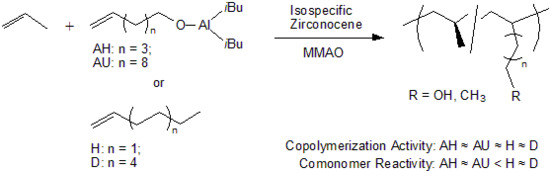
Reactivity Comparison of ω-Alkenols and Higher 1-Alkenes in Copolymerization with Propylene Using An Isospecific Zirconocene-MMAO Catalyst
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Polymerization Procedure
2.3. Analytical Procedure
3. Results and Discussion
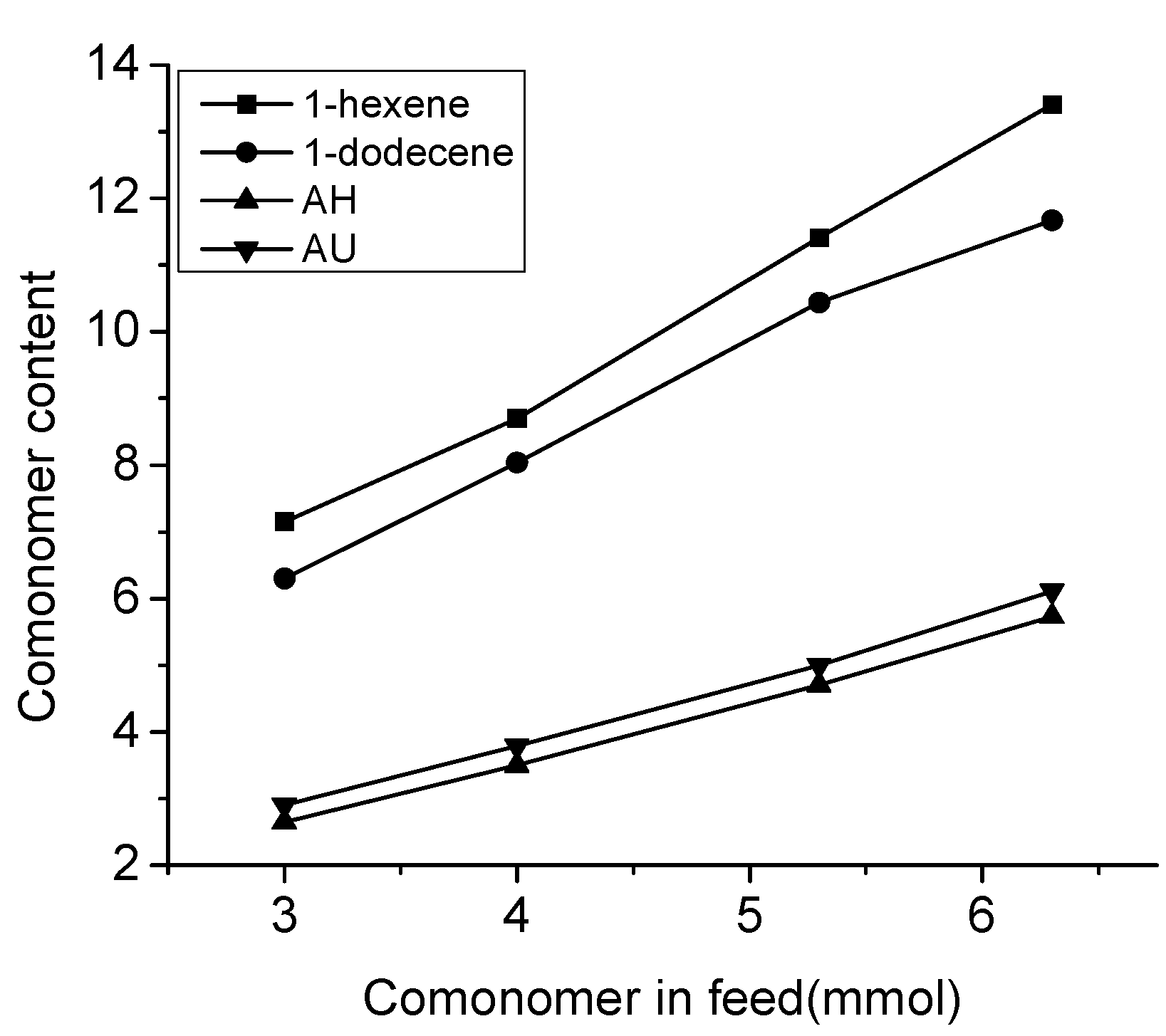
3.1. Copolymerization of Propylene with ω-Alkenols and Higher 1-Alkenes
| Entry | Comonomer b Feed (mmol) | Yield (g) | Activity c (103) | Mn d (104) | Mw/Mn d | Comonomer Content e (mol %) | Comonomer Conversion f (%) | Tm g (°C) |
|---|---|---|---|---|---|---|---|---|
| 1 | - | 0.70 | 25 | 19.5 | 2.1 | - | - | 160 |
| 2 | AH (3.0) | 0.28 | 10 | 18.0 | 2.2 | 2.7 | 4.9 | 143 |
| 3 | AH (4.0) | 0.23 | 8.5 | 16.4 | 2.1 | 3.5 | 3.8 | 136 |
| 4 | AH (5.3) | 0.17 | 6.5 | 11.0 | 2.3 | 4.7 | 2.8 | 132 |
| 5 | AH (6.3) | 0.14 | 5.1 | 8.5 | 2.0 | 5.7 | 2.0 | 131 |
| 6 | AU (3.0) | 0.30 | 11 | 18.5 | 2.2 | 2.9 | 6.5 | 121 |
| 7 | AU (4.0) | 0.26 | 9.3 | 18.2 | 2.1 | 3.8 | 5.1 | 117 |
| 8 | AU (5.3) | 0.20 | 7.2 | 16.5 | 2.3 | 5.0 | 3.7 | 107 |
| 9 | AU (6.3) | 0.16 | 5.8 | 9.7 | 2.2 | 6.1 | 2.8 | 98.6 |
| 10 | H (3.0) | 0.28 | 10 | 10.2 | 1.7 | 7.2 | 14 | 95.9 |
| 11 | H (4.0) | 0.25 | 9.0 | 9.1 | 1.7 | 8.7 | 12 | 80.5 |
| 12 | H (5.3) | 0.22 | 7.9 | 6.5 | 1.9 | 11 | 10 | 76.0 |
| 13 | H (6.3) | 0.17 | 6.1 | 5.4 | 1.9 | 13 | 7.6 | - h |
| 14 | D (3.0) | 0.32 | 12 | 12.9 | 1.7 | 6.3 | 13 | 107 |
| 15 | D (4.0) | 0.27 | 9.7 | 9.7 | 1.8 | 8.0 | 10 | 97.7 |
| 16 | D (5.3) | 0.24 | 8.6 | 6.9 | 1.9 | 10 | 8.6 | 84.9 |
| 17 | D (6.3) | 0.19 | 6.8 | 5.8 | 1.8 | 11 | 6.2 | 79.5 |
3.2. Microstructure of Copolymers

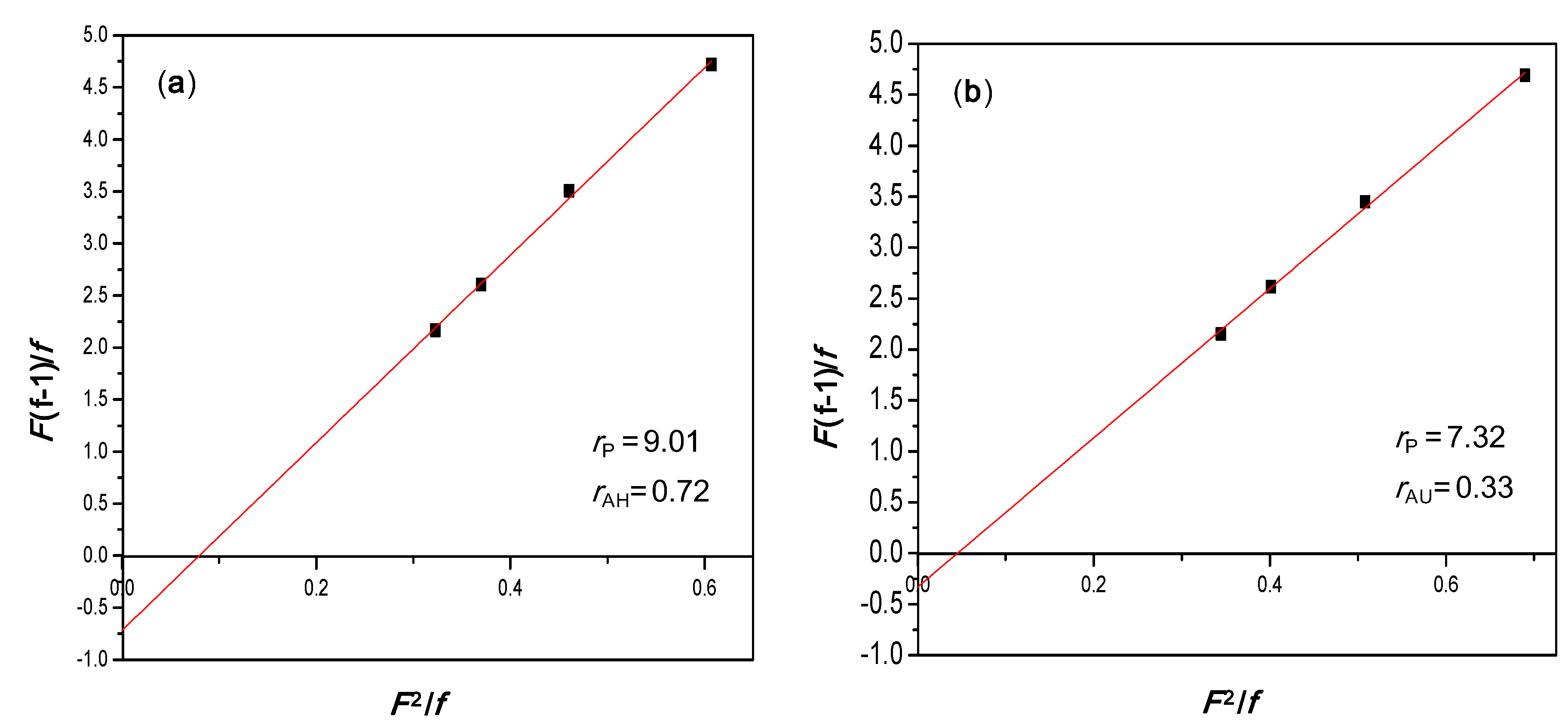
3.3. Comonomer Reactivity Ratios

3.4. Thermal Properties of Copolymers
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Franssen, N.M.G.; Reek, J.N.H.; Bruin, B.D. Synthesis of functional “polyolefins”: State of the art and remaining challenges. Chem. Soc. Rev. 2013, 42, 5809–5832. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Y.; Hu, Y. Design and synthesis of structurally well-defined functional polyolefins via transition metal-mediated olefin polymerization chemistry. Coord. Chem. Rev. 2006, 250, 47–65. [Google Scholar] [CrossRef]
- Boffa, L.S.; Novak, B.M. Copolymerization of polar monomers with olefins using transition-metal complexes. Chem. Rev. 2000, 100, 1479–1493. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.X. Coordination polymerization of polar vinyl monomers by single-site metal catalysts. Chem. Rev. 2009, 109, 5157–5214. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Ito, S.; Nozaki, K. Coordination-insertion copolymerization of fundamental polar monomers. Chem. Rev. 2009, 109, 5215–5244. [Google Scholar] [CrossRef] [PubMed]
- Carrow, B.P.; Nozaki, K. Transition-metal-catalyzed functional polyolefin synthesis: Effecting control through chelating ancillary ligand design and mechanistic insights. Macromolecules 2014, 47, 2541–2555. [Google Scholar] [CrossRef]
- Aaltonen, P.; Lofgren, B. Synthesis of functional polyethylenes with soluble metallocene/methylaluminoxane catalyst. Macromolecules 1995, 28, 5353–5357. [Google Scholar] [CrossRef]
- Aaltonen, P.; Lofgren, B. Functionalization of polyethylenes via metallocene/methylaluminoxane catalyst. Eur. Polym. J. 1997, 33, 1187–1190. [Google Scholar] [CrossRef]
- Hakala, K.; Lofgren, B.; Helaja, T. Copolymerizations of oxygen-functionalized olefins with propylene using metallocene/methylaluminoxane catalyst. Eur. Polym. J. 1998, 34, 1093–1097. [Google Scholar] [CrossRef]
- Hakala, K.; Helaja, T.; Lofgren, B. Metallocene/methylaluminoxane-catalyzed copolymerizations of oxygen-functionalized long-chain olefins with ethylene. J. Polym. Sci. Polym. Chem. 2000, 38, 1966–1971. [Google Scholar] [CrossRef]
- Marques, M.M.; Correia, S.G.; Ascenso, J.R.; Ribeiro, A.F.G.; Gomes, P.T.; Dias, A.R.; Foster, P.; Rausch, M.D.; Chien, J.C.W. Polymerization with TMA-protected polar vinyl comonomers. I. Catalyzed by group 4 metal complexes with η5-type ligands. J. Polym. Sci. Polym. Chem. 1999, 37, 2457–2469. [Google Scholar] [CrossRef]
- Hagihara, H.; Murata, M.; Uozumi, T. Alternating copolymerization of ethylene and 5-hexen-1-ol with [ethylene(1-indenyl)(9-fluorenyl)]-zirconium dichloride/methylaluminoxane as the catalyst. Macromol. Rapid Commun. 2001, 22, 353–357. [Google Scholar] [CrossRef]
- Hagihara, H.; Tsuchihara, K.; Takeuchi, K.; Murata, M.; Ozaki, H.; Shiono, T. Copolymerization of ethylene or propylene with α-olefins containing hydroxyl groups with zirconocene/methylaluminoxane catalyst. J. Polym. Sci. Polym. Chem. 2004, 42, 52–58. [Google Scholar] [CrossRef]
- Imuta, J.; Kashiwa, N.; Toda, Y. Catalytic regioselective introduction of allyl alcohol into the nonpolar polyolefins: Development of one-pot synthesis of hydroxyl-capped polyolefins mediated by a new metallocene IF catalyst. J. Am. Chem. Soc. 2002, 124, 1176–1177. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, N.; Kojoh, S.; Matsuo, S.; Kaneko, H.; Matsugi, T.; Kashiwa, N. Investigation of insertion reaction of 10-undecen-1-ol protected with alkylaluminum in En(Ind)2ZrCl2/MAO Catalyst System. J. Mol. Catal. A Chem. 2005, 241, 156–161. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Li, H.; Zhang, Z.; Lu, Y.; Wu, C.; Hu, Y. Highly active copolymerization of ethylene with 10-undecen-1-ol using phenoxy-based zirconium/methylaluminoxane catalysts. J. Polym. Sci. Polym. Chem. 2005, 43, 5944–5952. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Li, H.; Zhang, Z.; Lu, Y.; Wu, C.; Hu, Y. Copolymerizations of ethylene and polar comonomers with bis(phenoxyketimine) group IV complexes: Effects of the central metal properties. J. Polym. Sci. Polym. Chem. 2007, 45, 59–68. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, K.; Dong, J.Y. Copolymerization of ethylene and 10-undecen-1-olusing a montmorillonite-intercalated metallocene catalyst: Synthesis of polyethylene/montmorillonite nanocomposites with enhanced structural stability. Macromol. Rapid Commun. 2006, 27, 1278–1283. [Google Scholar] [CrossRef]
- Purgett, M.D.; Vogl, O. Functional Polymers. XLIX. Copolymerization of ω-alkenoates with α-olefins and Ethylene. J. Polym. Sci. Polym. Chem. 1989, 27, 2051–2063. [Google Scholar] [CrossRef]
- Zuo, W.; Zhang, M.; Sun, W.H. Imino-iindolate half-titanocene chlorides: Synthesis and their ethylene (co-)polymerization. J. Polym. Sci. Polym. Chem. 2009, 47, 357–372. [Google Scholar] [CrossRef]
- Fernandes, M.; Kaminsky, W. Copolymerization of ethylene with 2,7-octadienyl methyl ether in the presence of metallocene and nickel diimine catalysts. Macromol. Chem. Phys. 2009, 210, 585–593. [Google Scholar] [CrossRef]
- Terao, H.; Ishii, S.; Mitani, M.; Tanaka, H.; Fujita, T. Ethylene/polar monomer copolymerization behavior of bis(phenoxy-imine)ti complexes: Formation of polar monomer copolymers. J. Am. Chem. Soc. 2008, 130, 17636–17637. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Wang, Y.X.; Mu, H.L.; Li, Y.S. Efficient synthesis of hydroxylated polyethylene via copolymerization of ethylene with 5-norbornene-2-methanol using bis(β-enaminoketonato)titanium catalysts. Organometallics 2011, 30, 4678–4686. [Google Scholar] [CrossRef]
- Yang, X.H.; Liu, C.R.; Wang, C.; Sun, X.L.; Guo, Y.H.; Wang, X.K.; Wang, Z.; Xie, Z.; Tang, Y. [O−NSR]TiCl3-catalyzed copolymerization of ethylene with functionalized olefins. Angew. Chem. Int. Ed. 2009, 48, 8099–8102. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, J.F.; Tal, W.J.; Sun, X.J.; Yang, X.H.; Tang, Y. Copolymerization of ethylene with functionalized olefins by [onx] titanium complexes. Macromolecules 2013, 46, 2870–2875. [Google Scholar] [CrossRef]
- Aaltonen, P.; Fink, G.; Lofgren, B.; Seppala, J. Synthesis of hydroxyl group containing polyolefins with metallocene/methylaluminoxane catalysts. Macromolecules 1996, 29, 5255–5260. [Google Scholar] [CrossRef]
- Paavola, S.; Lofgren, B.; Seppala, J. Polymerization of hydroxyl functional polypropylene by metallocene catalysis. Eur. Polym. J. 2005, 41, 2861–2866. [Google Scholar] [CrossRef]
- Hagihara, H.; Tsuchihara, K.; Sugiyama, J.; Takeuchi, K.; Shiono, T. Copolymerization of 3-buten-1-ol and propylene with an isospecific zirconocene/methylaluminoxane catalyst. J. Polym. Sci. Polym. Chem. 2004, 42, 5600–5607. [Google Scholar] [CrossRef]
- Hagihara, H.; Ishihara, T.; Ban, H.T.; Shiono, T. Precise control of microstructure of functionalized polypropylene synthesized by the ansa-zirconocene/mao catalysts. J. Polym. Sci. Polym. Chem. 2008, 46, 1738–1748. [Google Scholar] [CrossRef]
- Busico, V.; Cipullo, R.; Cutillo, F.; Friederichs, N.; Ronca, S.; Wang, B. Improving the performance of methylalumoxane: A facile and efficient method to trap “free” trimethylaluminum. J. Am. Chem. Soc. 2003, 125, 12402–12403. [Google Scholar] [CrossRef] [PubMed]
- Kissin, Y.V.; Brandolini, A.J. 13C NMR spectra of propylene/1-hexene copolymer. Macromolecules 1991, 24, 2632–2633. [Google Scholar] [CrossRef]
- Fineman, R.; Ross, S.D. Linear method for determining monomer reactivity ratios in copolymerization. J. Polym. Sci. Polym. Chem. 1950, 5, 259–262. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyangoye, B.O.; Li, T.; Chen , L.; Cai, Z. Reactivity Comparison of ω-Alkenols and Higher 1-Alkenes in Copolymerization with Propylene Using An Isospecific Zirconocene-MMAO Catalyst. Polymers 2015, 7, 2009-2016. https://doi.org/10.3390/polym7101496
Nyangoye BO, Li T, Chen L, Cai Z. Reactivity Comparison of ω-Alkenols and Higher 1-Alkenes in Copolymerization with Propylene Using An Isospecific Zirconocene-MMAO Catalyst. Polymers. 2015; 7(10):2009-2016. https://doi.org/10.3390/polym7101496
Chicago/Turabian StyleNyangoye, Benard Oloo, Tianyou Li, Long Chen , and Zhengguo Cai. 2015. "Reactivity Comparison of ω-Alkenols and Higher 1-Alkenes in Copolymerization with Propylene Using An Isospecific Zirconocene-MMAO Catalyst" Polymers 7, no. 10: 2009-2016. https://doi.org/10.3390/polym7101496