A Systematic Study on the Self-Assembly Behaviour of Multi Component Fmoc-Amino Acid-Poly(oxazoline) Systems
Abstract
:1. Introduction
2. Results and Discussion
2.1. Single Component Fmoc-Amino Acid Functionalised PiPrOx Systems
2.1.1. Polymer Synthesis and Functionalisation
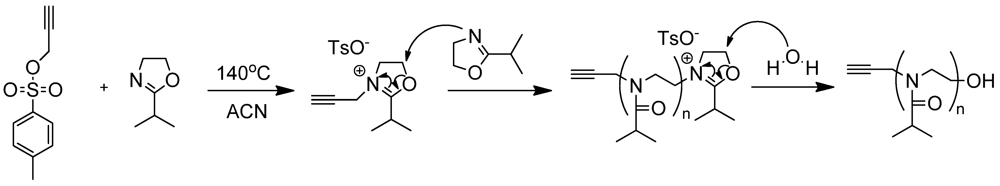
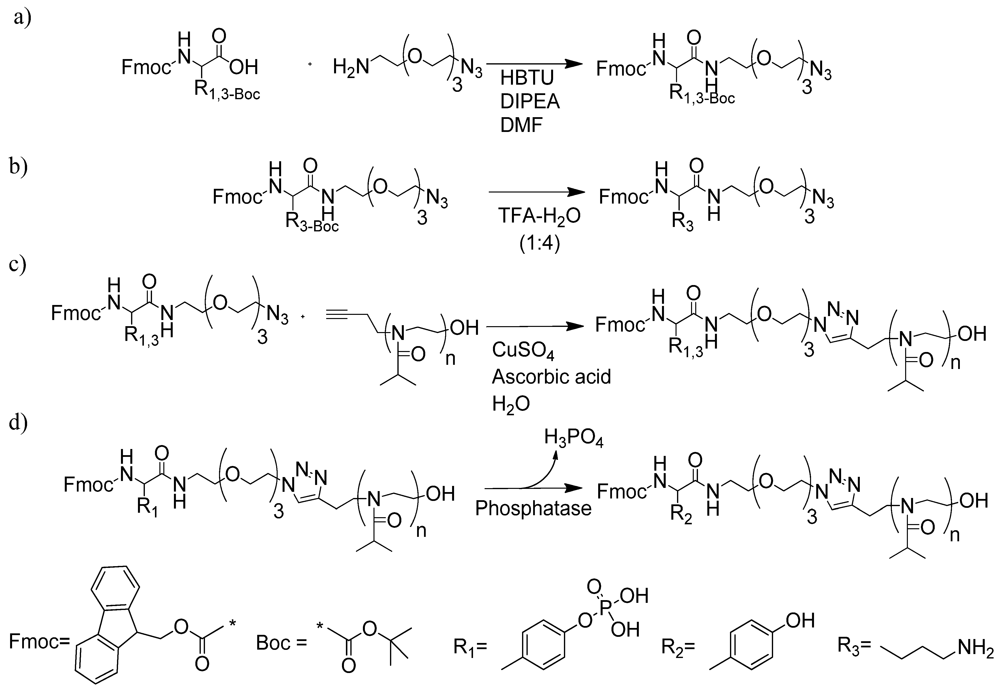
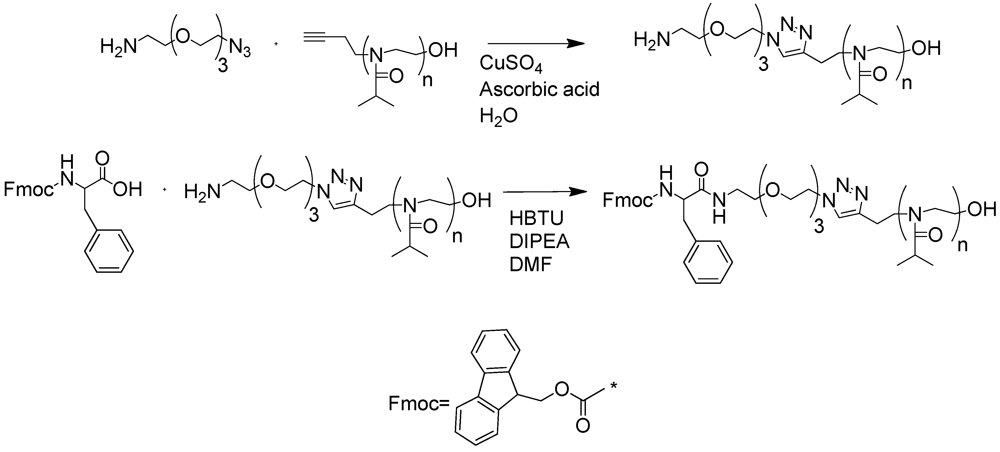
2.1.2. Phase Transition Temperature Studies
| Amino acid | CLogP* |
|---|---|
| pY | −3.561 |
| Y | −2.223 |
| F | −1.556 |
| K | −3.561 |
 ), 1 (■), 2 (□), 3 (●), 4 (▲) and 5 (●) (inset). (Polymer concentration used = 1 mg/mL).
), 1 (■), 2 (□), 3 (●), 4 (▲) and 5 (●) (inset). (Polymer concentration used = 1 mg/mL).
 ), 1 (■), 2 (□), 3 (●), 4 (▲) and 5 (●) (inset). (Polymer concentration used = 1 mg/mL).
), 1 (■), 2 (□), 3 (●), 4 (▲) and 5 (●) (inset). (Polymer concentration used = 1 mg/mL).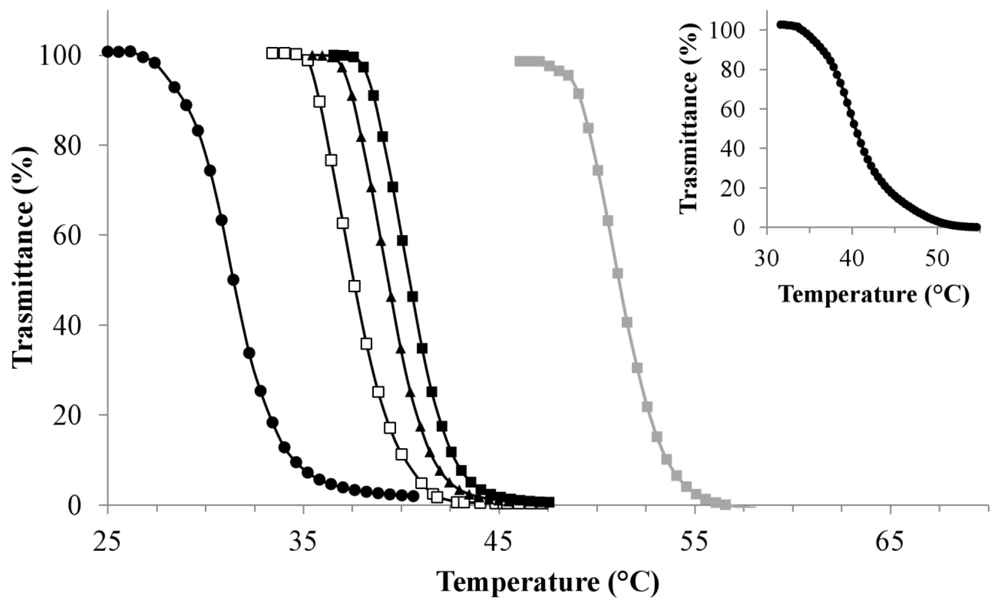
2.1.3. Dynamic Light Scattering Studies
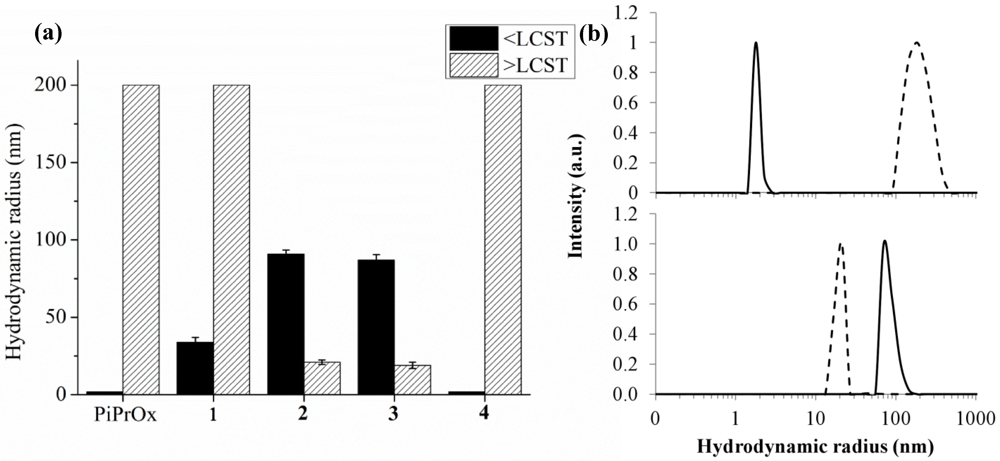
2.1.4. Critical Micelle Concentration Studies
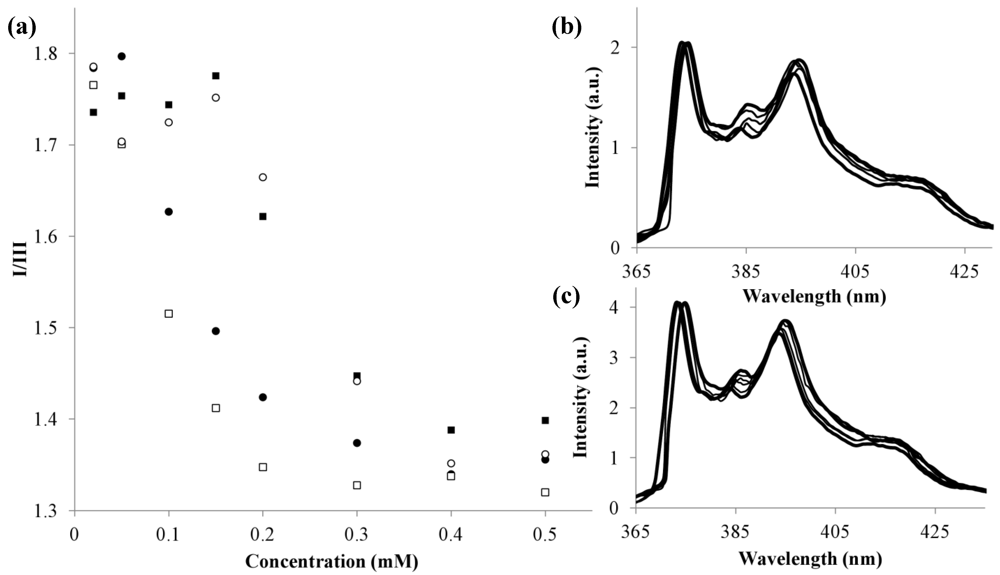
 ),1 (■), 2 (□), 3 (●) and 4 (▲); Total volume of each sample 1.050 mL; (b) Example of pyrene spectra where no changes are observed upon increase of polymer concentration (4); (c) Example of pyrene spectra where I/III ratio decreases due to self-assembly (3).
),1 (■), 2 (□), 3 (●) and 4 (▲); Total volume of each sample 1.050 mL; (b) Example of pyrene spectra where no changes are observed upon increase of polymer concentration (4); (c) Example of pyrene spectra where I/III ratio decreases due to self-assembly (3).
 ),1 (■), 2 (□), 3 (●) and 4 (▲); Total volume of each sample 1.050 mL; (b) Example of pyrene spectra where no changes are observed upon increase of polymer concentration (4); (c) Example of pyrene spectra where I/III ratio decreases due to self-assembly (3).
),1 (■), 2 (□), 3 (●) and 4 (▲); Total volume of each sample 1.050 mL; (b) Example of pyrene spectra where no changes are observed upon increase of polymer concentration (4); (c) Example of pyrene spectra where I/III ratio decreases due to self-assembly (3). 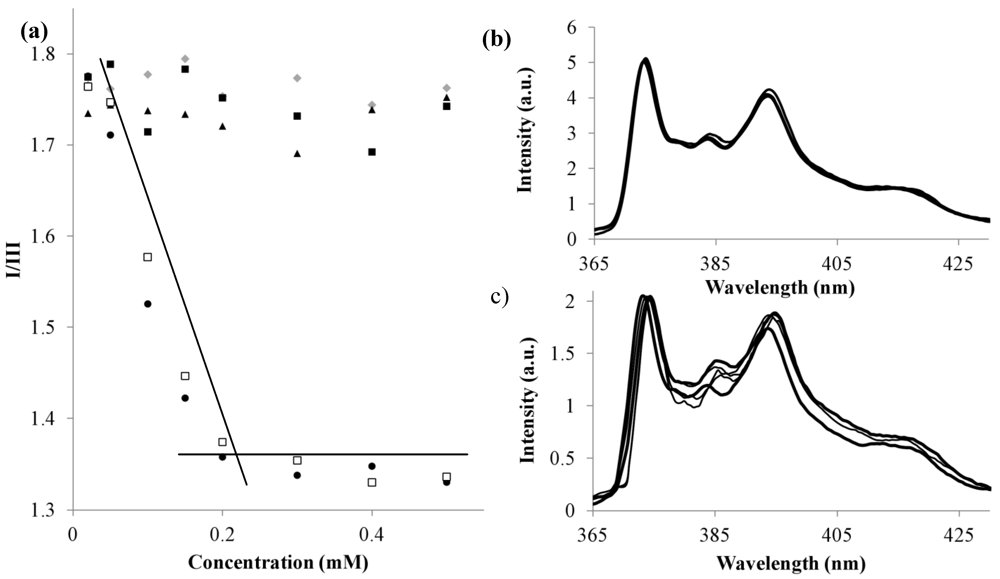
| Polymer | Functionalisation route | Functionalisation yield (%) | LCST (°C) | Radius (nm) | CMC (mM) | |
|---|---|---|---|---|---|---|
| <LCST | >LCST | |||||
| PiPrOx | / | / | 51.5 | 1–2 | >200 | PiPrOx |
| 1 | A | 96 | 40.7 | 35–40 | >200 | 1 |
| 2 | B | / | 37.7 | 80–90 | 15–20 | 2 |
| 3 | A | 94 | 31.4 | 75–85 | 15–20 | 3 |
| 4 | C | 93 | 39.2 | 1–2 | >200 | 4 |
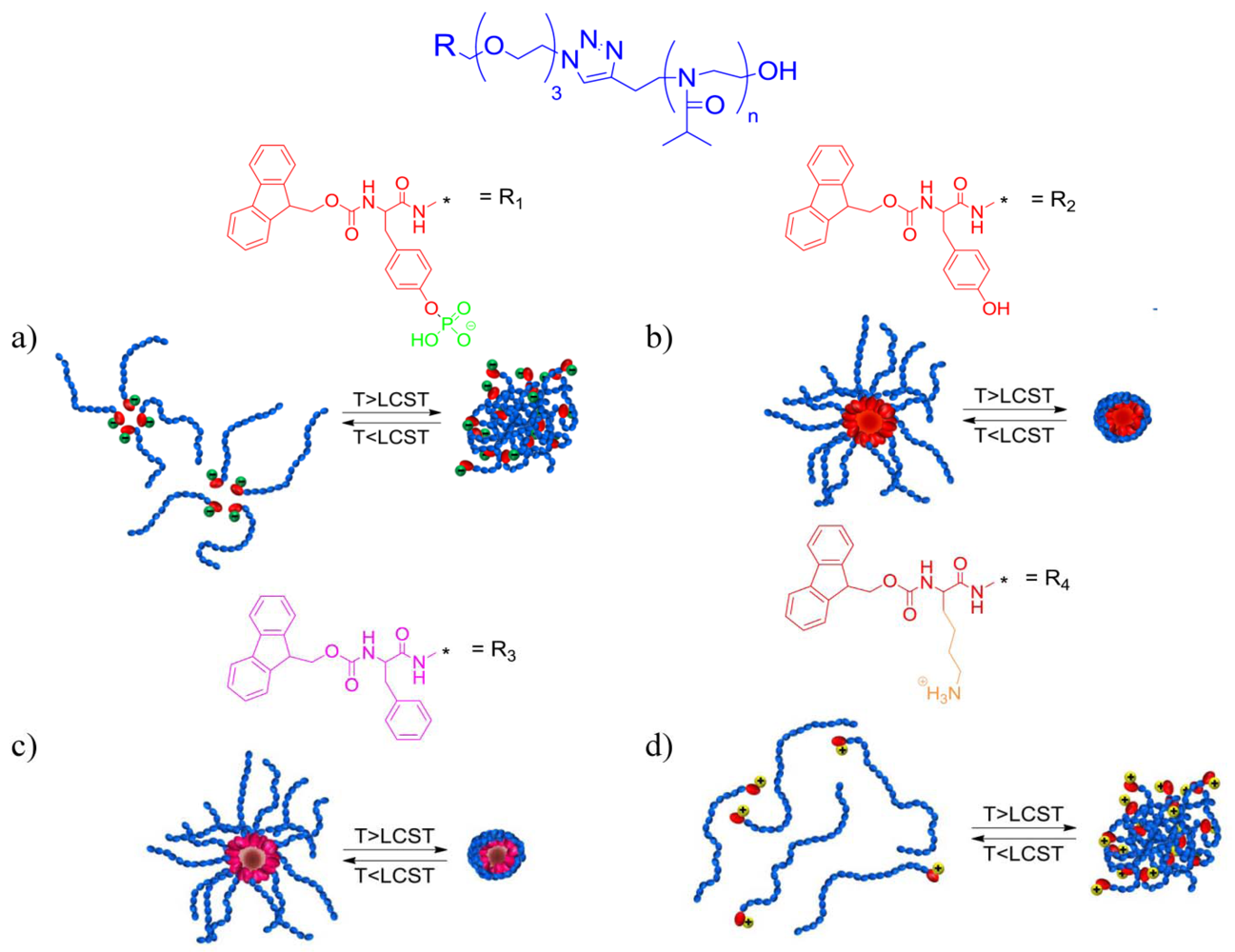
2.2. Two-Component Fmoc-Amino Acid Functionalised PiPrOx Systems
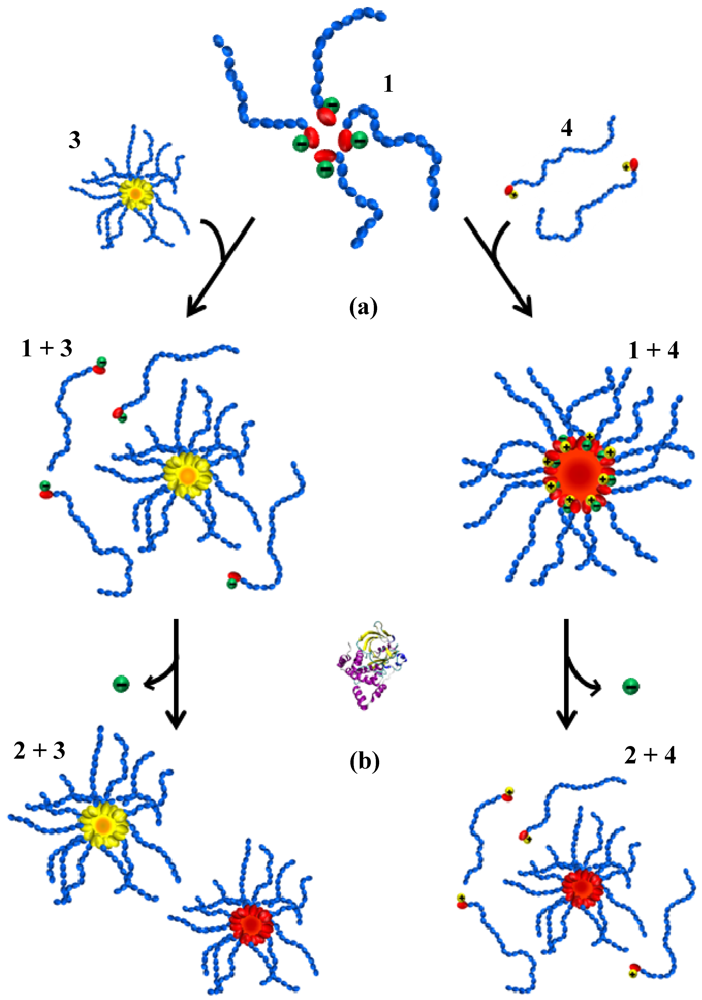
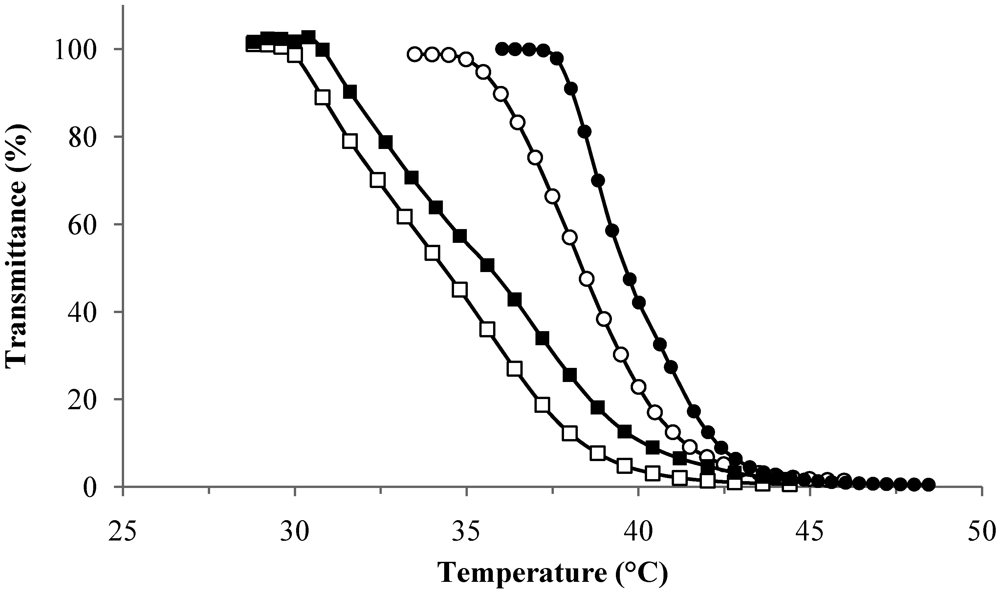
2.2.1. Phase Transition Temperature Studies
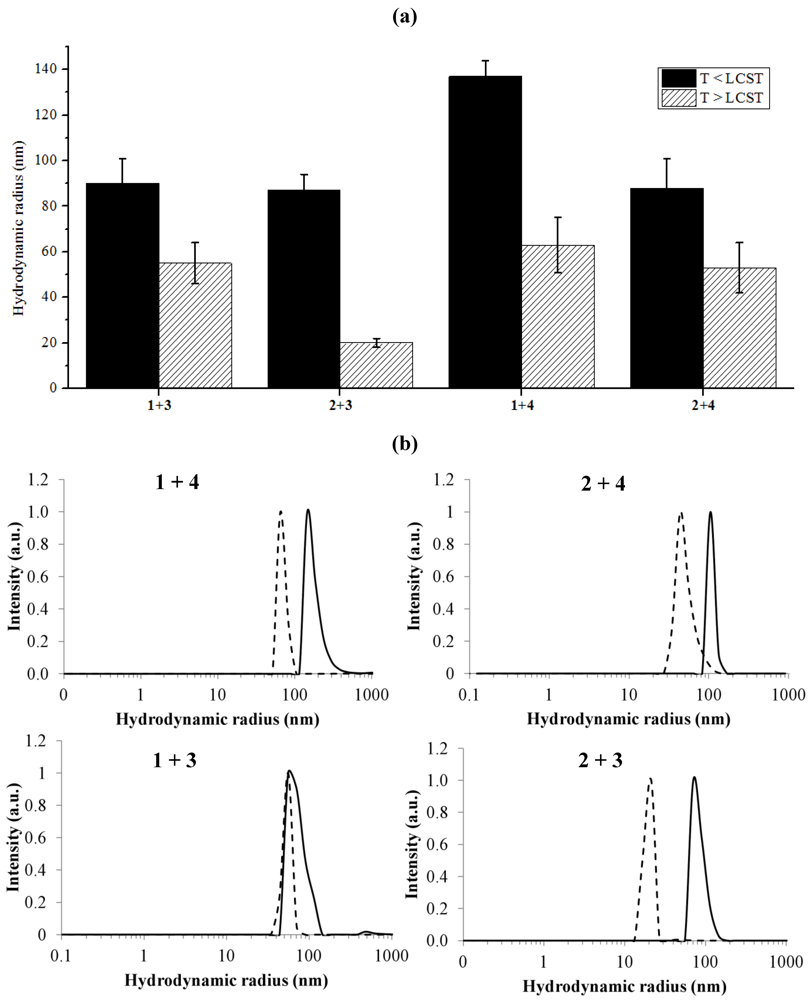
2.2.2. Dynamic Light Scattering Studies
2.2.3. Critical Micelle Concentration Studies
| Polymer | LCST (°C) | Radius (nm) | CMC (mM) | |
|---|---|---|---|---|
| <LCST | >LCST | |||
| 1 + 3 | 36.4 | 91 | 56 | 1 + 3 |
| 2 + 3 | 36.6 | 89 | 20 | 2 + 3 |
| 1 + 4 | 40.3 | 136 | 63 | 1 + 4 |
| 2 + 4 | 38.8 | 92 | 53 | 2 + 4 |
3. Experimental Section
3.1. Materials
3.2. Instrumentation
3.3. Polymer Synthesis
3.4. Synthesis and Characterisation of Fmoc-Amino Acids Bearing Terminal N3
3.4.1. Fmoc-pY-N3
3.4.2. Fmoc-K-N3
3.4.3. “Click” Reaction
4. Conclusions
References
- Grzybowski, B.A.; Wilmer, C.E.; Kim, J.; Browne, K.; Bishop, K.J.M. Self-assembly: From crystals to cells. Soft Matter 2009, 5, 1110–1128. [Google Scholar] [CrossRef]
- Zayed, J.M.; Nouvel, N.; Rauwald, U.; Scherman, O.A. Chemical complexity—Supramolecular self-assembly of synthetic and biological building blocks in water. Chem. Soc. Rev. 2010, 39, 2806–2816. [Google Scholar] [CrossRef]
- Börner, H.J.; Kühnle, H.; Hentschel, J. Making “smart polymers” smarter: Modern concepts to regulate functions in polymer science. J. Polym. Sci. 2010, 48, 1–14. [Google Scholar]
- Löwik, D.W.P.M.; Leunissen, E.H.P.; van den Heuvel, M.; Hansen, M.B.; van Hest, J.C.M. Stimulus responsive peptide based materials. Chem. Soc. Rev. 2010, 39, 3394–3412. [Google Scholar] [CrossRef]
- Ulijn, R.V. Enzyme-responsive materials: A new class of smart biomaterials. J. Mater. Chem. 2006, 16, 2217–2225. [Google Scholar] [CrossRef]
- Cohen Stuart, M.A.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; Winnik, F.; Zauscher, S.; Luzinov, I.; Minko, S. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar]
- Torchilin, V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Release 2001, 73, 137–172. [Google Scholar] [CrossRef]
- Amir, R.J.; Zhong, S.; Pochan, D.J.; Hawker, C.J. Enzymatically triggered self assembly of block copolymers. J. Am. Chem. Soc. 2009, 131, 13949–13952. [Google Scholar]
- Kühnle, H.; Börner, H.G. Biotransformation on polymer—Peptide conjugates: A versatile tool to trigger microstructure formation. Angew. Chem. Int. Ed. 2009, 48, 6431–6434. [Google Scholar] [CrossRef]
- Ku, T.H.; Chen, M.P.; Thompson, M.P.; Sinkovits, R.S.; Olson, N.H.; Baker, T.S.; Gianneschi, N.C. Controlling and switching the morphology of micellar nanoparticles with enzymes. J. Am. Chem. Soc. 2011, 133, 8392–8395. [Google Scholar]
- Wang, C.; Chen, Q.; Wang, Z.; Zhang, X. An enzyme-responsive polymeric superamphiphile. Angew. Chem. Int. Ed. 2010, 49, 8612–8615. [Google Scholar] [CrossRef]
- Caponi, P.F.; Qiu, X.P.; Vilela, F.; Winnik, F.M.; Ulijn, R.V. Phosphatase/temperature responsive poly(2-isopropyl-2-oxazoline). Polym. Chem. 2011, 2, 306–308. [Google Scholar] [CrossRef]
- Yang, Z.M.; Gu, H.W.; Fu, D.G.; Gao, P.; Lam, J.K.; Xu, B. Enzymatic formation of supramolecular hydrogels. Adv. Mater. 2004, 16, 1440–1444. [Google Scholar] [CrossRef]
- Caponi, P.F.; Winnik, F.M.; Ulijn, R.V. Charge complementary enzymatic reconfigurable polymeric nanostructures. Soft Matter 2012, 8, 5127–5130. [Google Scholar] [CrossRef]
- Aoi, K.; Okada, M. Polymerization of oxazolines. Prog. Polym. Sci. 1996, 21, 151–208. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Schlaad, H. Bioinspired poly(2-oxazoline)s. Polymers 2011, 3, 467–488. [Google Scholar] [CrossRef]
- Carbon-Nitrogen Backbone Chain Polymers. U.S. Patent 3,483,141, 9 December 1969.
- Wiesbrock, F.; Hoogenboom, R.; Abeln, C.H.; Schubert, U.S. Single-mode microwave ovens as new reaction devices: Accelerating the living polymerization of 2-ethyl-2-oxazoline. Macromol. Rapid Commun. 2004, 25, 1895–1899. [Google Scholar] [CrossRef]
- Wiesbrock, F.; Hoogenboom, R.; Leenen, M.A.M.; Meier, M.A.R.; Schubert, U.S. Investigation of the living cationic ring-opening polymerization of 2-methyl-, 2-ethyl-, 2-nonyl-, and 2-phenyl-2-oxazoline in a single-mode microwave reactor. Macromolecules 2005, 38, 5025–5034. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Wiesbrock, F.; Huang, H.; Leenen, M.A.A.; Thijs, H.M.L.; van Nispen, S.F.G.M.; van der Loop, M.; Fustin, C.A.; Jonas, A.M.; Gohy, J. F.; Schubert, U.S. Microwave-assisted cationic ring-opening polymerization of 2-oxazolines: A powerful method for the synthesis of amphiphilic triblock copolymers. Macromolecules 2006, 39, 4719–4725. [Google Scholar]
- Fijten, M.W.M.; Haensch, C.; van Lankvelt, B.M.; Hoogenboom, R.; Schubert, U.S. Clickable poly(2-Oxazoline)s as versatile building blocks. Macromol. Chem. Phys. 2008, 209, 1887–1895. [Google Scholar] [CrossRef]
- Kalyanasundaram, K.; Thomas, J.K. Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J. Am. Chem. Soc. 1977, 99, 2039–2044. [Google Scholar] [CrossRef]
- Konuk, R.; Cornelisse, J.; McGlynn, S.P. Fluorescence quenching of pyrene by Cu2+ and Co2+ in sodium dodecyl sulfate micelles. J. Phys. Chem. 1989, 93, 7405–7409. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Haldar, S.; Chattopadhyay, A. Organization and dynamics in micellar structural transition monitored by pyrene fluorescence. Biochem. Biophys. Res. Commun. 2009, 390, 728–732. [Google Scholar] [CrossRef]
- Yamazaki, I.; Winnik, F.M.; Winnik, M.A.; Tazuke, S. Excimer formatlon in pyrene labeled hydroxypropyl cellulose in water: Picosecond fluorescence studies. J. Phys. Chem. 1987, 91, 4213–4216. [Google Scholar] [CrossRef]
- Ieong, N.S.; Hasan, M.; Phillips, D.J.; Saaka, Y.; O’Reilly, R.K.; Gibson, M.I. Polymers with molecular weight dependent LCSTs are essential for cooperative behaviour. Polym. Chem. 2012, 3, 794–799. [Google Scholar] [CrossRef]
- Zhao, X.; Pan, F.; Xu, H.; Yaseen, M.; Shan, H.; Hauser, C.A.E.; Zhang, S.; Lu, J.R. Molecular self-assembly and applications of designer peptide amphiphiles. Chem. Soc. Rev. 2010, 39, 3480–3498. [Google Scholar] [CrossRef]
- Goddard, P.; Hutchinson, L.E.; Brown, J.; Brookman, L.J. Soluble polymeric carriers for drug delivery. Part 2. Preparation and in vivo behaviour of N-acylethylenimine copolymers. J. Control. Release 1989, 10, 5–16. [Google Scholar]
- Woodle, M.C.; Engbers, C.M.; Zalipsky, S. New amphipatic polymer-lipid conjugates forming long-circulating reticuloendothelial system-evading liposomes. Bioconjugate Chem. 1994, 5, 493–496. [Google Scholar] [CrossRef]
- Zalipsky, S.; Hansen, C.B.; Oaks, J.M.; Allen, T.M. Evaluation of blood clearance rates and biodistribution of poly(2-oxazoline)-grafted liposomes. J. Pharm. Sci. 1996, 85, 133–137. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Caponi, P.-F.; Ulijn, R.V. A Systematic Study on the Self-Assembly Behaviour of Multi Component Fmoc-Amino Acid-Poly(oxazoline) Systems. Polymers 2012, 4, 1399-1415. https://doi.org/10.3390/polym4031399
Caponi P-F, Ulijn RV. A Systematic Study on the Self-Assembly Behaviour of Multi Component Fmoc-Amino Acid-Poly(oxazoline) Systems. Polymers. 2012; 4(3):1399-1415. https://doi.org/10.3390/polym4031399
Chicago/Turabian StyleCaponi, Pier-Francesco, and Rein V. Ulijn. 2012. "A Systematic Study on the Self-Assembly Behaviour of Multi Component Fmoc-Amino Acid-Poly(oxazoline) Systems" Polymers 4, no. 3: 1399-1415. https://doi.org/10.3390/polym4031399




