Local Delivery of Antiproliferative Agents via Stents
Abstract
:1. Introduction
2. Antiproliferative Drugs
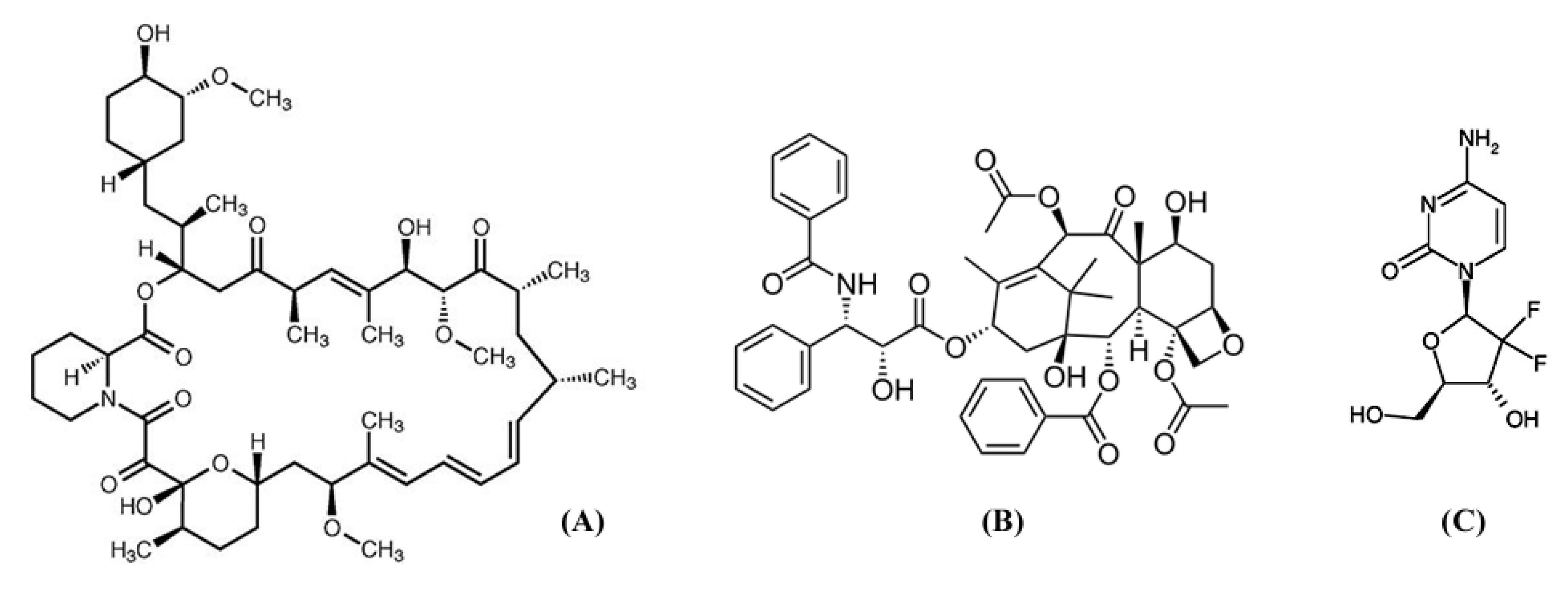
3. Vascular Drug Eluting Stents

| Product (Manufacturer) | Wire material (Specification) | Coating material | Drug |
|---|---|---|---|
| Cypher™ (Johnson & Johnson, New Brunswick, NJ, USA) | Stainless steel (316L) | Poly(ethylene vinyl acetate) (PEVA)–poly(n-butyl methacrylate) (PBMA) | Sirolimus |
| Taxus Express™ (Boston Scientific, Natick, MA, USA) | Stainless steel (316L) | Poly(styrene-b-isobutylene-b-styrene) (SIBS) | Paclitaxel |
| Nevo™ (Johnson & Johnson, New Brunswick, NJ, USA) | Cobalt–chromium (N/A) | Bioabsorbable poly(d,l-lactide-co-glycolide) (PLGA) | Sirolimus |
| Supralimus™ (Sahajanand Medical, Gujarat, India) | Stainless steel (316L) | Bioabsorbable poly(l-lactide) (PLLA)–poly(vinyl-pyrrolidone) (PVP)–PLGA | Sirolimus |
| Endeavor Resolute ZES™ (Medtronic Vascular, Minneapolis, MN, USA) | Cobalt–chromium (MP35N) | Bioabsorbable (BioLinx: C19-PVP-C10) | Zotarolimus |
| BioMatrix™ (Biosensors, Singapore) | Stainless steel (316L) | Bioabsorbable poly(lactic acid) (PLA) | Biolimus A9 |
| EXCEL™ (JW Medical, Weihai, China) | Stainless steel (316L) | Bioabsorbable poly(l-lactic acid) (PLLA) | Sirolimus |
4. Non-Vascular Stents
5. Drug Eluting Non-Vascular Stent
| Covering material | Carrier polymer | Drug | References |
|---|---|---|---|
| PTFE | Polyurethane | Gemcitabine | [7] |
| Acetylated pullulan | Gemcitabine | [8,58] | |
| Silicone | Nitric oxide | [59] | |
| Silicone | Abciximab | [60] | |
| Polyurethane | None | Paclitaxel | [57,61,62] |
| Gemcitabine | [9,37] | ||
| IN-1233 | [45] |
| Solvent | Swelling, % | |
|---|---|---|
| Organic | THF DMAc DMF | Soluble Soluble Soluble |
| Ethanol Methanol Acetone Acetonitrile | 38 26 86 30 | |
| Aqueous co-solvent | 95% Ethanol DMAc + H2O (9:1) DMAc + H2O (8:2) DMAc + H2O (7:3) | 13 70 26 6 |

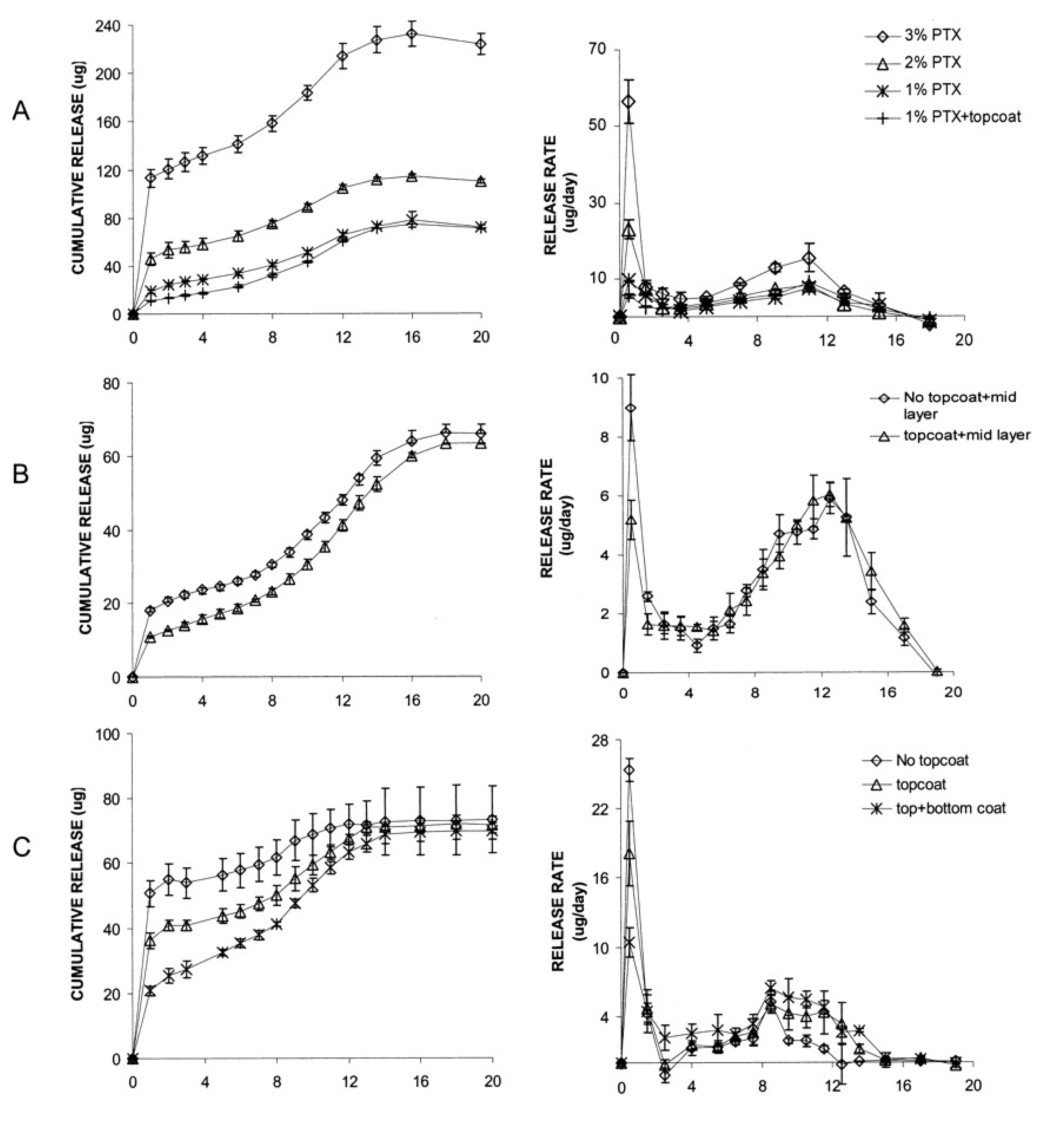

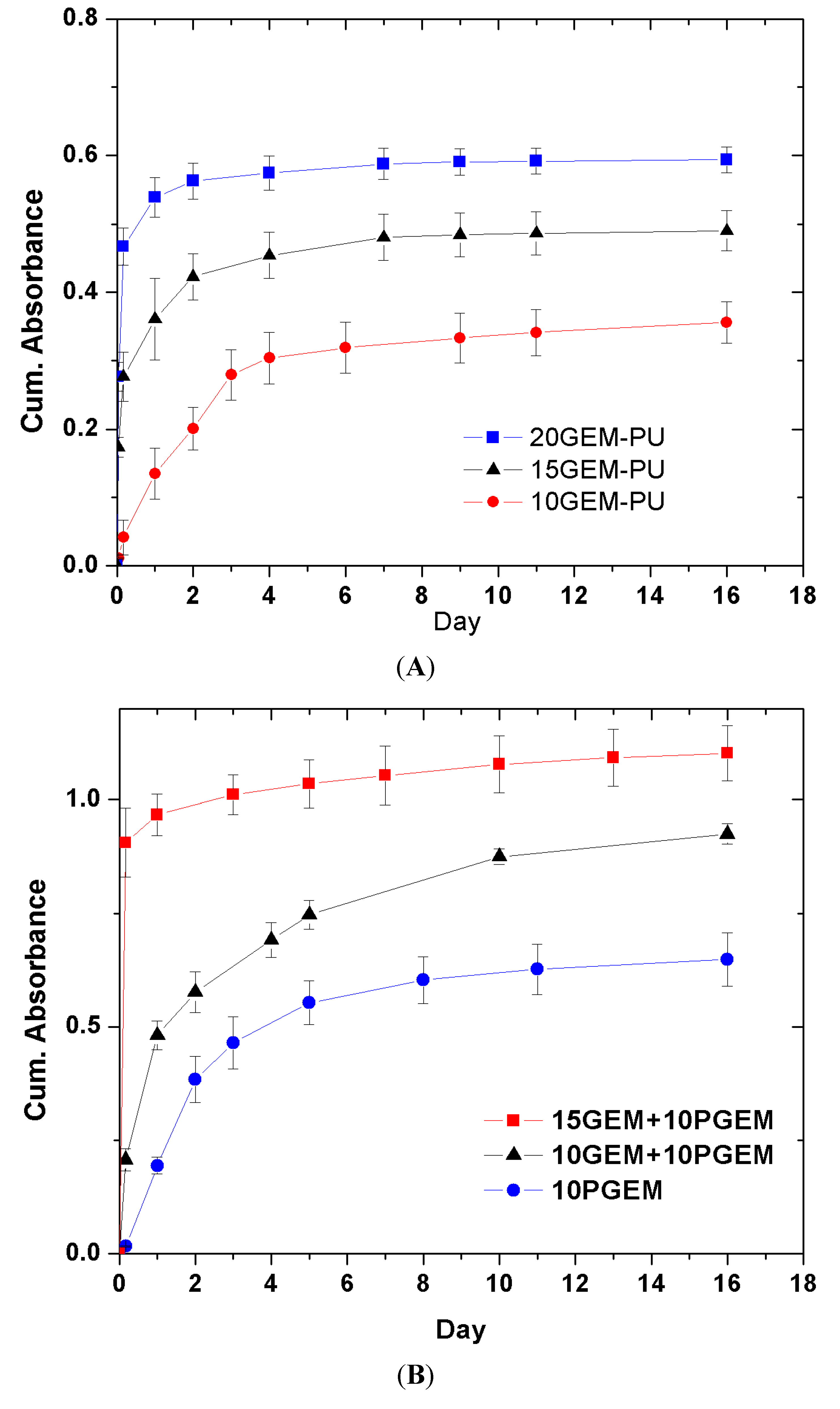

6. Small Interfering RNA Delivery Stent
7. Conclusions
Conflicts of Interest
References
- Yuan, J.G.; Marin, M.L.; Veith, F.J.; Ohki, T.; Sanchez, L.A.; Suggs, W.D.; Lyon, R.T. Endovascular grafts for noninfected aortoiliac anastomotic aneurysms. J. Vasc. Surg. 1997, 26, 210–221. [Google Scholar] [CrossRef]
- Bonello, L.; Com, O.; Gaubert, J.Y.; Sbraggia, P.; Paganelli, F. Covered stent for closure of symptomatic plexus-like coronary fistula. Int. J. Cardiol. 2006, 109, 408–410. [Google Scholar] [CrossRef]
- Bitigen, A.; Cevik, C.; Turan, B.; Otahbachi, M. Use of PTFE-covered stent in acute myocardial infarction of aneurysmatic coronary artery. Int. J. Cardiol. 2009, 132, 72–73. [Google Scholar] [CrossRef]
- Zhuang, Z.W.; Hoopes, P.J.; Koutras, P.C.; Ebbighausen, W.H.; Wagner, R.J.; Bettmann, M.A. Transjugular intrahepatic portosystemic shunt with an autologous vein-covered Stent: Results in a swine model. J. Vasc. Interv. Radiol. 2001, 12, 1333–1342. [Google Scholar] [CrossRef]
- Patel, J.V.; Rossbach, M.M.; Cleveland, T.J.; Gaines, P.A.; Beard, J.D. Endovascular stent-graft repair of traumatic carotid artery pseudoaneurysm. Clinic. Radiol. 2002, 57, 308–311. [Google Scholar] [CrossRef]
- Angeloni, S.; Merli, M.; Salvatori, F.M.; Santis, A.D.; Fanelli, F.; Pepino, D.; Attili, A.F.; Rossi, P.; Riggio, O. Polytetrafluoroethylene-covered stent grafts for TIPS procedure: 1-Year patency and clinical results. J. Hepatol. 2005, 42, 75–76. [Google Scholar]
- Lee, J.W.; Yang, S.G.; Na, K. Gemcitabine-releasing polymeric films for covered self-expandable metallic stent in treatment of gastrointestinal cancer. Int. J. Pharm. 2012, 427, 276–283. [Google Scholar] [CrossRef]
- Moon, S.; Yang, S.G.; Na, K. An acetylated polysaccharide-PTFE membrane-covered stent for the delivery of gemcitabine for treatment of gastrointestinal cancer and related stenosis. Biomaterials 2011, 32, 3603–3610. [Google Scholar] [CrossRef]
- Chung, M.J.; Kim, H.; Kim, K.S.; Park, S.; Chung, J.B.; Park, S.W. Safety evaluation of self-expanding metallic biliary stents eluting gemcitabine in a porcine model. J. Gastroen. Hepatol. 2012, 27, 261–267. [Google Scholar] [CrossRef]
- Nelson, D.B.; Silvis, S.E.; Ansel, H.J. Management of a tracheoesophageal fistula with a silicone-covered self-expanding metal stent. Gastrointest. Endosc. 1994, 40, 497–499. [Google Scholar] [CrossRef]
- Chae, E.Y.; Shin, J.H.; Song, H.Y.; Kim, J.H.; Shim, T.S.; Kim, D.K. Bronchopleural fistula treated with a silicone-covered bronchial occlusion stent. Ann. Thorac. Surg. 2010, 89, 293–296. [Google Scholar]
- Kim, J.H.; Shin, J.H.; Song, H.Y.; Lee, S.C.; Kim, K.R.; Park, J.H. Use of a retrievable metallic stent internally coated with silicone to treat airway obstruction. J. Vasc. Interv. Radiol. 2008, 19, 1208–1214. [Google Scholar] [CrossRef]
- Yoon, C.J.; Song, H.Y.; Kim, J.H.; Park, H.G.; Kang, H.S.; Ro, J.Y.; Hong, J.H. Temporary placement of a covered, retrievable, barbed stent for the treatment of hormone-induced benign prostatic hyperplasia: Technical feasibility and histologic changes in canine prostates. J. Vasc. Interv. Radiol. 2010, 21, 1429–1435. [Google Scholar] [CrossRef]
- Na, H.K.; Song, H.Y.; Yeo, H.J.; Park, J.H.; Kim, J.H.; Park, H.; Kim, C.S. Retrospective comparison of internally and externally covered retrievable stent placement for patients with benign urethral strictures caused by traumatic injury. Am. J. Roentgenol. 2012, 198, W55–W61. [Google Scholar] [CrossRef]
- Kastrati, A.; Schömig, A.; Elezi, S.; Schühlen, H.; Dirschinger, J.; Hadamitzky, M.; Wehinger, A.; Hausleiter, J.; Walter, H.; Neumann, F.J. Predictive factors of restenosis after coronary stent placement. J. Am. Coll. Cardiol. 1997, 30, 1428–1436. [Google Scholar] [CrossRef]
- Song, H.Y.; Do, Y.S.; Han, Y.M.; Sung, K.B.; Choi, E.K.; Sohn, K.H.; Kim, H.R.; Kim, S.H.; Min, Y.I. Covered, expandable esophageal metallic stent tubes: Experiences in 119 patients. Radiology 1994, 193, 689–696. [Google Scholar]
- Song, H.Y.; Park, S.I.; Do, Y.S.; Yoon, H.K.; Sung, K.B.; Sohn, K.H.; Min, Y.I. Expandable metallic stent placement in patients with benign esophageal strictures: Results of long-term follow-up. Radiology 1997, 203, 131–136. [Google Scholar]
- Ahmad, F.S.; Katsanos, K.; Fotiadis, N.; Gulati, M.; Sabharwal, T.; Adam, A. Management of complications following percutaneous biliary interventions. Cardiovasc. Intervent. Radiol. 2009, 32, S1–S27. [Google Scholar]
- Serruys, P.W.; Foley, D.P.; Jackson, G.; Bonnier, H.; Macaya, C.; Vrolix, M.; Branzi, A.; Shepherd, J.; Suryapranata, H.; de Feyter, P.J.; et al. A randomized placebo-controlled trial of fluvastatin for prevention of restenosis after successful coronary balloon angioplasty; final results of the fluvastatin angiographic restenosis (FLARE) trial. Eur. Heart. J. 1999, 20, 58–69. [Google Scholar] [CrossRef]
- Holmes, D.R., Jr.; Savage, M.; LaBlanche, J.M.; Grip, L.; Serruys, P.W.; Fitzgerald, P.; Fischman, D.; Goldberg, S.; Brinker, J.A.; Zeiher, A.M.; et al. Results of prevention of restenosis with tranilast and its outcomes (PRESTO) trial. Circulation 2002, 106, 1243–1250. [Google Scholar] [CrossRef]
- Stettler, C.; Allemann, S.; Wandel, S.; Kastrati, A.; Morice, M.C.; Schömig, A.; Pfisterer, M.; Stone, G.; Leon, M.; Lezo, J.S.; et al. Drug eluting and bare metal stents in people with and without diabetes: Collaborative network meta-analysis. Br. Med. J. 2008, 337. [Google Scholar]
- Marx, S.O.; Marks, A.R. Bench to bedside the development of rapamycin and its application to stent restenosis. Circulation 2001, 104, 852–855. [Google Scholar] [CrossRef]
- Gregory, C.R.; Huie, P.; Billingham, M.E.; Morris, R.E. Rapamycin inhibits arterial intimal thickening caused by both alloimmune and mechanical injury: Its effect on cellular, growth factor, and cytokine response in injured vessels. Transplantation 1993, 55, 1409–1418. [Google Scholar] [CrossRef]
- Poon, M.; Marx, S.O.; Gallo, R.; Badimon, J.J.; Taubman, M.B.; Marks, A.R. Rapamycin inhibits vascular smooth muscle cell migration. J. Clin. Invest. 1996, 98, 2277–2283. [Google Scholar] [CrossRef]
- Wessely, R.; Schömig, A.; Kastrati, A. Sirolimus and paclitaxel on polymer-based drug-eluting stents: Similar but different. J. Am. Coll. Cardiol. 2006, 47, 708–714. [Google Scholar] [CrossRef]
- Moreno, R. Drug-eluting stents and other anti-restenosis devices. Rev. Esp. Cardiol. 2005, 58, 842–862. [Google Scholar] [CrossRef]
- Ribamar Coata, J.; Abizaid, A.; Feres, F.; Costa, R.; Seixas, A.C.; Maia, F.; Eduardo Sousa, J. EXCELLA first-in-man (FIM) study: Safety and efficacy of novolimus-eluting stent in de novo coronary lesions. EuroIntervention 2008, 4, 53–58. [Google Scholar] [CrossRef]
- Ormiston, J.A.; Serruys, P.W.; Regar, E.; Dudek, D.; Thuesen, L.; Webster, M.W.; Garcia-Garcia, H.M.; McGreevy, R.; Veldhof, S. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): A prospective open-label trial. Lancet 2008, 371, 899–907. [Google Scholar] [CrossRef]
- Hamada, N.; Miyata, M.; Eto, H.; Shirasawa, T.; Akasaki, Y.; Nagaki, A.; Tei, C. Tacrolimus-eluting stent inhibits neointimal hyperplasia via calcineurin/NFAT signaling in porcine coronary artery model. Atherosclerosis 2010, 208, 97–103. [Google Scholar] [CrossRef]
- Herdeg, C.; Oberhoff, M.; Baumbach, A.; Blattner, A.; Axel, D.I.; Schröder, S.; Heinle, H.; Karsch, K.R. Local paclitaxel delivery for the prevention of restenosis: Biological effects and efficacy in vivo. J. Am. Coll. Cardiol. 2000, 35, 1969–1976. [Google Scholar] [CrossRef]
- Heldman, A.W.; Cheng, L.; Jenkins, G.M.; Heller, P.F.; Kim, D.W.; Ware, M., Jr.; Nater, C.; Hruban, R.H.; Rezai, B.; Abella, B.S.; et al. Paclitaxel stent coating inhibits neointimal hyperplasia at 4 weeks in a porcine model of coronary restenosis. Circulation 2001, 103, 2289–2295. [Google Scholar] [CrossRef]
- Abizaid, A.; Albertal, M.; Costa, M.A.; Abizaid, A.S.; Staico, R.; Feres, F.; Sousa, J.E. First human experience with the 17-β-estradiol-eluting stent: The estrogen and stents to eliminate restenosis (EASTER) trial. J. Am. Coll. Cardiol. 2004, 43, 1118–1121. [Google Scholar] [CrossRef]
- Adriaenssens, T.; Mehilli, J.; Wessely, R.; Ndrepepa, G.; Seyfarth, M.; Wieczorek, A.; Schömig, A. Does addition of estradiol improve the efficacy of a rapamycin-eluting stent? Results of the ISAR-PEACE randomized trial. J. Am. Coll. Cardiol. 2007, 49, 1265–1271. [Google Scholar] [CrossRef]
- Patti, G.; Pasceri, V.; Carminati, P.; D’Ambrosio, A.; Carcagnì, A.; di Sciascio, G. Effect of dexamethasone-eluting stents on systemic inflammatory response in patients with unstable angina pectoris or recent myocardial infarction undergoing percutaneous coronary intervention. J. Am. Cardiol. 2005, 95, 502–505. [Google Scholar] [CrossRef]
- Ferrero, V.; Ribichini, F.; Rognoni, A.; Marino, P.; Brunelleschi, S.; Vassanelli, C. Comparison of efficacy and safety of lower-dose to higher-dose oral prednisone after percutaneous coronary interventions (the IMPRESS-LD study). Am. J. Cardiol. 2007, 99, 1082–1086. [Google Scholar] [CrossRef]
- Pesarini, G.; Ferrero, V.; Tomai, F.; Paloscia, L.; de Cesare, N.; Tamburino, C.; Ribichini, F. Steroid-eluting stents in patients with acute coronary syndromes. Angiographic results of DESIRE: Dexamethasone-eluting stent Italian registry. J. Invasive Cardiol. 2009, 21, 86–91. [Google Scholar]
- Shin, M.S.; Hong, J.Y.; Park, S. Gemcitabine release behavior of polyurethane matrixes designed for local anti-cancer drug delivery via stent. J. Drug Deliv. Sci. Technol. 2012, 22, 301–306. [Google Scholar]
- Moses, J.W.; Leon, M.B.; Popma, J.J.; Fitzgerald, P.J.; Holmes, D.R.; O’Shaughnessy, C.; Kuntz, R.E. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 2003, 349, 1315–1323. [Google Scholar] [CrossRef]
- Stone, G.W.; Ellis, S.G.; Cox, D.A.; Hermiller, J.; O’Shaughnessy, C.; Mann, J.T. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N. Engl. J. Med. 2004, 350, 221–231. [Google Scholar] [CrossRef]
- Foerst, J.; Vorpahl, M.; Engelhardt, M.; Koehler, T.; Tiroch, K.; Wessely, R. Evolution of coronary stents: From bare-metal stents to fully biodegradable, drug-eluting stents. Comb. Prod. Ther. 2013, 3, 9–24. [Google Scholar] [CrossRef]
- Krucoff, M.W.; Kereiakes, D.J.; Petersen, J.L.; Mehran, R.; Hasselblad, V.; Lansky, A.J. A novel bioresorbable polymer paclitaxel-eluting stent for the treatment of single and multivessel coronary disease: Primary results of the COSTAR (cobalt chromium stent with antiproliferative for restenosis) II study. J. Am. Coll. Cardiol. 2008, 51, 1543–1552. [Google Scholar] [CrossRef]
- Dani, S.; Kukreja, N.; Parikh, P.; Joshi, H.; Prajapati, J.; Jain, S.; Thanvi, S.; Shah, B.; Dutta, J.P. Biodegradable-polymer-based, sirolimus-eluting supralimus stent: 6-Month angiographic and 30-month clinical follow-up results from the series I prospective study. Eur. Intervention. 2008, 4, 59–63. [Google Scholar]
- Gutiérrez-Chico, J.L.; van Geuns, R.J.; Regar, E.; Giessen, W.J.; Kelbæk, H.; Saunamäki, K.; Escaned, J.; Gonzalo, N.; Mario, C.; Borgia, F.; et al. Tissue coverage of a hydrophilic polymer-coated zotarolimus-eluting stent vs. a fluoropolymer-coated everolimus-eluting stent at 13-month follow-up: An optical coherence tomography substudy from the resolute all comers trial. Eur. Heart J. 2011, 32, 2454–2463. [Google Scholar] [CrossRef]
- Huang, Y.; Venkatraman, S.S.; Boey, F.Y.; Lahti, E.M.; Umashankar, P.R.; Mohanty, M.; Arumugam, S.; Khanolkar, L.; Vaishnav, S. In vitro and in vivo performance of a dual drug-eluting stent (DDES). Biomaterials 2010, 31, 4382–4391. [Google Scholar] [CrossRef]
- Kim, E.Y.; Song, H.Y.; Kim, J.H.; Fan, Y.; Park, S.; Kim, D.K.; Na, H.K. IN-1233–eluting covered metallic stent to prevent hyperplasia: Experimental study in a rabbit esophageal model. Radiology 2013, 267, 396–404. [Google Scholar] [CrossRef]
- Guo, Q.; Guo, S.; Wang, Z. A type of esophageal stent coating composed of one 5-fluorouracil-containing EVA layer and one drug-free protective layer: In vitro release, permeation and mechanical properties. J. Control. Release 2007, 118, 318–324. [Google Scholar] [CrossRef]
- Wikol, M.; Hartmann, B.; Brendle, J.; Crane, M.; Beuscher, U.; Brake, J.; Gore, W.L. Expanded polytetrafluoroethylenemembranes and their applications. Drugs Pharm. Sci. 2008, 174, 619–640. [Google Scholar]
- Melissano, G.; Civilini, E.; Bertoglio, L.; Setacci, F.; Chiesa, R. Endovascular treatment of aortic arch aneurysms. Eur. J. Vasc. Endovasc. 2005, 29, 131–138. [Google Scholar] [CrossRef]
- Shimono, T.; Kato, N.; Yasuda, F.; Suzuki, T.; Yuasa, U.; Onoda, K.; Yada, I. Transluminal stent-graft placements for the treatments of acute onset and chronic aortic dissections. Circulation 2002, 106, 241–247. [Google Scholar]
- Curtis, J.; Colas, A. Silicone Biomaterials: History and Chemistry. In Biomaterials Science: An Introduction to Materials in Medicine, 2nd ed.; Rutner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Elsevier Academic Press: Melville, NY, USA, 2004. [Google Scholar]
- Curtis, J.; Colas, A. Medical Applications of Silicones. In Biomaterials Science: An Introduction to Materials in Medicine, 2nd ed.; Rutner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Elsevier Academic Press: Melville, NY, USA, 2004; pp. 697–707. [Google Scholar]
- Hron, P. Hydrophilisation of silicone rubber for medical applications. Polym. Int. 2003, 52, 1531–1539. [Google Scholar] [CrossRef]
- Mashak, A.; Rahimi, A. Silicone polymers in controlled drug delivery systems: A review. Iran. Polym. J. 2009, 18, 279–295. [Google Scholar]
- Nelson, D.B.; Axelrad, A.M.; Fleischer, D.E.; Kozarek, R.A.; Silvis, S.E.; Benjamin, S.B.; Freeman, M.L. Silicone-covered wallstent prototypes for palliation of malignant esophageal obstruction and digestive-respiratory fistulas. Gastrointest. Endosc. 1997, 45, 31–37. [Google Scholar] [CrossRef]
- Tsang, T.K.; Pollack, J.; Chodash, H.B. Silicone-covered metal stents (An in vitro evaluation for biofilm formation and patency). Dig. Dis. Sci. 1999, 44, 1780–1785. [Google Scholar] [CrossRef]
- Bang, B.W.; Jeong, S.; Lee, D.H.; Lee, J.I.; Lee, S.C.; Kang, S.G. The biodurability of covering materials for metallic stents in a bile flow phantom. Dig. Dis. Sci. 2012, 57, 1056–1063. [Google Scholar] [CrossRef]
- Kang, S.G.; Lee, S.C.; Choi, S.H.; Park, S.S.; Jeong, S.; Lee, D.H.; Kim, M. Paclitaxel-polyurethane film for anti-cancer drug delivery: Film characterization and preliminary in vivo study. Macromol. Res. 2010, 18, 680–685. [Google Scholar] [CrossRef]
- Park, T.H.; Jo, E.A.; Na, K. Development of polymeric coating material for effective drug-elutingstent. Polymer (Korea) 2011, 35, 483–487. [Google Scholar]
- Hou, D.; Narciso, H.; Kamdar, K.; Zhang, P.; Barclay, B.; March, K.L. Stent-based nitric oxide delivery reducing neointimal proliferation in a porcine carotid overstretch injury model. Cardiovasc. Intervent. Radiol. 2005, 28, 60–65. [Google Scholar]
- Fontaine, A.B.; Borsa, J.J.; Passos, S.D.; Hoffer, E.K.; Bloch, R.D.; Starr, F.; So, C. Evaluation of local abciximab delivery from the surface of a polymer-coated covered stent: In vivo canine studies. J. Vasc. Interv. Radiol. 2001, 12, 487–492. [Google Scholar] [CrossRef]
- Shin, J.H.; Song, H.Y.; Cho, C.G.; Yuk, S.H.; Kim, J.S.; Kim, Y.M.; Yoon, C.J.; Kim, T.H.; Suh, J.Y.; He, X. Tissue hyperplasia: Influence of a paclitaxel-eluting covered stent-preliminary study in a canine urethral model. Radiology 2005, 234, 438–444. [Google Scholar] [CrossRef]
- Lee, D.K. Drug-eluting stent in malignant biliary obstruction. J. Hepatobiliary Pancreat. Surg. 2009, 16, 628–632. [Google Scholar]
- Jeon, S.R.; Eun, S.H.; Shim, C.S.; Ryu, C.B.; Kim, J.O.; Cho, J.Y.; Lee, J.S.; Lee, M.S.; Jin, S.Y. Effect of drug-eluting metal stents in benign esophageal stricture: An in vivo animal study. Endoscopy 2009, 41, 449–456. [Google Scholar] [CrossRef]
- Lao, L.L.; Venkatraman, S.S. Paclitaxel release from single and double-layered poly(d,l-lactide-co-glycolide)/poly(l-lactide) film for biodegradable coronary stent application. J. Biomed. Mater. Res. A 2008, 87, 1–7. [Google Scholar]
- Finkelstein, A.; McClean, D.; Kar, S.; Takizawa, K.; Varghese, K.; Baek, N.; Park, K.; Fishbein, M.C.; Makkar, R.; Litvack, F.; et al. Local drug delivery via a coronary stent with programmable release pharmacokinetics. Circulation 2003, 107, 777–784. [Google Scholar] [CrossRef]
- Wessely, R.; Hausleiter, J.; Michaelis, C.; Jaschke, B.; Vogeser, M.; Milz, S.; Behnisch, B.; Schratzenstaller, T.; Renke-Gluszko, M.; Stover, M.; et al. Inhibition of neointima formation by a novel drug-eluting stent system that allows for dose-adjustable, multiple, and on-site stent coating. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 748–753. [Google Scholar] [CrossRef]
- Hamada, H.; Ishihara, K.; Masuoka, N.; Mikuni, K.; Nakajima, N. Enhancement of water-solubility and bioactivity of paclitaxel using modified cyclodextrins. J. Biosci. Bioeng. 2006, 102, 369–371. [Google Scholar] [CrossRef]
- Gupta, B.P.; Thakur, N.; Jain, N.P.; Banweer, J.; Jain, S. Osmotically controlled drug delivery system with associated drugs. J. Pharm. Pharm. Sci. 2010, 13, 571–588. [Google Scholar]
- Khan, I.; Smith, N.; Jones, E.; Finch, D.S.; Cameron, R.E. Analysis and evaluation of a biomedical polycarbonate urethane tested in an in vitro study and an ovine arthroplasty model. Part I: Materials selection and evaluation. Biomaterials 2005, 26, 621–631. [Google Scholar] [CrossRef]
- Song, T.J.; Lee, S.S.; Yun, S.C.; Park, D.H.; Seo, D.W.; Lee, S.K.; Kim, M.H. Paclitaxel-eluting covered metal stents versus covered metal stents for distal malignant biliary obstruction: A prospective comparative pilot study. Gastrointest. Endosc. 2011, 73, 727–733. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Dorsett, Y.; Tuschl, T. SiRNAs: Applications in functional genomics and potential as therapeutics. Nat. Rev. Drug. Discov. 2004, 3, 318–329. [Google Scholar] [CrossRef]
- Rana, T.M. Illuminating the silence: Understanding the structure and function of small RNAs. Nat. Rev. Mol. Cell Biol. 2007, 8, 23–36. [Google Scholar] [CrossRef]
- San Juan, A.; Bala, M.; Hlawaty, H.; Portes, P.; Vranckx, R.; Feldman, L.J.; Letourneur, D. Development of a functionalized polymer for stent coating in the arterial delivery of small interfering RNA. Biomacromolecules 2009, 10, 3074–3080. [Google Scholar] [CrossRef]
- Che, H.L.; Bae, I.H.; Lim, K.S.; Song, I.T.; Lee, H.; Muthiah, M.; Namgung, R.; Kim, W.J.; Kim, D.G.; Ahn, Y.; et al. Suppression of post-angioplasty restenosis with an Akt1 siRNA-embeddedcoronary stent in a rabbit model. Biomaterials 2012, 33, 8548–8556. [Google Scholar] [CrossRef]
- Dimitrova, M.; Affolter, C.; Meyer, F.; Nguyen, I.; Richard, D.G.; Schuster, C.; Bartenschlager, R.; Voegel, J.C.; Ogier, J.; Baumert, T.F. Sustained delivery of siRNAs targeting viral infection by cell-degradable multilayered polyelectrolyte films. Proc. Natl. Acad. Sci. 2008, 105, 16320–16325. [Google Scholar] [CrossRef]
- Flessner, R.M.; Jewell, C.M.; Anderson, D.G.; Lynn, D.M. Degradable polyelectrolyte multilayers that promote the release of siRNA. Langmuir 2011, 27, 7868–7876. [Google Scholar] [CrossRef]
- Hossfeld, S.; Nolte, A.; Hartmann, H.; Recke, M.; Schaller, M.; Walker, T.; Kjems, J.; Schlosshauer, B.; Stoll, D.; Wendel, H.P.; et al. Bioactive coronary stent coating based on layer-by-layer technology for siRNA release. Acta Biomater. 2013, 9, 6741–6752. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kwon, H.J.; Park, S. Local Delivery of Antiproliferative Agents via Stents. Polymers 2014, 6, 755-775. https://doi.org/10.3390/polym6030755
Kwon HJ, Park S. Local Delivery of Antiproliferative Agents via Stents. Polymers. 2014; 6(3):755-775. https://doi.org/10.3390/polym6030755
Chicago/Turabian StyleKwon, Hyuck Joon, and Sangsoo Park. 2014. "Local Delivery of Antiproliferative Agents via Stents" Polymers 6, no. 3: 755-775. https://doi.org/10.3390/polym6030755





