and
Figure 5. The results showed that the tensile strength, elongation at break, and the elastic modulus of the plasticized PVC blends increased with increasing concentration of the alkyd as the co-plasticizer. The PVC blends plasticized with only DOP or DiNP served as controls in this study.
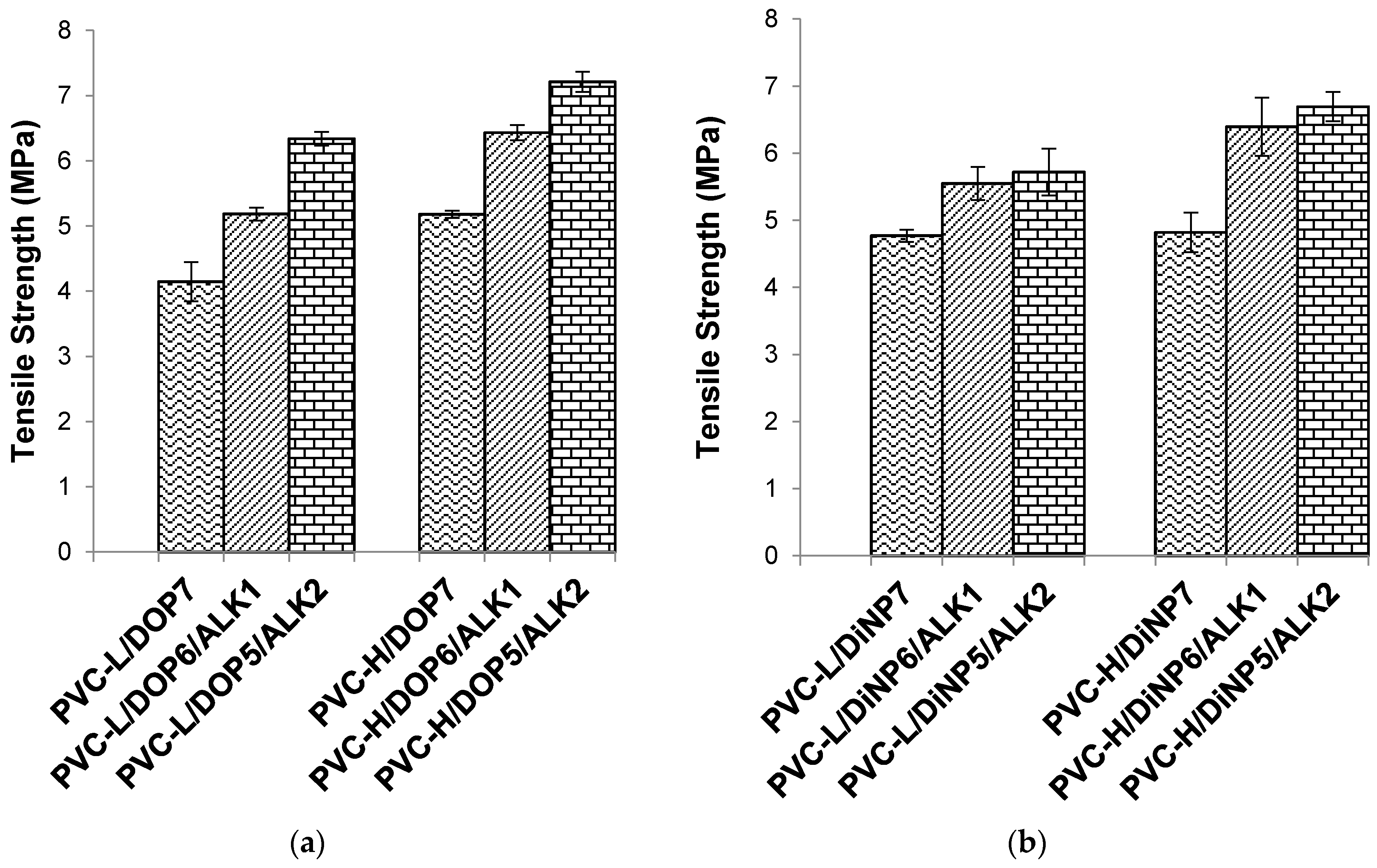
Figure 3.
Effect of palm oil-based alkyd on the tensile strength of (a) PVC/DOP blend and (b) PVC/DiNP blend.
Figure 3.
Effect of palm oil-based alkyd on the tensile strength of (a) PVC/DOP blend and (b) PVC/DiNP blend.
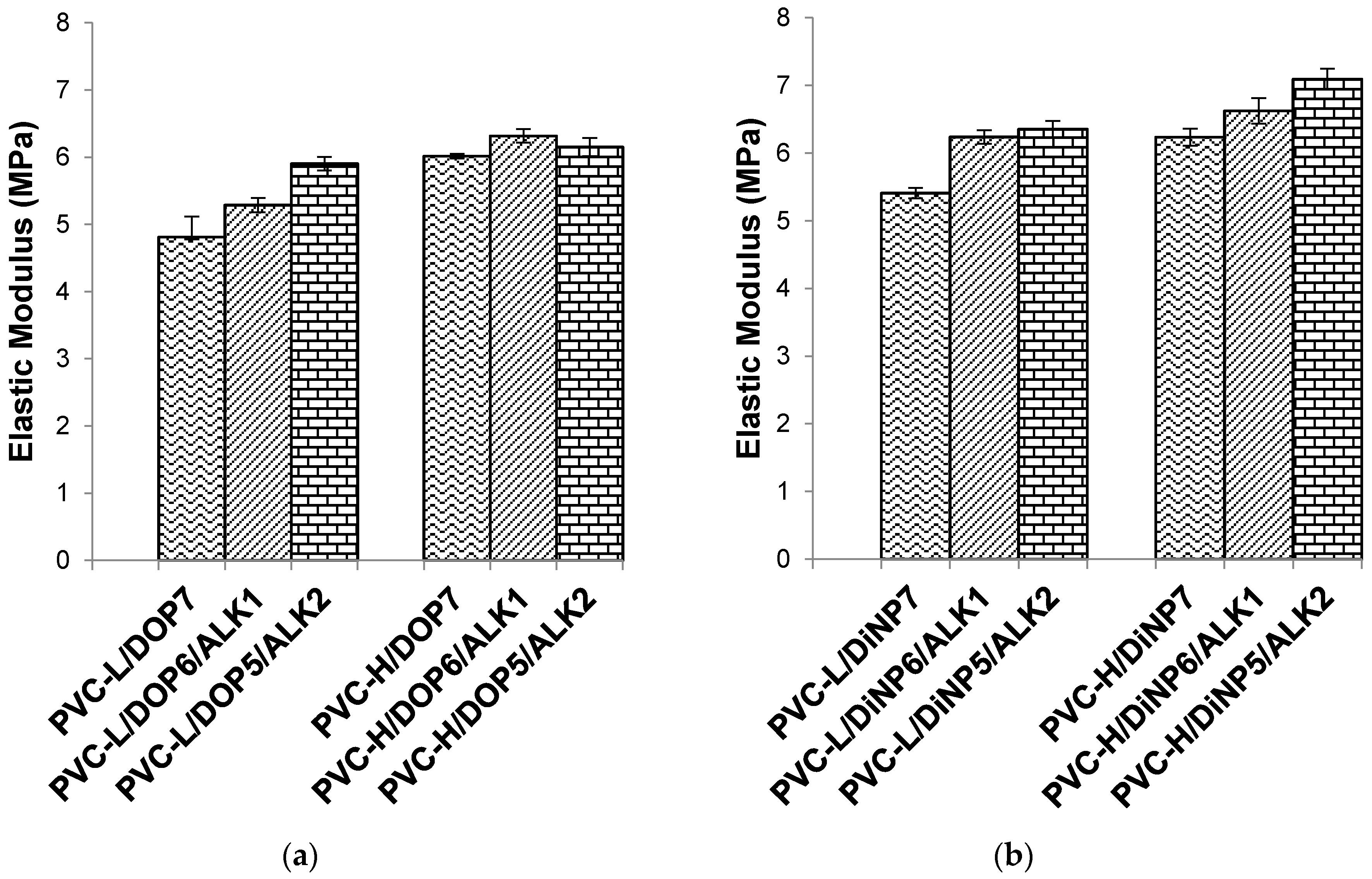
Figure 4.
Effect of palm oil-based alkyd on the elastic modulus of (a) PVC/DOP blend and (b) PVC/DiNP blend.
Figure 4.
Effect of palm oil-based alkyd on the elastic modulus of (a) PVC/DOP blend and (b) PVC/DiNP blend.
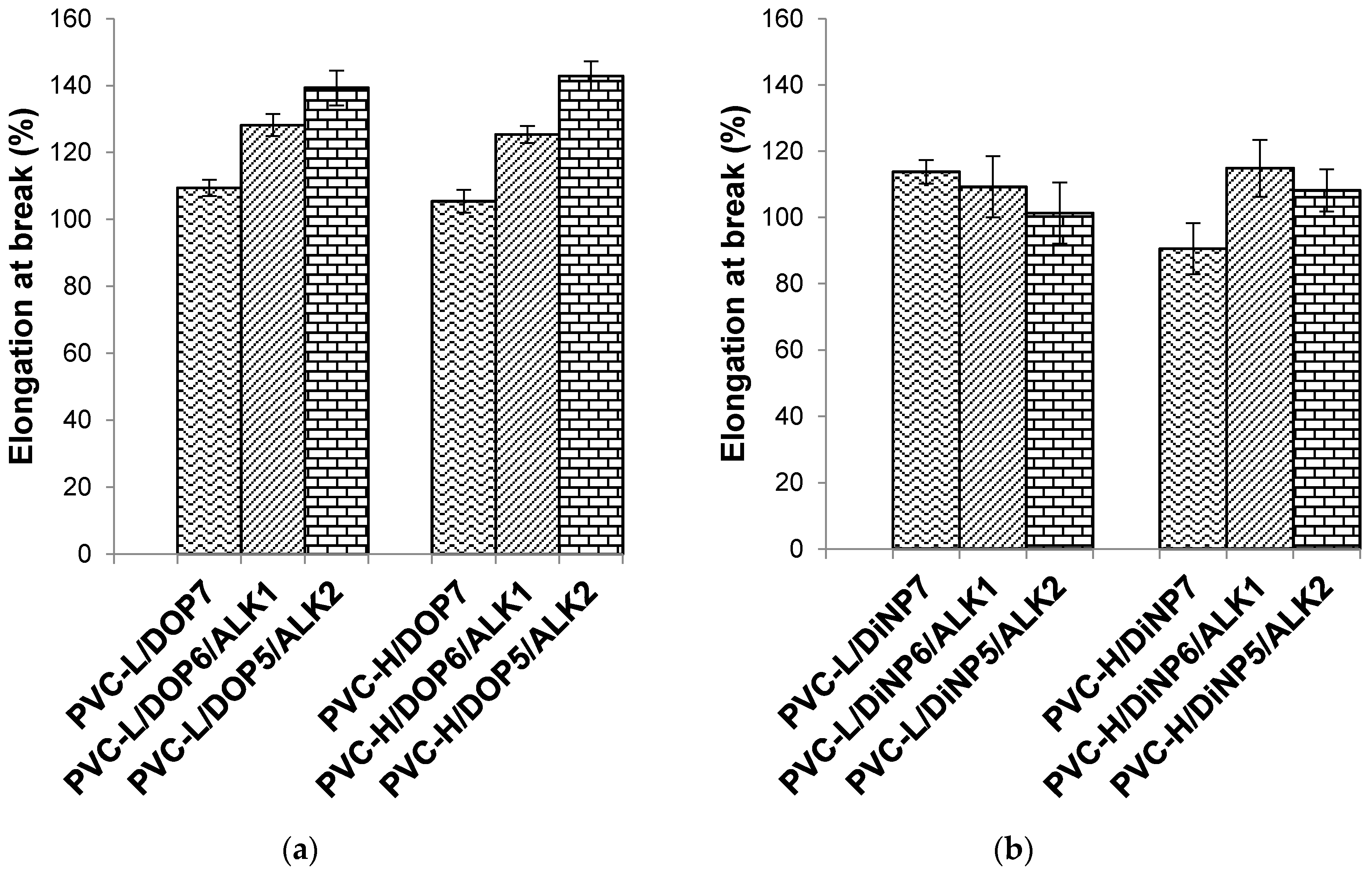
Figure 5.
Effect of palm oil-based alkyd on the elongation at break of (a) PVC/DOP blend and (b) PVC/DiNP blend.
Figure 5.
Effect of palm oil-based alkyd on the elongation at break of (a) PVC/DOP blend and (b) PVC/DiNP blend.
PVC-H/DOP plasticized with 20 phr of the palm oil-based alkyd showed the highest tensile strength and elongation at break among the series. The tensile strength improved with increasing alkyd content, from 10 to 20 phr, which effectively increased the overall plasticizing efficiency of the PVC. In this study, plasticized PVC-H with higher K-value and molecular weight showed significantly higher tensile strength compared to plasticized PVC-L blends with lower K-value and molecular weight. High K-value PVC is more difficult to process compared to low K-value PVC due to its higher molecular weight. The introduction of the palm oil-based alkyd as the co-plasticizer in this study has successfully improved the tensile strength of the plasticized PVC. The improved tensile strength is caused by the higher intermolecular interaction and chain entanglement of the alkyd with the PVC. PVC-H with higher molecular weight could have more entanglement within the PVC matrix resulting in higher mechanical properties.
DOP and DiNP are small molecules, having molecular weights of 398 and 418 respectively. The alkyd is a polyester with an average molecular weight in the range of 3600. It can entangle with the PVC chains. The amount of entanglements increases with the alkyd content, hence leading to higher tensile strength and elastic modulus of the PVC samples. While the chain entanglement can increase the tensile strength and can also increase the elongation at break (as shown in
Figure 5). PVC-L/DiNP/ALK appears to be the exception, showing a slight decrease in elongation at break. The error analysis (as indicated by the error bars) has shown that perhaps this set of samples was not uniformly mixed.
Tensile strength improved by approximately 20% from the PVC-L/DOP control sample to PVC-L/DOP6/ALK1 with 10 phr alkyd. A further 18.3% improvement was achieved at 20 phr of the palm oil-based alkyd. Correspondingly, the improvement of tensile strength from the PVC-H/DOP control sample to PVC-H/DOP6/ALK1 at 10 phr of alkyd was 19.4%; further improvement of 10.8% was observed at 20 phr of the alkyd.
Generally an increase in tensile strength would lead to a decrease in percentage elongation [
26,
27,
28]. Comparatively, the increase of elongation at break is usually accompanied by a decrease of the elastic modulus [
29,
30,
31,
32]. However, using the alkyd as a co-plasticizer has resulted in some interesting observations. As the alkyd is increased from 10 to 20 phr, the percentage elongation has increased together with tensile strength. Consequently, elastic modulus and elongation at break have increased, and this could be attributed to the ability of the alkyd to entangle with the PVC chain and reducing the close-packing of repeating units, thereby increasing the chain segmental motion, generating a greater amount of amorphous region, resulting in higher flexibility of the PVC matrix.
Increasing alkyd content could enhance the tensile strength, increase the elastic modulus, and also increase the percentage elongation. This trend implies that the combined effect of DOP or DiNP with the alkyd can lead to an overall improvement in physical properties.
3.3. Morphological Studies of PVC/Plasticizer Blends
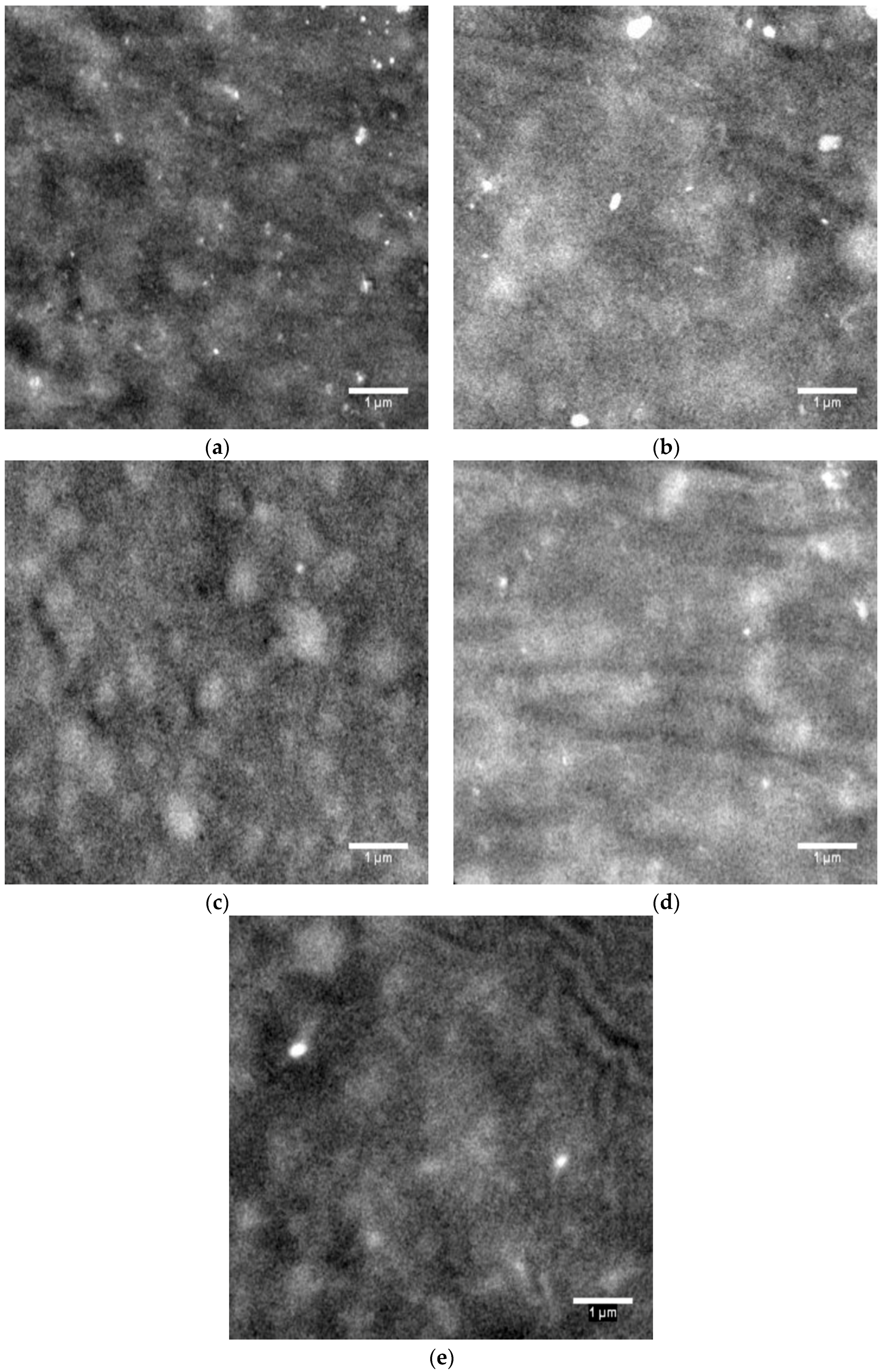
FESEM is used to study the morphology of the plasticized PVC blends and examine the contrast difference between phases of varying compositions in the blends.
Figure 6 shows the micrographs of blends with varying proportions of the alkyd at 5000× magnification.
Figure 6a shows the surface of the PVC blend exhibiting some uniformly dispersed white spots, presumably due to some crystalline regions of the PVC matrix. With increasing content of the alkyd, the amount of white spots decreased (
Figure 6b,c). At the same time, incorporation of plasticizers has led to larger amorphous phases, where the segmental chain movement increases, leading to higher flexibility of the PVC chains [
9,
16,
19,
33].
Figure 6d,e illustrates that PVC-H/DiNP6 and PVC-L/DOP6 blended with 10 phr of the alkyd showed similar morphology to PVC-H/DOP6 blended with 10 phr of the alkyd (
Figure 6b). The formation of overlapping aggregates with a smaller quantity of white spots was observed.
3.4. Thermal Stability of PVC/Plasticizer Blends
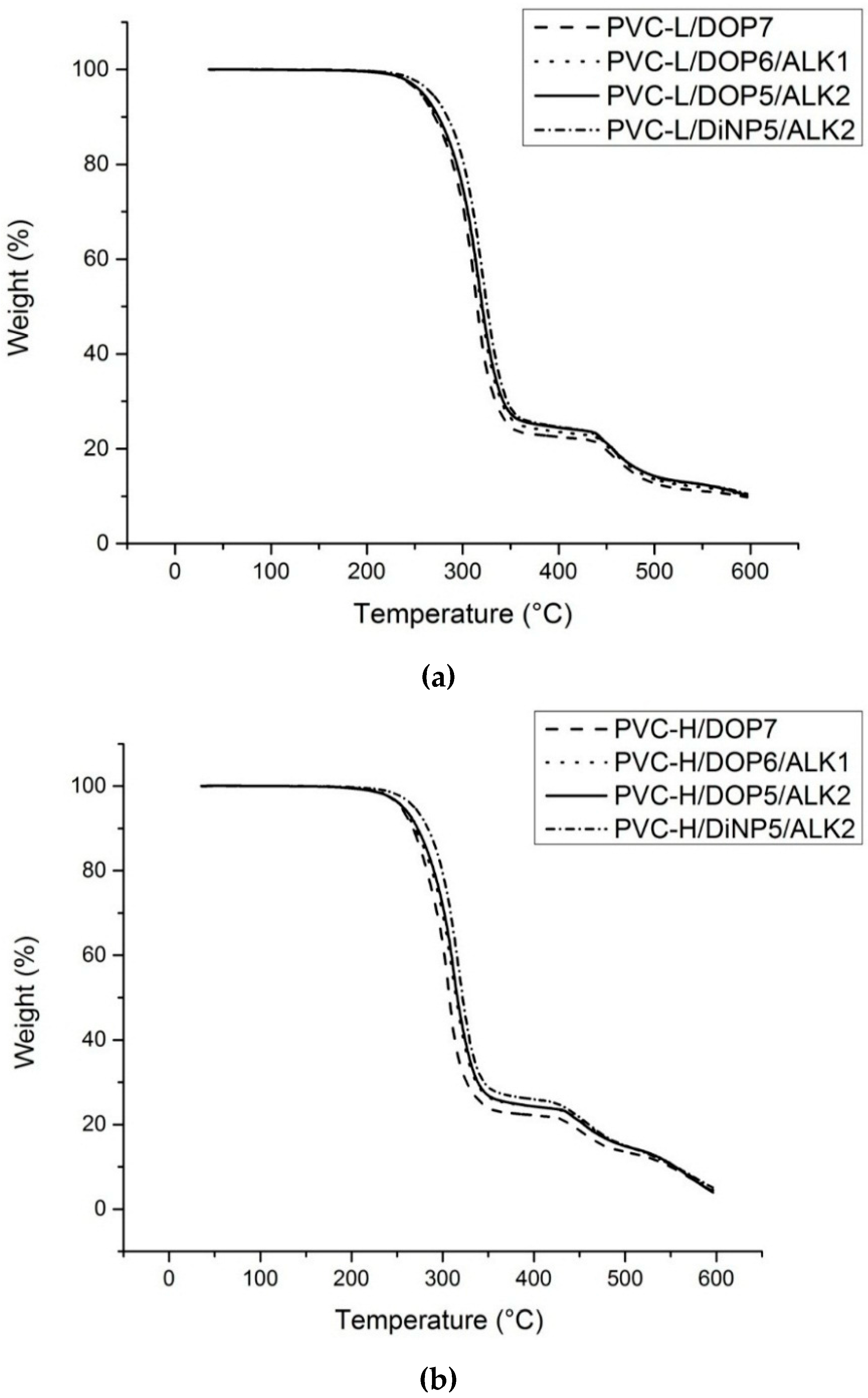
TGA was used to evaluate the thermal stability and thermal behavior of plasticized PVC blends. The dynamic thermogravimetric curves of plasticized PVC blends with varying proportions of DOP or DiNP with the alkyd are shown in
Figure 7. Meanwhile,
Table 2 summarizes the TGA results based on the content of the alkyd with
T5%,
T10%, and
T50%, which are the temperatures at 5%,10%, and 50% weight loss, respectively, as well as the weight loss at stage 1 (up to 230 °C), stage 2 (up to 320 °C), and stage 3 (up to 520 °C), respectively.
Figure 6.
FESEM micrographs of (a) PVC-H plasticized with DOP; (b) PVC-H plasticized with DOP/palm oil-based alkyd (10 phr); (c) PVC-H plasticized with DOP/palm oil-based alkyd (20 phr); (d) PVC-H plasticized with DiNP/palm oil-based alkyd (10 phr); and (e) PVC-L plasticized with DOP/palm oil-based alkyd (10 phr). Field Emission Scanning Electron Microscopy (FESEM).
Figure 6.
FESEM micrographs of (a) PVC-H plasticized with DOP; (b) PVC-H plasticized with DOP/palm oil-based alkyd (10 phr); (c) PVC-H plasticized with DOP/palm oil-based alkyd (20 phr); (d) PVC-H plasticized with DiNP/palm oil-based alkyd (10 phr); and (e) PVC-L plasticized with DOP/palm oil-based alkyd (10 phr). Field Emission Scanning Electron Microscopy (FESEM).
Figure 7.
Thermogravimetric thermograms of varying proportions of palm oil-based alkyd in (a) PVC-L blends and (b) PVC-H blends.
Figure 7.
Thermogravimetric thermograms of varying proportions of palm oil-based alkyd in (a) PVC-L blends and (b) PVC-H blends.
Table 2.
Thermogravimetric (TGA) results for PVC plasticized sample with different proportions of palm oil-based alkyd. dW1 = wt loss from 35 to 230 °C, dW2 = wt loss from 230 to 320 °C, dW3 = wt loss from 320 to 520 °C.
Table 2.
Thermogravimetric (TGA) results for PVC plasticized sample with different proportions of palm oil-based alkyd. dW1 = wt loss from 35 to 230 °C, dW2 = wt loss from 230 to 320 °C, dW3 = wt loss from 320 to 520 °C.
| Blends | Alkyd content (phr) | T5% | T10% | T50% | Weight loss (%) |
|---|
| Step 1 (dW1) | Step 2 (dW2) | Step 3 (dW3) |
|---|
| PVC-L/DOP7 | 0 | 255.2 | 270.8 | 315.0 | 1.33 | 56.37 | 30.53 |
| PVC-L/DOP6/ALK1 | 10 | 255.7 | 272.5 | 319.1 | 1.37 | 50.03 | 35.99 |
| PVC-L/DOP5/ALK2 | 20 | 258.2 | 274.6 | 320.2 | 1.30 | 48.34 | 37.05 |
| PVC-L/DiNP5/ALK2 | 20 | 267.4 | 284.0 | 325.3 | 0.86 | 41.59 | 44.38 |
| PVC-H/DOP7 | 0 | 253.7 | 267.2 | 307.2 | 1.50 | 65.11 | 20.86 |
| PVC-H/DOP6/ALK1 | 10 | 254.2 | 269.6 | 314.6 | 1.32 | 55.62 | 29.42 |
| PVC-H/DOP5/ALK2 | 20 | 256.6 | 272.5 | 316.0 | 1.55 | 53.47 | 31.29 |
| PVC-H/DiNP5/ALK2 | 20 | 268.8 | 283.9 | 321.9 | 0.77 | 46.34 | 39.40 |
The results showed that all the plasticized PVC blends underwent three stages of degradation, and this was in agreement with earlier observations reported in the literature [
2,
21,
23,
34,
35]. In the first stage of degradation, the weight loss was due to plasticizer evaporation. The second stage involves the dechlorination of PVC, as well as the formation and volatilization of HCl with the subsequent formation of double bonds. As the temperature increases further, degradation proceeded by the free radical mechanism to release more HCl, and the formation of the polyene structure. The third stage of degradation is short, due to the thermal cracking and pyrolysis of the polyene sequence formed in stage 2.
The results showed that T5%, T10% and T50% shifted toward higher temperatures for all plasticized PVC blends containing the alkyd compared to the control samples. The value of T50% indicates the temperature where thermal degradation was more pronounced, in which significant stabilization of thermal degradation was observed with the increasing content of the alkyd. PVC-H/DOP7 which was composed of DOP only showed T50% at 307.2 °C. Subsequently, the T50% increased from 314.6 to 316 °C with the incorporation of 10 to 20 phr of the alkyd. This result indicates an improvement in thermal stability due to the incorporation of the alkyd into the PVC matrix. Plasticized PVC-L/DOP blends showed the same trend. The degradation temperature has increased with the added alkyd. Thus, a significant improvement of thermal stability was observed when the content of the alkyd was increased.
The extent of weight loss is the most significant during the first and second stages of decomposition. The decomposition rate decreased with increasing alkyd content in the plasticized PVC. In the second decomposition stage, the control samples (PVC-L/DOP7 and PVC-H/DOP7) resulted in maximum decomposition of 56.37% and 65.11% respectively, compared with the other plasticized PVC blends with varying proportions of the alkyd among the series. The second stage of weight loss was reduced to around 41.59% with the incorporation of 20 phr alkyd. Therefore, the alkyd plays dual actions in the PVC structure. Firstly, it can increase the plasticizing efficiency; and secondly, it can hinder decomposition, improving the thermal stability.
4. Conclusions
The palm oil-based alkyd as a bio-based co-plasticizer, along with DOP or DiNP as the primary plasticizer for PVC, shows improved results compared with those with only DOP or DiNP. Results show good incorporation of the alkyd into the plasticized PVC, with enhanced mechanical and thermal properties. FTIR results showed that the polar –OH and –C=O groups of the alkyd have good interaction with the –CH–Cl group in PVC via polar interaction. Improved mechanical properties are observed with the increased the alkyd content, effectively enhancing the overall plasticizing efficiency of the PVC blends. DOP and DiNP are small molecules while the alkyd is large molecule that can entangle with the PVC chains. The amount of entanglements increases with the alkyd content and hence leads to higher tensile strength and elastic modulus of the PVC samples. The chain entanglement can increase the tensile strength and can also increase the elongation at break; PVC-L/DiNP/ALK appears to be the exception, showing a slight decrease in elongation at break. PVC-H/DOP5/ALK2 with 20 phr of the palm oil-based alkyd exhibited the highest tensile strength and elongation at break among the series. FESEM results lend support to good incorporation of the alkyd into the PVC matrix. The introduction of the alkyd also successfully increased the degradation temperature, improving the thermal stability of plasticized PVC blends.