Temperature-Responsive Polymer Modified Surface for Cell Sheet Engineering
Abstract
:1. Introduction

2. Temperature-Responsive Cell Culture Surface
2.1. Ultrathin Poly(N-isopropylacrylamide) Gel Modified Surface for Cell Culture
| Amount of grafted PIPAAm (µg/cm2) a | 1.4 ± 0.1 | 2.9 ± 0.1 | |
| Thickness of PIPAAm layer (nm) b | 15.5 ± 7.2 | 29.3 ± 8.4 | |
| Contact angle (cosθ) c | 20 °C | 0.42 | 0.50 |
| 37 °C | 0.20 | 0.35 | |
| Cell adhesion | 20 °C | non-adhesive | – d |
| 37 °C | adhesive | non-adhesive | |

2.2. Cell Sheet Engineering

3. Rapid Recovery of Cell Sheets and Cell Sheet Manipulation Technology

3.1. Poly(N-isopropylacrylamide) Grafted on Porous Membranes
3.2. Poly(N-isopropylacrylamide) Co-Grafted with Poly(ethylene glycol) onto Porous Membranes
| PIPAAm grafted surface | Contact angle (cosθ) a | |
|---|---|---|
| 20 °C | 37 °C | |
| PIPAAm-PM | 0.633 ± 0.025 | 0.528 ± 0.031 |
| P(IPAAm-co-PEG0.1)-PM | 0.711 ± 0.017 | 0.543 ± 0.025 |
| P(IPAAm-co-PEG0.5)-PM | 0.754 ± 0.023 | 0.536 ± 0.016 |

3.3. Comb-Type Grafted Poly(N-isopropylacrylamide) Gel Modified Surface
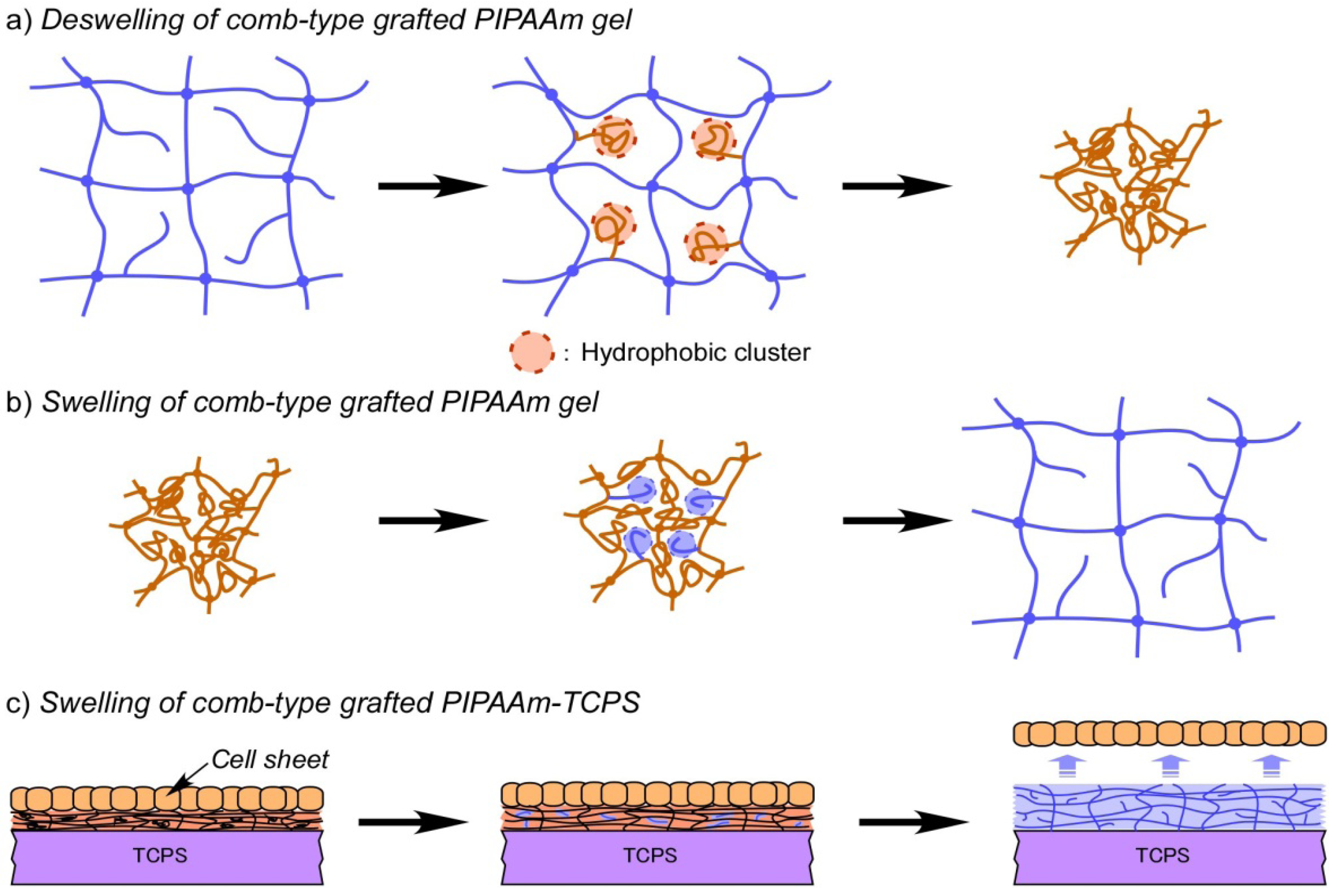
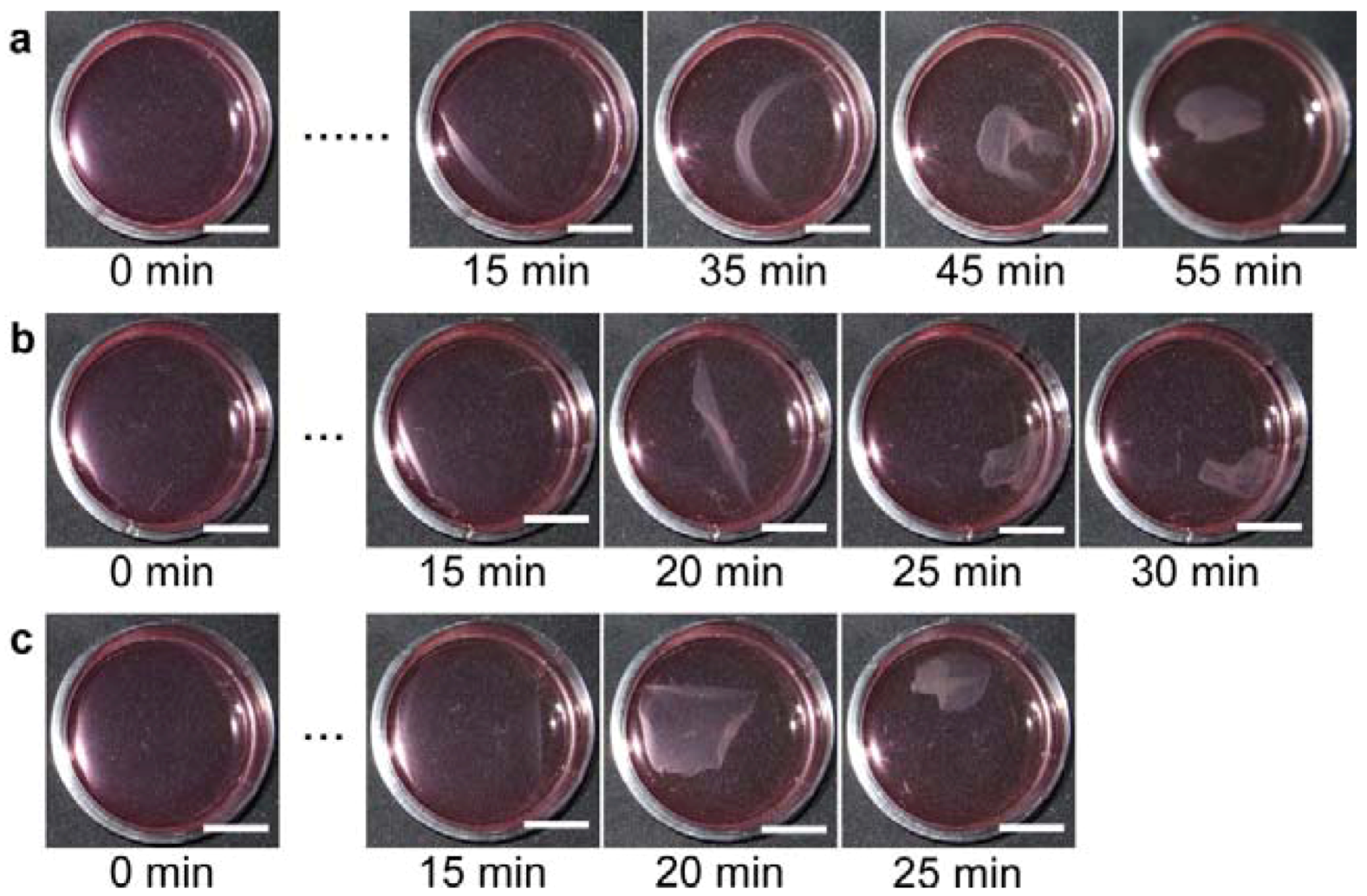
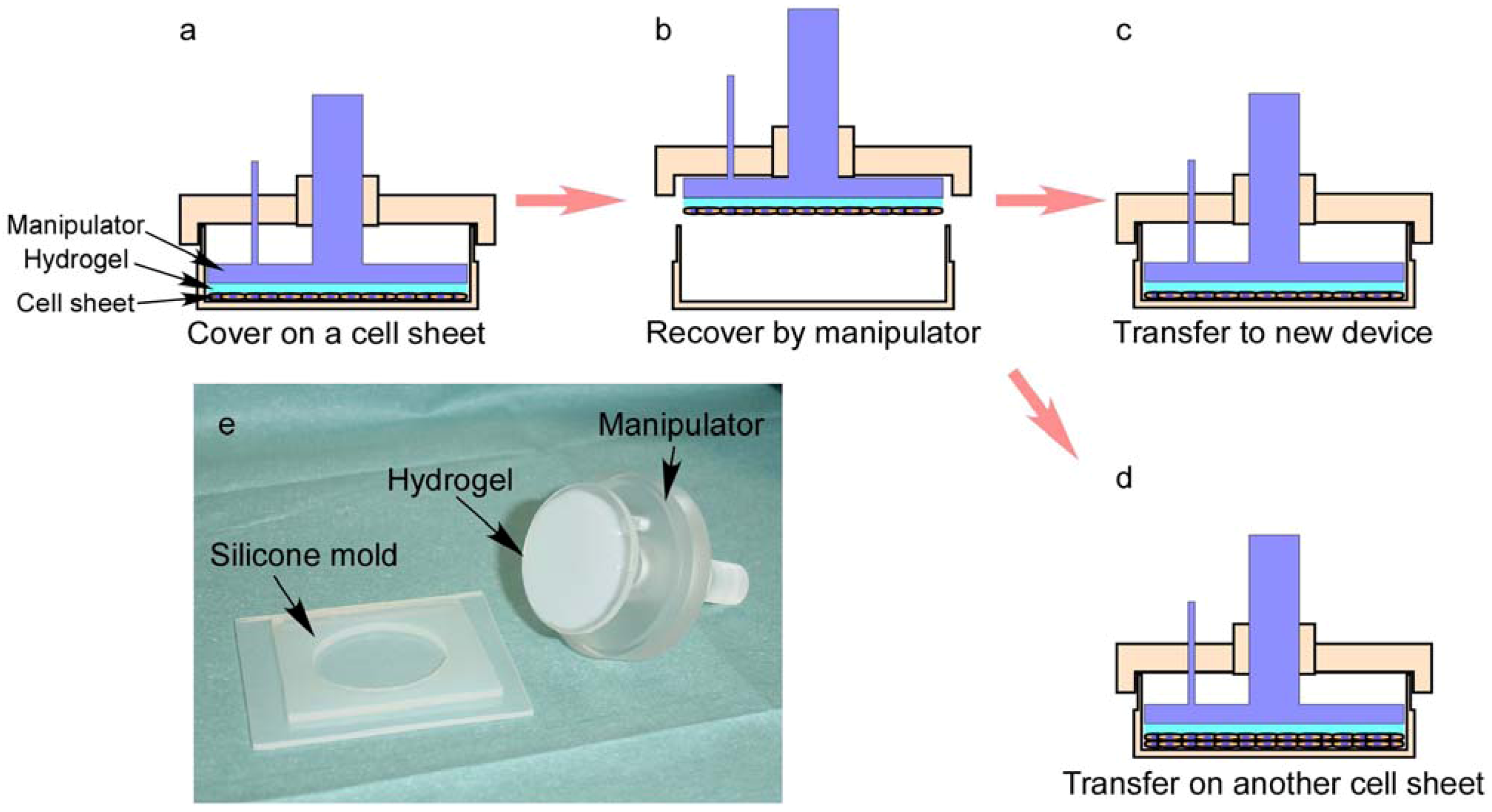
3.4. Fabrication of 3D Cell-Dense Tissue Using a Manipulator
4. Functionalization of Temperature-Responsive Cell Culture Surfaces
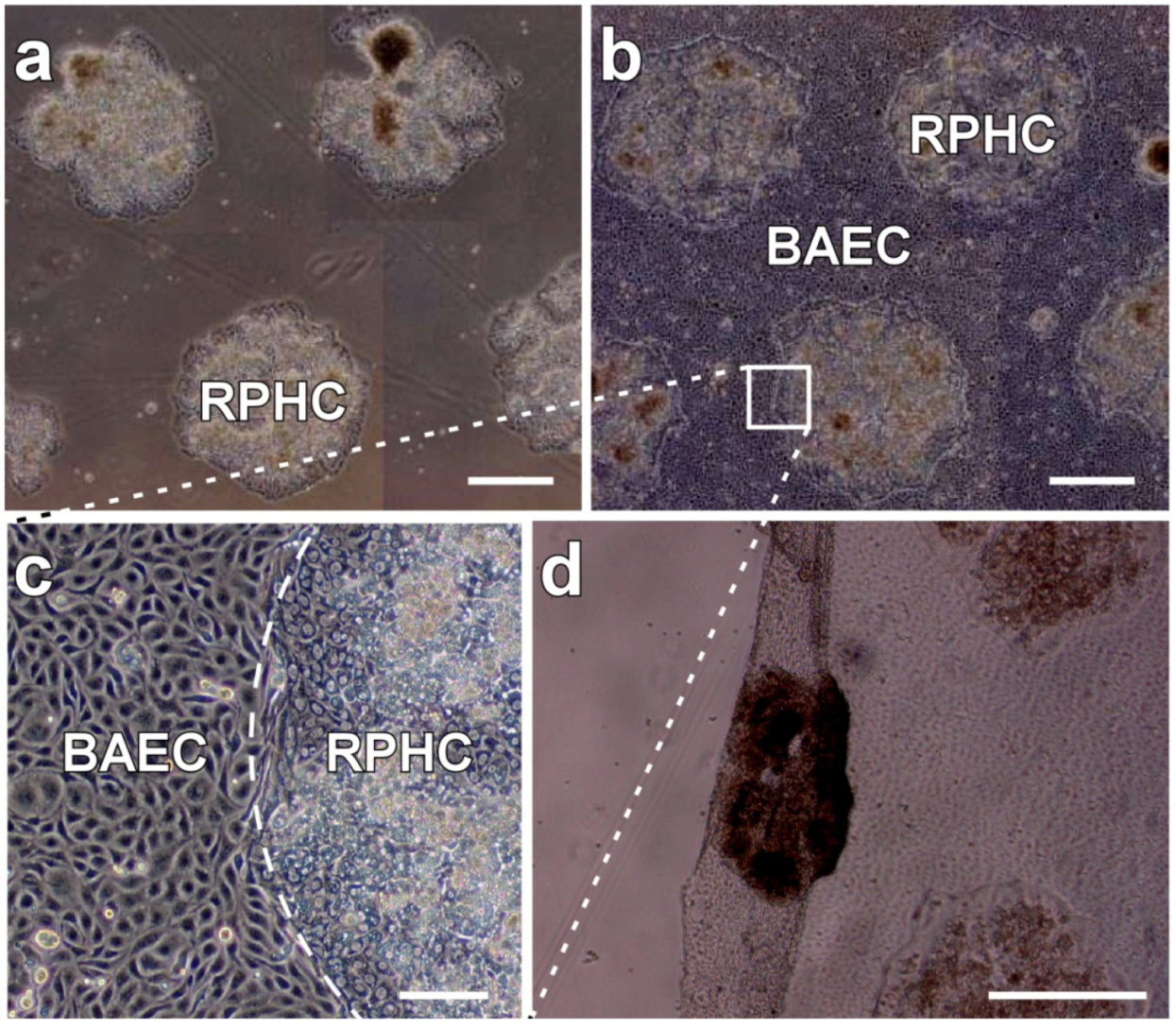
4.1. Patterned Temperature-Responsive Polymer Modified Surface
4.2. Fabricating Biomolecule on Temperature-Responsive Polymer Modified Surface
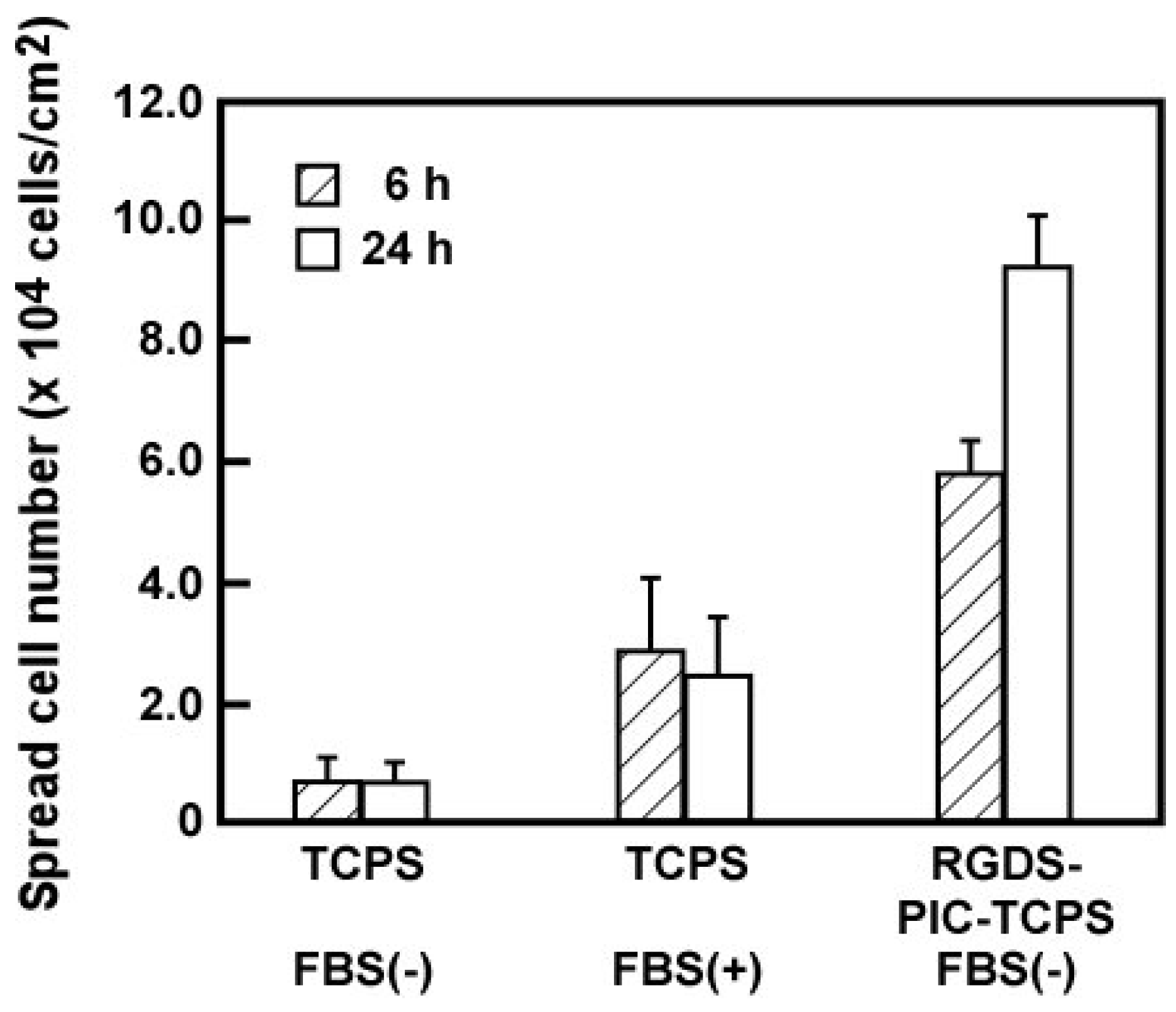
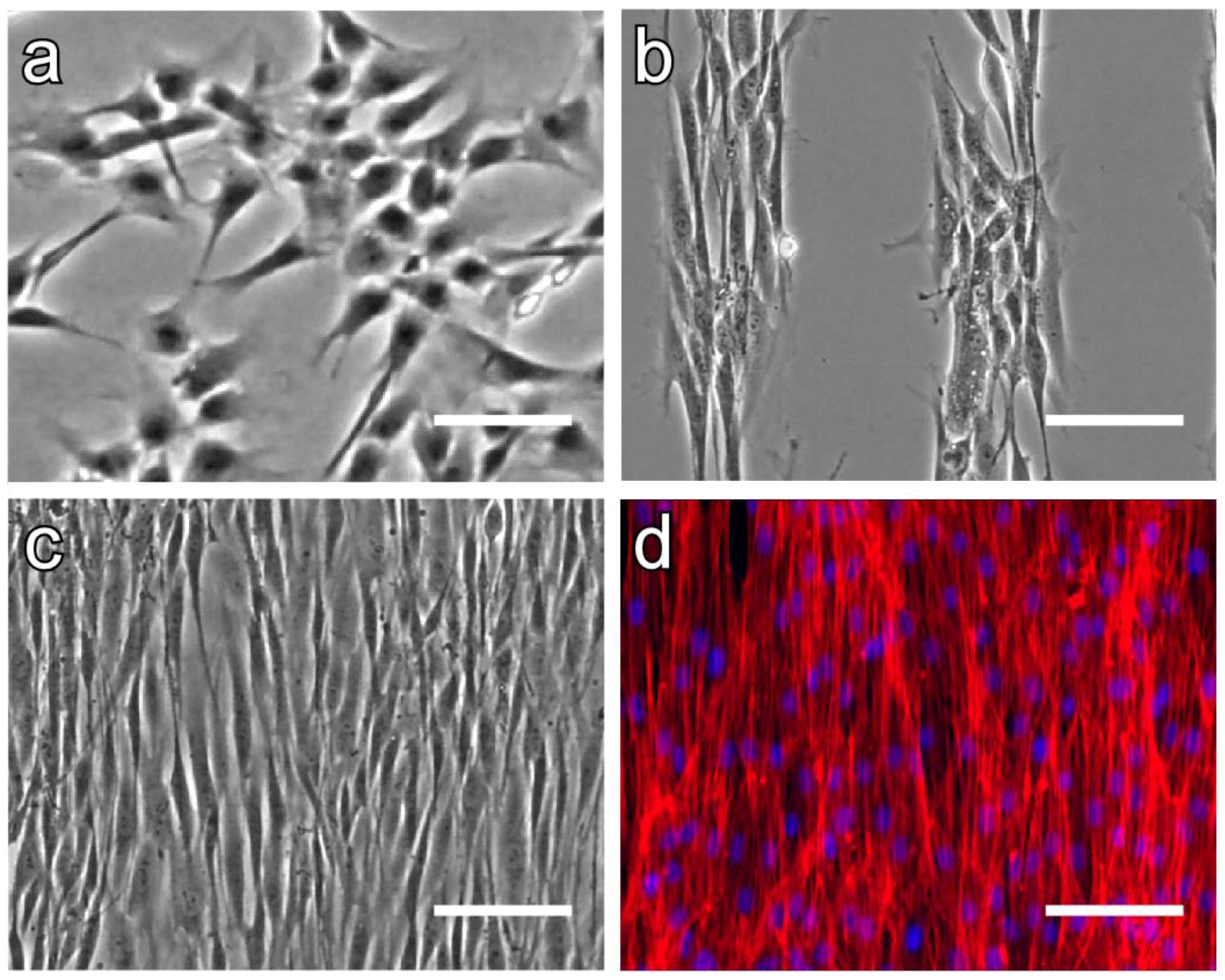
4.3. Micropatterned Temperature-Responsive Polymer Brush Surfaces for Fabricating Cell Sheets with Well-Controlled Orientational Structures

4. Conclusions
Acknowledgements
References
- Kopecek, J.; Vacik, J.; Lim, D. Permeability of membranes containing ionogenic groups. J. Polym. Sci. A Polym. Chem. 1971, 9, 2801–2815. [Google Scholar] [CrossRef]
- Tanaka, T.; Nishino, I.; Sun, S.-T.; Ueno-Nishio, S. Collapse of gels in an electric field. Science 1981, 218, 467–469. [Google Scholar]
- Ishihara, K.; Muramoto, N.; Shinohara, I. Controlled release of organic substances using polymer membrane with responsive function for amino compounds. J. Appl. Polym. Sci. 1984, 29, 211–217. [Google Scholar] [CrossRef]
- Bae, Y.H.; Okano, T.; Kim, S.W. Temperature dependence of swelling of crosslinked poly(N,N-alkyl substituted acrylamides) in water. J. Polym. Sci. Polym. Phys. 1990, 28, 923–936. [Google Scholar] [CrossRef]
- Heskins, M.; Guillet, J.E. Solution properties of poly(N-isopropylacrylamide). J. Macromol. Sci. Chem. 1968, 2, 1441–1445. [Google Scholar] [CrossRef]
- Nakayama, M.; Okano, T. Intelligent thermoresponsive polymeric micelles for targeted drug delivery. J. Drug Del. Sci. Tech. 2006, 16, 35–44. [Google Scholar]
- Kanazawa, H.; Yamamoto, K.; Matsushima, Y.; Takai, N.; Kikuchi, A.; Sakurai, Y.; Okano, T. Temperature-responsive chromatography using poly(N-isopropylacrylamide)-modified silicacells. Anal. Chem. 1996, 68, 100–105. [Google Scholar] [CrossRef]
- Kikuchi, A.; Okano, T. Intelligent thermoresponsive polymeric stationary phases for aqueous chromatography for biological compounds, Prog. Polym. Sci. 2002, 27, 1165–1193. [Google Scholar]
- Nagase, K.; Kobayashi, J.; Okano, T. Temperature-responsive intelligent interfaces for biomolecular separation and cell sheet engineering. J. R. Soc. Interface 2009, 6, S293–S309. [Google Scholar] [CrossRef]
- Yamada, N.; Okano, T.; Sakai, H.; Karikusa, F.; Sawasaki, Y.; Sakurai, Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Makromol. Chem. Rapid Commun. 1990, 11, 571–576. [Google Scholar] [CrossRef]
- Yang, J.; Yamato, M.; Kohno, C.; Nishimoto, A.; Sekine, H.; Fukai, F.; Okano, T. Cell sheet engineering: recreating tissues without biodegradable scaffolds. Biomaterials 2005, 26, 6415–6422. [Google Scholar] [CrossRef]
- Yamato, M.; Okano, T. Cell sheet engineering. Mater. Today 2004, 7, 42–47. [Google Scholar] [CrossRef]
- Matsuda, N.; Shimizu, T.; Yamato, M.; Okano, T. Tissue engineering based on cell sheet technology. Adv. Mater. 2007, 19, 3089–3099. [Google Scholar] [CrossRef]
- Yamato, M.; Akiyama, Y.; Kobayashi, J.; Yang, J.; Kikuchi, A.; Okano, T. Temperature-responsive cell culture surfaces for regenerative medicine with cell sheet engineering. Prog. Polym. Sci. 2007, 32, 1123–1133. [Google Scholar] [CrossRef]
- Shimizu, T.; Yamato, M.; Kikuchi, A.; Okano, T. Two-dimensional manipulation of cardiac myocyte sheets utilizing temperature-responsive culture dishes augments the pulsatile amplitude. Tissue Eng. 2001, 7, 141–151. [Google Scholar] [CrossRef]
- Kikuchi, A.; Okuhara, M.; Karikusa, F.; Sakurai, Y.; Okano, T. Two-dimensional manipulation of confluently cultured vascular endothelial cells using temperature-responsive poly(N-isopropyl-acrylamide)-grafted surfaces. J. Biomater. Sci. Polym.Ed. 1998, 9, 1331–1348. [Google Scholar] [CrossRef]
- Okano, T.; Yamada, N.; Sakai, H.; Sakurai, Y. A novel recovery system for cultured cells using plasma treated polystyrene dishes grafted with poly (N-isopropylacrylamide). J. Biomed. Mater. Res. 1993, 27, 1243–1251. [Google Scholar] [CrossRef]
- Yamato, M.; Utsumi, M.; Kushida, A.; Konno, C.; Kikuchi, A.; Okano, T. Thermo-responsive culture dishes allow the intact harvest of multilayered keratinocyte sheets without dispase by reducing temperature. Tissue Eng. 2001, 7, 473–480. [Google Scholar] [CrossRef]
- Yamato, M.; Okuhara, M.; Karikusa, F.; Kikuchi, A.; Sakurai, Y.; Okano, T. Signal transduction and cytoskeletal reorganization are required for cell detachmentfrom cell culture surfaces grafted with a temperature-responsive polymer. J. Biomed. Mater. Res. 1999, 44, 44–52. [Google Scholar] [CrossRef]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Maeda, N.; Watanabe, H.; Yamamoto, K.; Nagai, S.; Kikuchi, A.; Tano, Y.; Okano, T. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation 2004, 77, 379–385. [Google Scholar] [CrossRef]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, H.; Okano, T.; Tano, Y. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. New Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar]
- Kushida, A.; Yamato, M.; Isoi, Y.; Kikuchi, A.; Okano, T. A noninvasive transfer system for polarized renal tubule epithelial cell sheets using temperatureresponsive culture dishes. Eur. Cells Mater. 2005, 10, 23–30. [Google Scholar]
- Kushida, A.; Yamato, M.; Kikuchi, A.; Okano, T. Two-dimensional manipulation of differentiated Madin-Darby canine kidney (MDCK) cell sheets: The noninvasive harvest from temperature-responsive culture dishes and transfer to other surfaces. J. Biomed. Mater. Res. 2001, 54, 37–46. [Google Scholar] [CrossRef]
- Akizuki, T.; Oda, S.; Komaki, M.; Tsuchioka, H.; Kawakatsu, N.; Kikuchi, A.; Yamato, M.; Okano, T.; Ishikawa, I. Application of periodontal ligament cell sheet for periodontal regeneration: a pilot study in beagle dogs. J. Periodontal Res. 2005, 40, 245–251. [Google Scholar] [CrossRef]
- Hasegawa, M.; Yamato, M.; Kikuchi, A.; Okano, T.; Ishikawa, I. Human periodontal ligament cell sheets can regenerate periodontal ligament tissue in an athymic rat model. Tissue Eng. 2005, 11, 469–478. [Google Scholar] [CrossRef]
- Ohashi, K.; Yokoyama, T.; Yamato, M.; Kuge, H.; Kanehiro, H.; Tsutsumi, M.; Amanuma, T.; Iwata, H.; Yang, J.; Okano, T.; Nakajima, Y. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat. Med. 2007, 13, 880–885. [Google Scholar] [CrossRef]
- Shimizu, T.; Yamato, M.; Akutsu, T.; Shibata, T.; Isoi, Y.; Kikuchi, A.; Umezu, M.; Okano, T. Electrically communicating three-dimensional cardiac tissue mimic fabricated by layered cultured cardiomyocyte sheets. J. Biomed. Mater. Res. 2002, 60, 110–117. [Google Scholar] [CrossRef]
- Akiyama, Y.; Kikuchi, A.; Yamato, M.; Okano, T. Ultrathin poly(N-isopropylacrylamide) grafted layer on pulystyrene surfaces for cell adhesion/detachment control. Langmuir 2004, 20, 5506–5511. [Google Scholar] [CrossRef]
- Kikuchi, A.; Okano, T. Nanostructured designs of biomedical materials: Applications of cell sheet engineering to functional regenerative tissues and organs. J. Control. Release 2005, 101, 69–84. [Google Scholar] [CrossRef]
- Takei, Y.G.; Aoki, T.; Sanui, K.; Ogata, N.; Sakurai, Y.; Okano, T. Dynamic contact angle measurement of temperature-responsive surface properties for poly(N-isopropylacrylamide) grafted surfaces. Macromolecules 1994, 27, 6163–6166. [Google Scholar] [CrossRef]
- Yakushiji, T.; Sakai, K.; Kikuchi, A.; Aoyagi, T.; Sakurai, Y.; Okano, T. Graft architectural effects on thermo-responsive wettability changes of poly(N-isopropylacrylamide)-modified surfaces. Langmuir 1998, 14, 4657–4662. [Google Scholar] [CrossRef]
- Fukumori, K.; Akiyama, Y.; Yamato, M.; Kobayashi, J.; Sakai, K.; Okano, T. Temperature-responsive glass coverslips with an ultrathin poly(N-isopropyl-acrylamide) layer. Acta Biomater. 2009, 5, 470–476. [Google Scholar] [CrossRef]
- Waymouth, C. To disaggregate or not to disaggregate injury and cell disaggregation, transient or permanent? In Vitro 1974, 10, 97–111. [Google Scholar] [CrossRef]
- Osunkoya, B.O.; Mottram, F.C.; Isoun, M.J. Synthesis and fate of immunological surface receptors on cultured Burkitt lymphoma cells. Int. J. Cancer 1969, 4, 159–165. [Google Scholar] [CrossRef]
- Revel, J.P.; Hoch, P.; Ho, D. Adhesion of cultured cells to their substratum. Exp. Cell Res. 1974, 84, 207–218. [Google Scholar] [CrossRef]
- Kushida, A.; Yamato, M.; Konno, C.; Kikuchi, A.; Sakurai, Y.; Okano, T. Decrease in culture temperature releases monolayer endothelial cell sheets together with deposited fibronectin matrix from temperature-responsive culture surfaces. J. Biomed. Mater. Res. 1999, 45, 355–362. [Google Scholar] [CrossRef]
- Kondoh, H.; Sawa, Y.; Miyagawa, S.; Sakakida, K.; Memon, I.A.; Kawaguchi, N.; Matsuura, N.; Shimizu, T.; Okano, T.; Matsuda, H. Longer preservation of cardiac performance by sheet-shaped myoblast implantation in dilated cardiomyopathic hamsters. Cardiovasc. Res. 2006, 69, 466–475. [Google Scholar] [CrossRef]
- Memon, A.I.; Sawa, Y.; Fukushima, N.; Matsumiya, G.; Miyagawa, S.; Taketani, S.; Sakakida, S.K.; Kondoh, H.; Aleshin, A.N.; Shimizu, T.; Okano, T.; Matsuda, H. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. J. Thorac. Cardiovasc. Surg. 2005, 130, 1333–1341. [Google Scholar] [CrossRef]
- Kwon, O.H.; Kikuchi, A.; Yamato, M.; Sakurai, Y.; Okano, T. Rapid cell sheet detachment from poly(N-isopropylacrylamide)-grafted porous cell culture membranes. J. Biome. Mater. Res. 2000, 50, 82–89. [Google Scholar] [CrossRef]
- Kaneko, Y.; Nakamura, S.; Sakai, K.; Kikuchi, A.; Aoyagi, T.; Sakurai, Y.; Okano, T. Deswelling mechanism for comb-type grafted poly(N-isopropylacrylamide) hydrogels with rapid temperature responses. Polym. Gels Netw. 1998, 6, 333–345. [Google Scholar] [CrossRef]
- Kaneko, Y.; Nakamura, S.; Sakai, K.; Aoyagi, T.; Kikuchi, A.; Sakurai, Y.; Okano, T. Rapid deswelling response of poly(N-isopropylacrylamide) hydrogels by the formation of water release channels using poly(ethylene oxide) graft chains. Macromolecules 1998, 31, 6099–6105. [Google Scholar] [CrossRef]
- Kwon, O.H.; Kikuchi, A.; Yamato, M.; Okano, T. Accelerated cell sheet recovery by co-grafting of PEG with PIPAAm onto porous cell culture membranes. Biomaterials 2003, 24, 1223–1232. [Google Scholar] [CrossRef]
- Yoshida, R.; Uchida, K.; Kaneko, Y.; Sakai, K.; Kikuchi, A.; Sakurai, Y.; Okano, T. Comb-type grafted hydrogels with rapid de-swelling response to temperature changes. Nature 1994, 374, 240–242. [Google Scholar]
- Kaneko, Y.; Sakai, K.; Kikuchi, A.; Sakurai, Y.; Okano, T. Fast swelling/deswelling kinetics of comb-type grafted poly(N-isopropylacrylamide) hydrogels. Macromol. Symp. 1996, 109, 41–53. [Google Scholar] [CrossRef]
- Tang, Z.; Akiyama, Y.; Yamato, M.; Okano, T. Comb-type grafted poly(N-isopropylacrylamide) gel modified surfaces for rapid detachment of cell sheet. Biomaterials 2010, 31, 7435–7443. [Google Scholar] [CrossRef]
- Haraguchi, Y.; Shimizu, T.; Sasagawa, T.; Sekine, H.; Sakaguchi, K.; Kikuchi, T.; Sekine, W.; Sekiya, S.; Yamato, M.; Umezu, M.; Okano, T. Fabrication of functional three-dimensional tissues by stacking cell sheets in vitro. Nat. Protoc. 2012, 7, 850–858. [Google Scholar] [CrossRef]
- Bhatis, S.; Balis, U.; Yarmush, M.; Toner, M. Effect of cell-cell interactions in preservation of cellular phenotype: Cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999, 13, 1883–1900. [Google Scholar]
- Harimoto, M.; Yamato, M.; Kikuchi, A.; Okano, T. Cell sheet engineering: Intelligent polymer patterned surfaces for tissue engineered liver. Macromol. Symp. 2003, 195, 231–235. [Google Scholar] [CrossRef]
- Bae, Y.H.; Okano, T.; Kim, S.W. Temperature dependence of swelling of crosslinked poly(N,N-alkyl substituted acrylamides) in water. J. Polym. Sci. Polym. Phys. 1990, 28, 923–936. [Google Scholar] [CrossRef]
- Takei, Y.G.; Aoki, T.; Sanui, K.; Ogata, N.; Okano, T.; Sakurai, Y. Temperature-responsive bioconjugates. 2. Molecular design for temperature-modulated bioseparations. Bioconjugate Chem. 1993, 4, 341–346. [Google Scholar] [CrossRef]
- Iwata, H.; Oodate, M.; Uyama, Y.; Amemiya, H.; Ikada, Y. Preparation of temperature-sensitive membranes by graft polymerization onto a porous membrane. J. Membr. Sci. 1991, 55, 119–130. [Google Scholar] [CrossRef]
- Feil, H.; Bae, Y.H.; Feijen, J.; Kim, S.W. Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers. Macromolecules 1993, 26, 2496–2500. [Google Scholar] [CrossRef]
- Tsuda, Y.; Kikuchi, A.; Yamato, M.; Sakurai, Y.; Umezu, M.; Okano, T. Control of cell Adhesion and detachment using temperature and thermo-responsive copolymer grafted culture surfaces. J. Biomed. Mater. Res. 2004, 69A, 70–78. [Google Scholar] [CrossRef]
- Tsuda, Y.; Kikuchi, A.; Yamato, M.; Nakao, A.; Sakurai, Y.; Umezu, M.; Okano, T. The use of patterned dual thermoresponsive surfaces for the cullective recovery as co-cultured cell sheets. Biomaterials 2005, 26, 1885–1893. [Google Scholar] [CrossRef]
- Ebara, M.; Yamato, M.; Nagai, S.; Aoyagi, T.; Kikuchi, A.; Sakai, K.; Okano, T. Incorporation of new carboxylate functionalized co-monomers to temperature-responsive polymer-grafted cell surfaces. Surf. Sci. 2004, 570, 134–141. [Google Scholar] [CrossRef]
- Ebara, M.; Yamato, M.; Aoyagi, T.; Kikuchi, A.; Sakai, K.; Okano, T. Temperature-responsive cell culture surfaces enable “on-off” affinity control between cell integrins and RGDS ligands. Biomacromolecules 2004, 5, 505–510. [Google Scholar] [CrossRef]
- Yamada, K.M. Adhesive recognition sequences. J. Biol. Chem. 1991, 266, 12809–12812. [Google Scholar]
- Hatakeyama, H.; Kikuchi, A.; Yamato, M.; Okano, T. Bio-functionalized thermoresponsive interfaces facilitating cell adhesion and proliferation. Biomaterials 2006, 27, 5069–5078. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Kikuchi, A.; Yamato, M.; Okano, T. Patterned biofunctional designs of thermoresponsive surfaces for spatiotemporally controlled cell adhesion, growth, and thermally induced detachment. Biomaterials 2007, 28, 3632–3643. [Google Scholar] [CrossRef]
- Nishi, M.; Kobayashi, J.; Pechmann, S.; Yamato, M.; Akiyama, Y.; Kikuchi, A.; Uchida, K.; Textor, M.; Yajima, H.; Okano, T. The use of biotin-avidin binding to facilitate biomodification of thermoresponsive culture surfaces. Biomaterials 2007, 28, 5471–5476. [Google Scholar] [CrossRef]
- Takahashi, H.; Matsuzaka, N.; Nakayama, M.; Kikuchi, A.; Yamato, M.; Okano, T. Terminally functionalized thermoresponsive polymer brushes for simultaneously promoting cell adhesion and cell sheet harvest. Biomacromolecules 2012, 13, 253–260. [Google Scholar] [CrossRef]
- Nakayama, M.; Okano, T. Polymer terminal group effects on properties of thermoresponsive polymeric micelles with controlled outer-shell chain lengths. Biomacromolecules 2005, 6, 2320–2327. [Google Scholar] [CrossRef]
- McCormick, C.L.; Sumerlin, B.S.; Lokitz, B.S.; Stempka, J.E. RAFT-synthesized diblock and triblock copolymers: Thermallyinduced supramolecular assembly in aqueous media. Soft Matter 2008, 4, 1760–1773. [Google Scholar] [CrossRef]
- Takahashi, H.; Nakayama, M.; Itoga, K.; Yamato, M.; Okano, T. Micropatterned thermoresponsive polymer brush surfaces for fabricating cell sheets with well-controlled orientational structures. Biomacromolecules 2011, 12, 1414–1418. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tang, Z.; Akiyama, Y.; Okano, T. Temperature-Responsive Polymer Modified Surface for Cell Sheet Engineering. Polymers 2012, 4, 1478-1498. https://doi.org/10.3390/polym4031478
Tang Z, Akiyama Y, Okano T. Temperature-Responsive Polymer Modified Surface for Cell Sheet Engineering. Polymers. 2012; 4(3):1478-1498. https://doi.org/10.3390/polym4031478
Chicago/Turabian StyleTang, Zhonglan, Yoshikatsu Akiyama, and Teruo Okano. 2012. "Temperature-Responsive Polymer Modified Surface for Cell Sheet Engineering" Polymers 4, no. 3: 1478-1498. https://doi.org/10.3390/polym4031478




