Influence of Surface Treatment on Tensile Properties of Low-Density Polyethylene/Cellulose Woven Biocomposites: A Preliminary Study
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
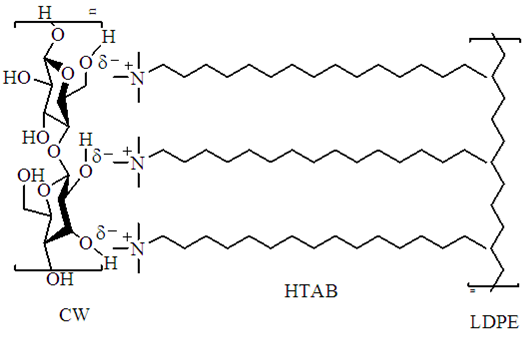
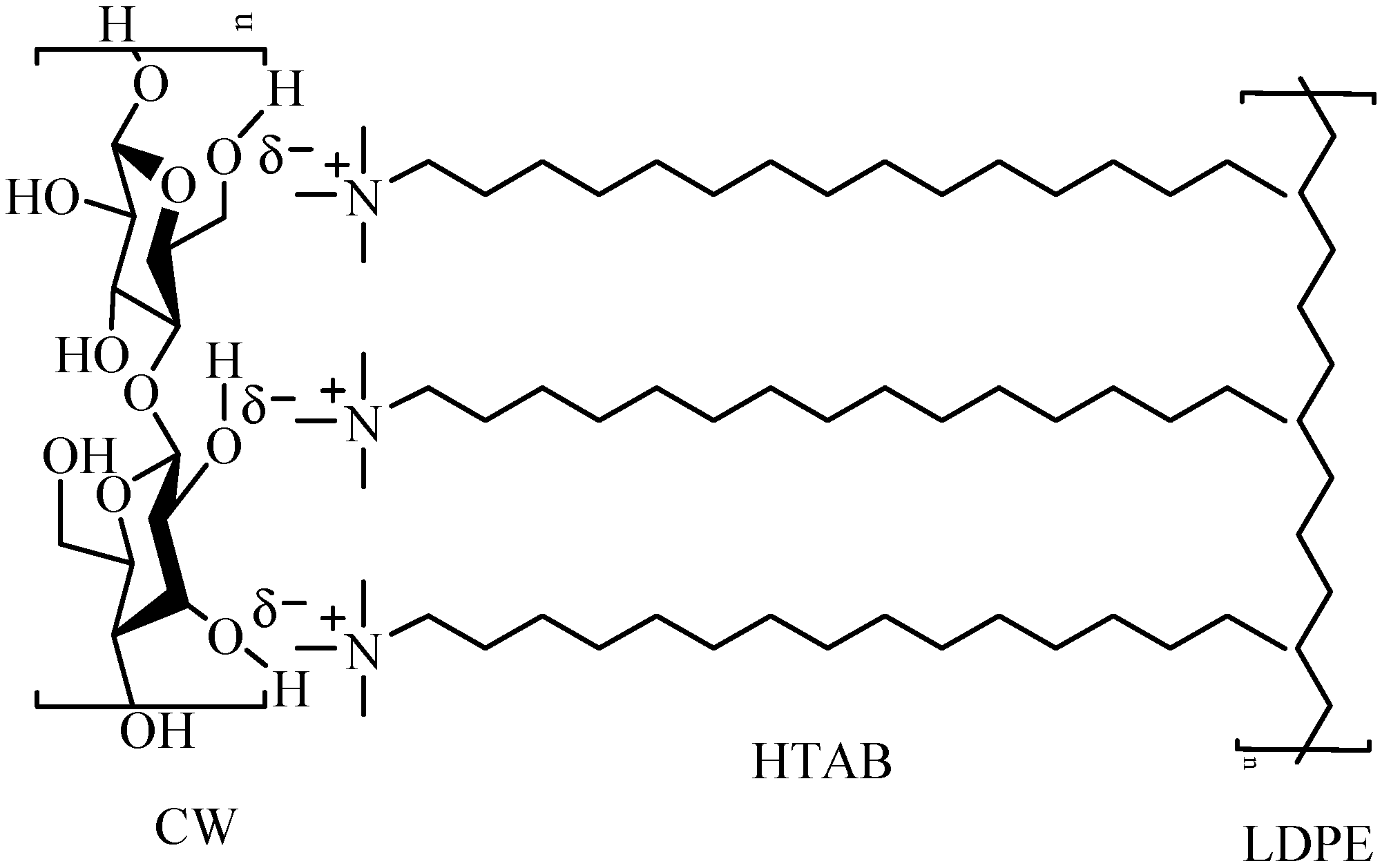
2.2. Surface Treatment of CW
2.3. Preparation of LDPE/CW Biocomposites
2.4. Characterization of LDPE/CW Biocomposites
3. Results and Discussion
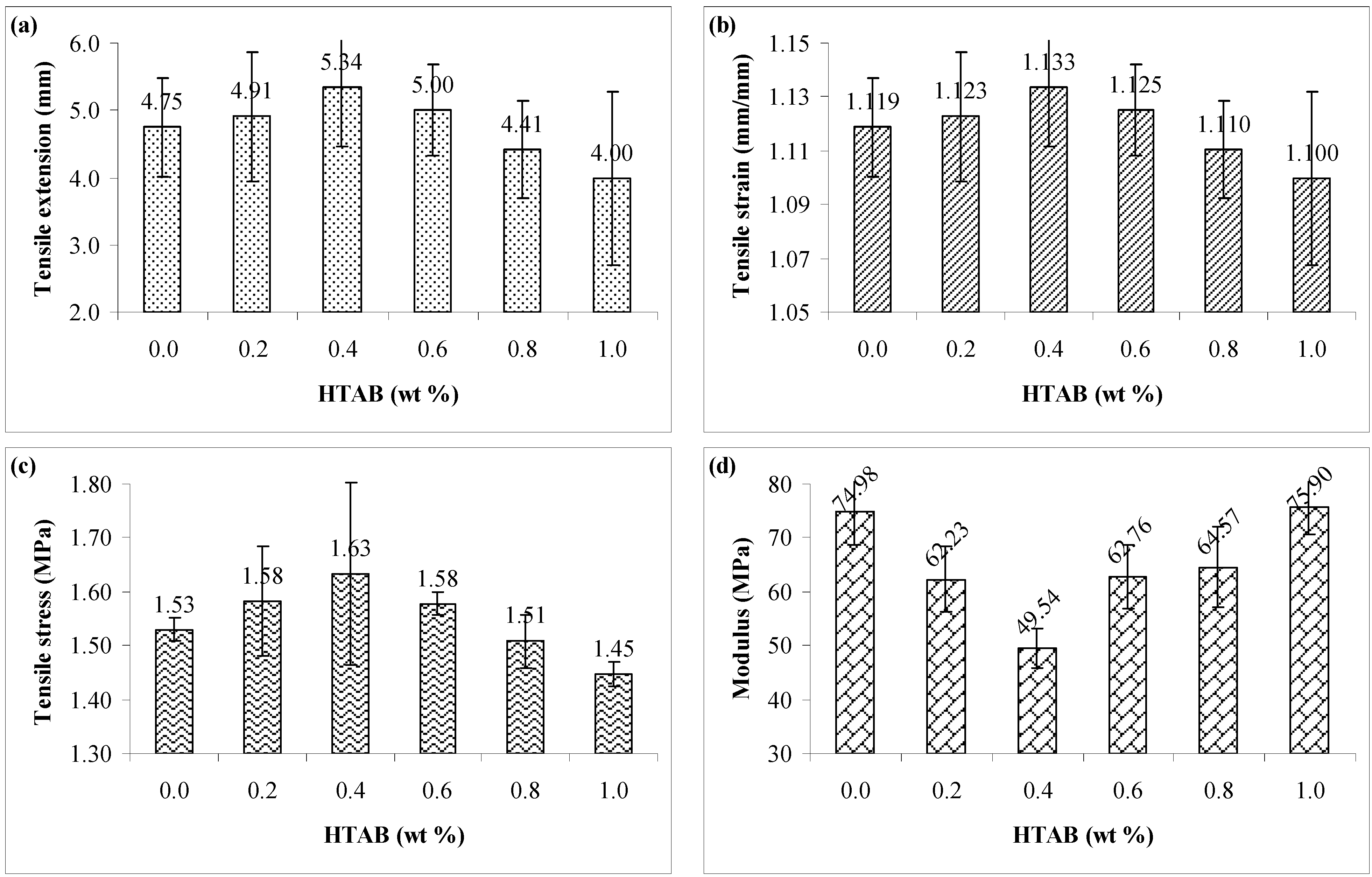
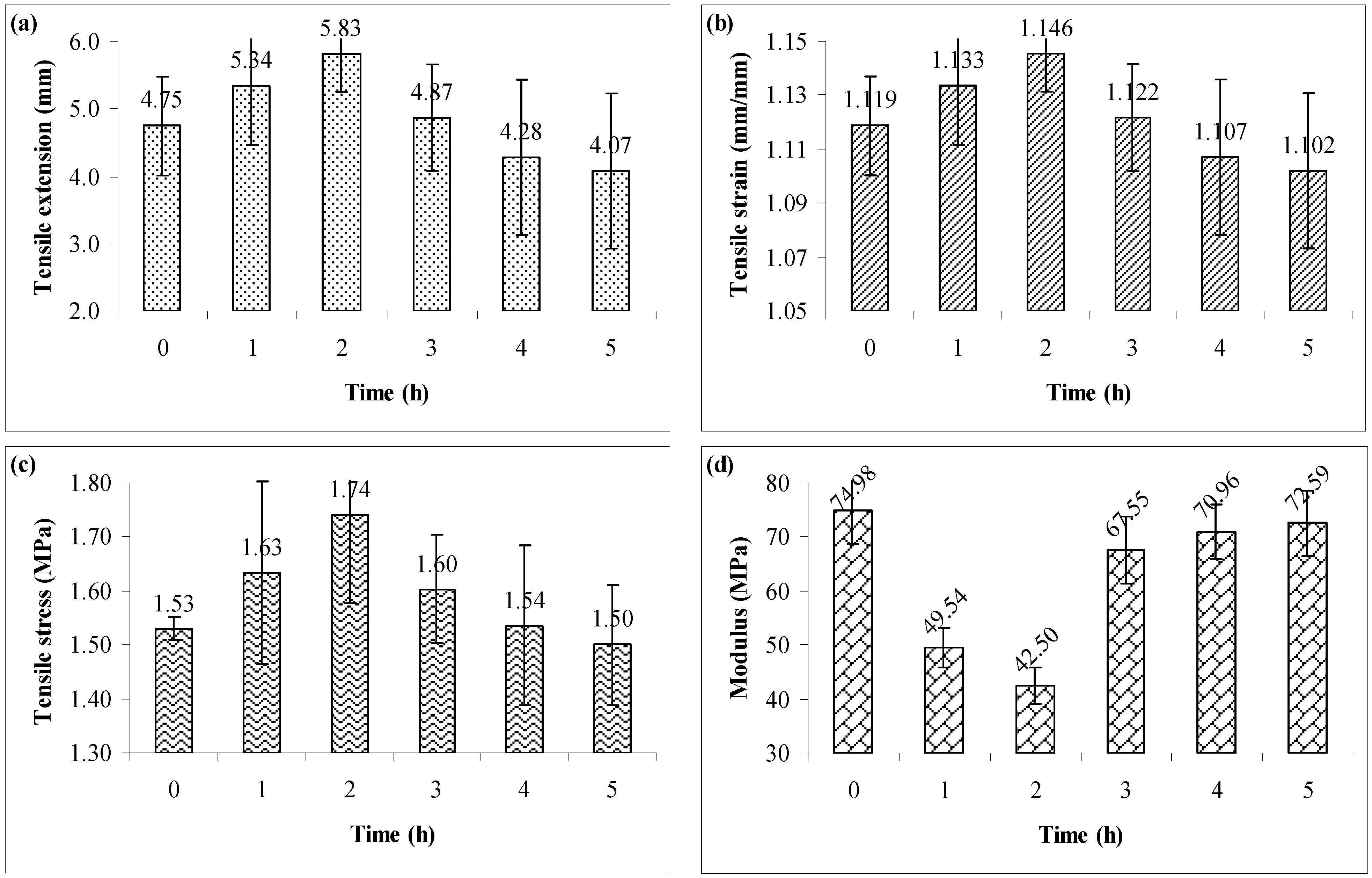
3.1. Tensile Testing
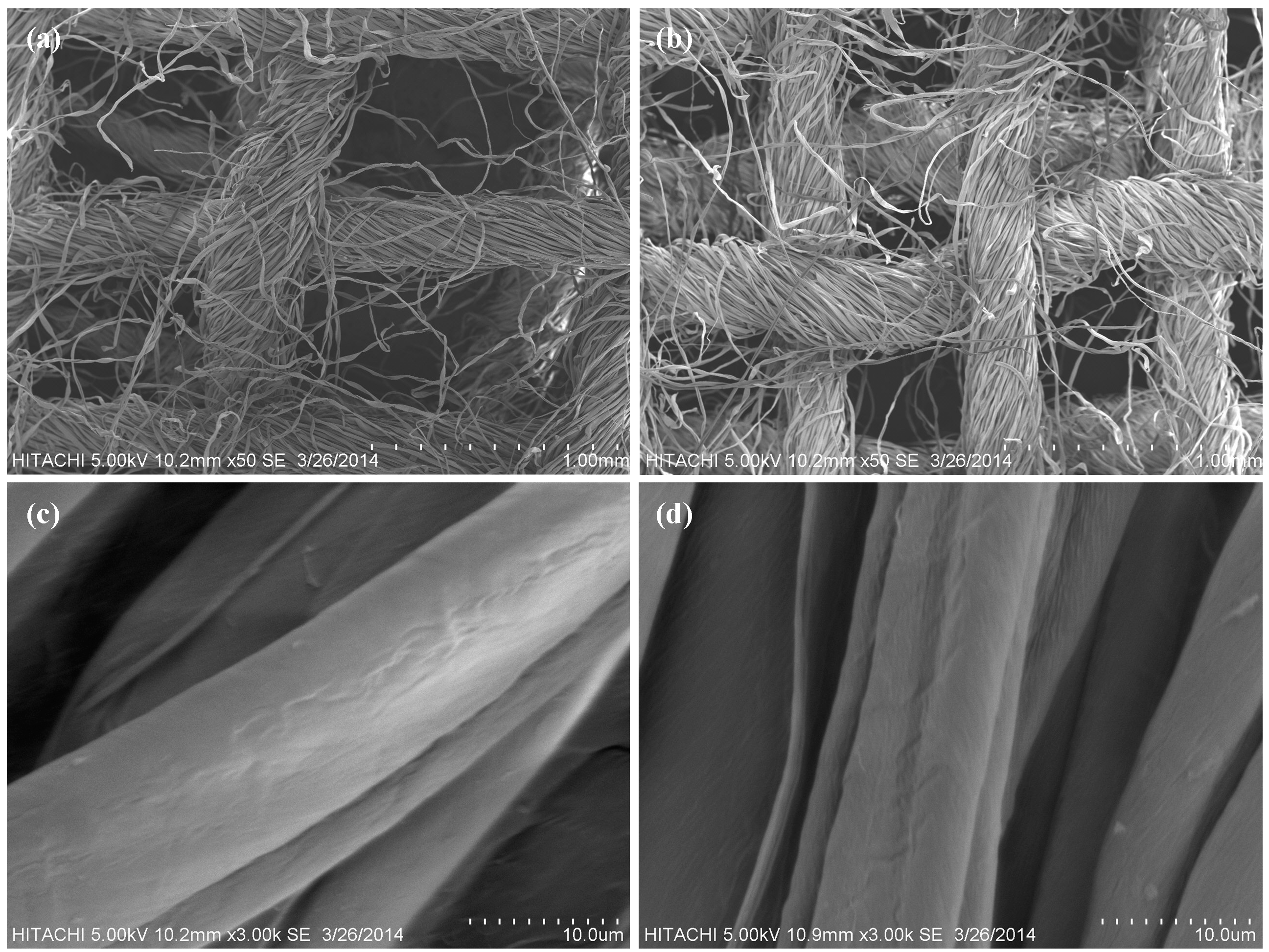
3.2. Morphology Examination

3.3. FTIR Study
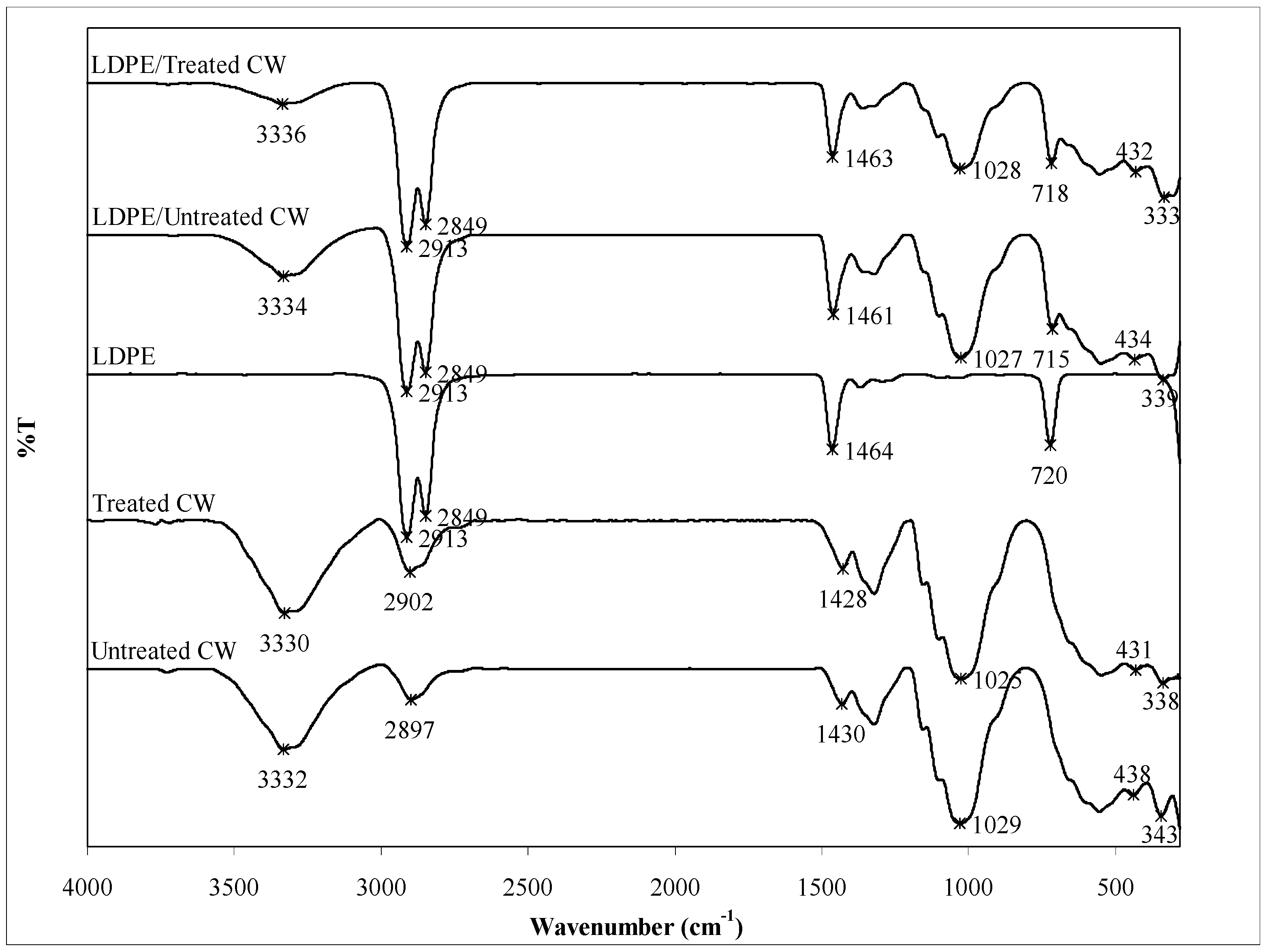
| Sample | Wavenumber (cm−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| O–H stretching | CH2 stretching | C–H stretching | CH2 stretching | C–H bending | O–H bending | C–O stretching | CH2 rocking | CH3 wagging | C–C bending | |
| Untreated CW | 3332 | - | 2897 | - | - | 1430 | 1029 | - | 438 | 343 |
| Treated CW | 3330 | - | 2902 | - | - | 1428 | 1025 | - | 431 | 338 |
| LDPE | - | 2913 | - | 2849 | 1464 | - | - | 720 | - | - |
| LDPE/untreated CW | 3334 | 2913 | - | 2849 | 1461 | - | 1027 | 715 | 434 | 339 |
| LDPE/treated CW | 3336 | 2913 | - | 2849 | 1463 | - | 1028 | 718 | 432 | 333 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic bionanocomposites: A review of preparation, properties and applications. Polymers 2010, 2, 728–765. [Google Scholar] [CrossRef]
- Qu, P.; Gao, Y.; Wu, G.; Zhang, L. Nanocomposites of poly(lactic acid) reinforced with cellulose nanofibrils. BioResources 2010, 5, 1811–1823. [Google Scholar]
- Ten, E.; Vermerris, W. Functionalized polymers from lignocellulosic biomass: State of the art. Polymers 2013, 5, 600–642. [Google Scholar]
- Hanifawati, I.N.; Azmah Hanim, M.A.; Sapuan, S.M.; Zainudin, E.S. Tensile and flexural behaviour of hybrid banana pseudostem/glass fiber reinforced polyester composites. Key Eng. Mater. 2011, 471–472, 686–691. [Google Scholar]
- Shamsuri, A.A.; Abdullah, D.K.; Daik, R. Fabrication of agar/biopolymer blend aerogels in ionic liquid and co-solvent mixture. Cell. Chem. Technol. 2012, 46, 45–52. [Google Scholar]
- Pracella, M.; Haque, M.U.; Alvarez, V. Functionalization, compatibilization and properties of polyolefin composites with natural fibers. Polymers 2010, 2, 554–574. [Google Scholar] [CrossRef]
- Nozari, O.; Madanipour, M.; Farsi, M.; Tabei, A. Mechanical properties and water uptake of nanoclay/wood flour/LDPE composites after fiber surface mercerization. Cell. Chem. Technol. 2013, 47, 295–301. [Google Scholar]
- Mei, C.; Fan, Y.; Mei, L.; Luo, G. Surface characteristics of modified poplar wood-flour and its effect on the mechanical properties of wood polymer composites. Rev. Adv. Mater. Sci. 2013, 33, 203–210. [Google Scholar]
- Chen, J.; Wang, Y.; Gu, C.; Liu, J.; Liu, Y.; Li, M.; Lu, Y. Enhancement of the mechanical properties of basalt fiber-wood-plastic composites via maleic anhydride grafted high-density polyethylene (MAPE) addition. Materials 2013, 6, 2483–2496. [Google Scholar] [CrossRef]
- Fan, Y.; Mei, C.; Liu, Y.; Mei, L. Effect of surface free energy of wood-flour and its polar component on the mechanical and physical properties of wood-thermoplastic composites. Rev. Adv. Mater. Sci. 2013, 33, 211–218. [Google Scholar]
- Wang, X.; Cui, Y.; Xu, Q.; Xie, B.; Li, W. Effects of alkali and silane treatment on the mechanical properties of jute-fiber-reinforced recycled polypropylene composites. J. Vinyl Addit. Technol. 2010, 16, 183–188. [Google Scholar] [CrossRef]
- Dzulkefly, K.; Khoh, H.F.; Ahmad, F.B.H.; Ahmad, S.A.; Lim, W.H. Solvent-free esterification process for the synthesis of glucose bolaform surfactants. Orient. J. Chem. 2010, 26, 747–752. [Google Scholar]
- Shamsuri, A.A.; Daik, R.; Zainudin, E.S.; Tahir, P.M. Compatibilization of HDPE/agar biocomposites with eutectic-based ionic liquid containing surfactant. J. Reinf. Plast. Compos. 2014, 33, 440–453. [Google Scholar] [CrossRef]
- Daik, R.; Yee, L.C. Preparation of LDPE/LNR blend via emulsion dispersion. Sains Malays. 2007, 36, 183–188. [Google Scholar]
- Shamsuri, A.A.; Daik, R.; Ahmad, I.; Jumali, M.H.H. Nylon-6/liquid natural rubber blends prepared via emulsion dispersion. J. Polym. Res. 2009, 16, 381–387. [Google Scholar] [CrossRef]
- Mondragón, M.; Arroyo, K.; Romero-García, J. Biocomposites of thermoplastic starch with surfactant. Carbohydr. Polym. 2008, 74, 201–208. [Google Scholar]
- Islam, M.N.; Islam, M.S. Mechanical properties of chemically treated sawdust-reinforced recycled polyethylene composites. Ind. Eng. Chem. Res. 2011, 50, 11124–11129. [Google Scholar] [CrossRef]
- Silviya, E.K.; Unnikrishnan, G.; Varghese, S.; Guthrie, J.T. Surfactant effects on poly(ethylene-co-vinyl acetate)/cellulose composites. Compos. B Eng. 2013, 47, 137–144. [Google Scholar] [CrossRef]
- Standard Test Method for Tensile Properties of Plastics; ASTM D638-10; American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2010.
- Shamsuri, A.A.; Abdullah, D.K. A preliminary study of oxidation of lignin from rubber wood to vanillin in ionic liquid medium. Oxid. Commun. 2012, 35, 767–775. [Google Scholar]
- Alavudeen, A.; Thiruchitrambalam, M.; Venkateshwaran, N.; Athijayamani, A. Review of natural fiber reinforced woven composite. Rev. Adv. Mater. Sci. 2011, 27, 146–150. [Google Scholar]
- Ariffin, A.; Mansor, A.S.; Jikan, S.S.; Mohd Ishak, Z.A. Evaluation of hybridizing talc and surface-treated kaolin on the properties of PP hybrid composites. J. Reinf. Plast. Compos. 2010, 29, 3429–3441. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Daik, R. Plasticizing effect of choline chloride/urea eutectic-based ionic liquid on physicochemical properties of agarose films. BioResources 2012, 7, 4760–4775. [Google Scholar]
- Wu, J.; Zheng, Y.; Yang, Z.; Cui, Q.; Wang, Q.; Gao, S.; Ding, X. Chemical modifications and characteristic changes in bacterial cellulose treated with different media. J. Polym. Res. 2012, 19, 1–8. [Google Scholar] [CrossRef]
- Flores-Hernández, C.G.; Colín-Cruz, A.; Velasco-Santos, C.; Castaño, V.M.; Rivera-Armenta, J.L.; Almendarez-Camarillo, A.; García-Casillas, P.E.; Martínez-Hernández, A.L. All green composites from fully renewable biopolymers: Chitosan-starch reinforced with keratin from feathers. Polymers 2014, 6, 686–705. [Google Scholar]
- Shamsuri, A.A.; Daik, R. Utilization of an ionic liquid/urea mixture as a physical coupling agent for agarose/talc composite films. Materials 2013, 6, 682–698. [Google Scholar] [CrossRef]
- Huang, F.Y. Thermal properties and thermal degradation of cellulose tri-stearate (CTs). Polymers 2012, 4, 1012–1024. [Google Scholar] [CrossRef]
- Mani, P.; Suresh, S. Vibrational spectra and normal coordinate analysis of acetic acid cyclohexyl ester. Rasayan J. Chem. 2009, 2, 307–311. [Google Scholar]
- Poptoshev, E. Polyelectrolyte Moderated Interactions between Glass and Cellulose Surfaces. P.h.D Thesis, Royal Institute of Technology, Stockholm, Sweden, 13 November 2001. [Google Scholar]
- Sood, Y.V.; Tyagi, R.; Tyagi, S.; Pande, P.C.; Tondon, R. Surface charge of different paper making raw materials and its influence on paper properties. J. Sci. Ind. Res. 2010, 69, 300–304. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shamsuri, A.A.; Azid, M.K.A.; Ariff, A.H.M.; Sudari, A.K. Influence of Surface Treatment on Tensile Properties of Low-Density Polyethylene/Cellulose Woven Biocomposites: A Preliminary Study. Polymers 2014, 6, 2345-2356. https://doi.org/10.3390/polym6092345
Shamsuri AA, Azid MKA, Ariff AHM, Sudari AK. Influence of Surface Treatment on Tensile Properties of Low-Density Polyethylene/Cellulose Woven Biocomposites: A Preliminary Study. Polymers. 2014; 6(9):2345-2356. https://doi.org/10.3390/polym6092345
Chicago/Turabian StyleShamsuri, Ahmad Adlie, Muhammad Kamarul Azroy Azid, Azmah Hanim Mohamed Ariff, and Ahmad Khuzairi Sudari. 2014. "Influence of Surface Treatment on Tensile Properties of Low-Density Polyethylene/Cellulose Woven Biocomposites: A Preliminary Study" Polymers 6, no. 9: 2345-2356. https://doi.org/10.3390/polym6092345