Spiral Assembly of the 1D Chain Sheet of Fe(NCBH3)2(bpa)2·(biphenyl) (bpa = 1,2-bis(4-pyridyl)ethane) and its Stepwise Spin-Crossover Phenomenon
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthsis
2.2. Single Crystal X-Ray Structural Analysis
2.3. 57Fe Mössbauer Spectra
2.4. Magnetic Measurement
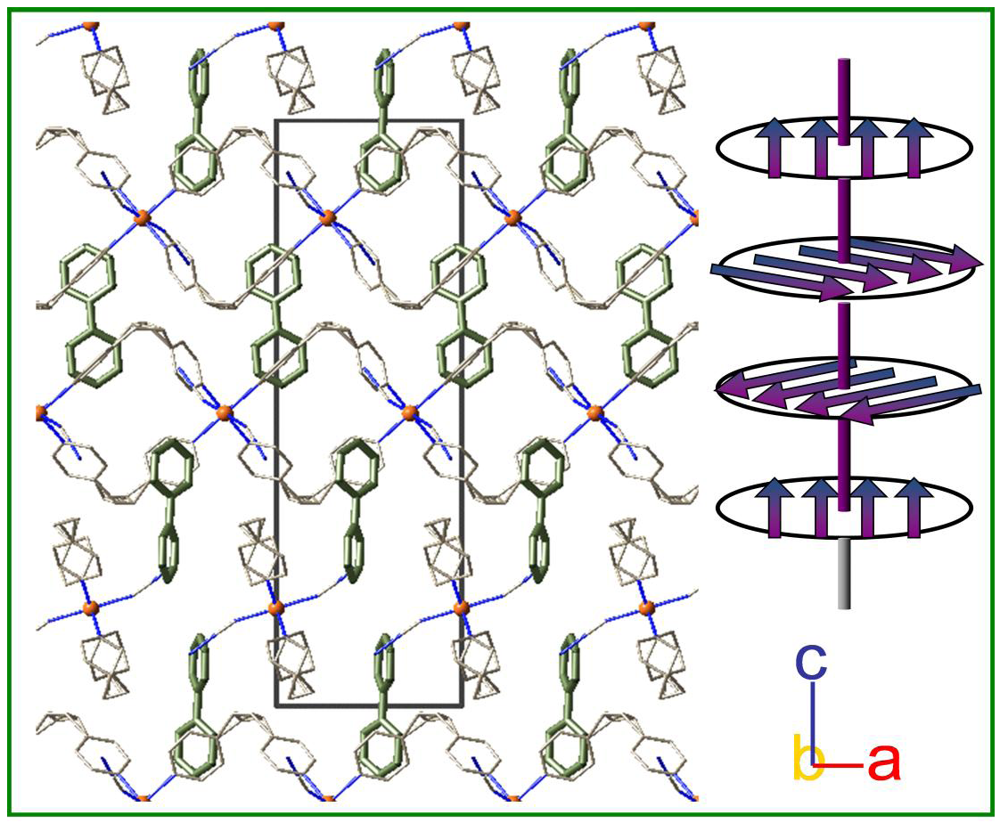
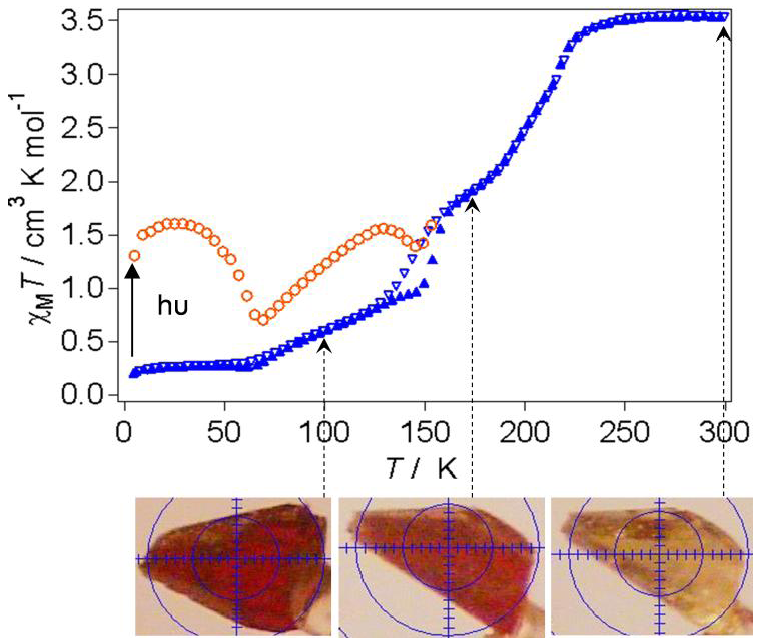
3. Results and Discussion
| Chemical formula | C38H40B2FeN6 | C38H40B2FeN6 | C38H40B2FeN6 |
|---|---|---|---|
| Formula weight | 658.23 | 658.23 | 658.23 |
| Crystal system | trigonal | trigonal | trigonal |
| Space group | P3121 | P3121 | P3121 |
| a, b/Å | 10.167(5) | 10.2293(3) | 10.378(3) |
| c/Å | 29.245(5) | 29.499(5) | 29.967(12) |
| α, β/° | 90 | 90 | 90 |
| γ/° | 120 | 120 | 120 |
| V/Å3 | 2,618.0(19) | 2,673.2(7) | 2,795.3(16) |
| T/K | 100 | 175 | 298 |
| Z | 3 | 3 | 3 |
| Dcalc/g·cm−3 | 1.253 | 1.227 | 1.173 |
| R | 0.0495 | 0.0471 | 0.0569 |
| Rw | 0.1133 | 0.1146 | 0.1305 |
| Goodness of fit | 1.054 | 1.085 | 1.037 |

4. Conclusions
References
- Cambi, L.; Gagnasso, A. Iron dithiocarbamates and nitrosodithiocarbamates. Atti. Accad. Naz. Lincei 1931, 13, 809–813. [Google Scholar]
- Kahn, O.; Martinez, C.J. Spin-transition polymers: From molecular materials toward memory devices. Science 1998, 297, 44–48. [Google Scholar]
- Real, J.A.; Gaspar, A.B.; Niel, N.; Muñoz, M.C. Communication between iron(II) building blocks in cooperative spin transition phenomena. Coord. Chem. Rev. 2003, 236, 121–141. [Google Scholar]
- Real, J.A.; Andrés, E.; Muñoz, M.C.; Julve, M.; Granier, J.; Bousseksou, A.; Varret, V. Spin crossover in a catenane supramolecular system. Science 1995, 268, 265–267. [Google Scholar]
- Halder, G.J.; Kepert, C.J.; Moubaraki, B.; Murray, K.S.; Cashion, J.D. Guest-dependent spin crossover in a nanoporous molecular framework material. Science 2002, 298, 1762–1765. [Google Scholar]
- Gütlich, P.; Garcia, Y.; Goodwin, H.A. Spin crossover phenomena in Fe(II) complexes. Chem. Soc. Rev. 2000, 29, 419–427. [Google Scholar]
- Garcia, Y.; Ksenofontov, V.; Gütlich, P. Spin transition molecular materials: New sensors. Hyperfine Interact 2009, 139/140, 543–551. [Google Scholar]
- Quesada, M.; Koojiman, H.; Gomez, P.; Costa, J.S.; Koningsbruggen, P.J.; Weinberger, P.; Reissner, M.; Spek, A.L.; Haasnoot, J.G.; Reedjik, J. Fe(μ-btzmp)2(btzmp)2](ClO4)2: A doubly-bridged 1D spin-transition bistetrazole-based polymer showing thermal hysteresis behavior. Dalton. Trans. 2007, 5434–5440. [Google Scholar]
- Kitazawa, T.; Gomi, Y.; Takahashi, M.; Takeda, M.; Enomoto, M.; Miyazaki, A.; Enoki, T. Spin-crossover behavior of the coordination polymer FeII(C5H5N)2NiII(CN)4. J. Mater. Chem. 1996, 6, 117–118. [Google Scholar]
- Agustí, G.; Muñoz, M.C.; Gaspar, A.B.; Real, J.A. Spin-crossover behavior in cyanide-bridged iron(II)-gold(I) bimetallic 2D Hofmann-like metal-organic frameworks. Inorg. Chem. 2008, 47, 2552–2561. [Google Scholar]
- Białońska, A.; Bronisz, R.; Weseiski, M. A new family of spin-crossover complexes based on a FeII(tetrazolyl)4(MeCN)2-type core. Inorg. Chem. 2008, 47, 4436–4438. [Google Scholar]
- Zhang, Y.; Chen, B.; Fronczek, F.R.; Maverick, A.W. A nanoporous Ag-Fe mixed-metal-organic framework exhibiting single-crystal-to-single-crystal transformations upon guest exchange. Inorg. Chem. 2008, 47, 4433–4435. [Google Scholar]
- Hayami, S.; Juháez, G.; Maeda, Y.; Yokoyama, T.; Sato, O. Novel structural and magnetic properties of a 1-D iron(II)-manganese(II) LIESST compound bridged by cyanide. Inorg. Chem. 2005, 44, 7289–7291. [Google Scholar]
- Gütlich, P. Spin crossover in iron(II)-complexes. In Structure and Bonding; Springer: Heidelberg, Germany, 1981; Volume 44, pp. 83–195. [Google Scholar]
- Nakashima, S.; Yamamoto, A.; Asada, Y.; Koga, N.; Okuda, T. New assembled Fe-trans-1,2-bis(4-pyridyl)ethylene-NCS(NCSe) complexes—hydrogen bonded and π-π interacted structure and grid structure enclathrating ligand. Inorg. Chim. Acta 2005, 358 , 257–364. [Google Scholar]
- Hernández, M.L.; Barandika, M.G.; Urtiga, M.K.; Cortés, R.; Lezama, L.; Arriotua, M.I.; Rojo, T. Structural analysis and magnetic properties of the 1-D compounds [M(NCS)2bpa2] [M = Fe, Co, Ni and bpa = 1,2-bis(4-pyridyl)ethane]. J. Chem. Soc., Dalton Trans. 1999, 1401–1406. [Google Scholar]
- Morita, T.; Asada, Y.; Okuda, T.; Nakashima, S. Isomerism of assembled iron complex bridged by 1,2-di(4-pyridyl)ethane and its solid-to-solid transformation accompanied by a change of electronic state. Bull. Chem. Soc. Jpn. 2006, 79, 738–744. [Google Scholar]
- Atsuchi, M.; Higashikawa, H.; Yoshida, Y.; Nakashima, S.; Inoue, K. Novel 2D interpenetrated structure and occurrence of the spin-crossover phenomena of assembled complexes, Fe(NCX)2(bpp)2 (X = S, Se, BH3; bpp = 1,3-bis(4-pyridyl)propane. Chem. Lett. 2007, 36, 1064–1065. [Google Scholar] [CrossRef]
- Atsuchi, M.; Inoue, K.; Nakashima, S. Reversible structural change of host framework triggered by desorption and adsorption of guest benzene molecules in Fe(NCS)2(bpp)2·2(benzene) (bpp = 1,3-bis(4-pyridyl)propane). Inorg. Chim. Acta 2011, 370, 82–88. [Google Scholar] [CrossRef]
- Morita, T.; Nakashima, S.; Yamada, K.; Inoue, K. Occurrence of the spin-crossover phenomenon of assembled complexes, Fe(NCX)2(bpa)2 (X = S ,BH3; bpa = 1,2-bis(4-pyridyl)ethane by enclathrating organic guest molecule. Chem. Lett. 2006, 35, 1042–1043. [Google Scholar] [CrossRef]
- Gütlich, P.; Garcia, Y.; Spiering, H. Spin transition phenomena. In Magnetism: Molecules to Materials IV: Nanosized Magnetic Materials; Miller, J.S., Drillon, M., Eds.; Wiley-VCH: Weinheim, Germany, 2003; pp. 271–344. [Google Scholar]
- Nakashima, S.; Morita, T.; Inoue, K. Spin-crossover phenomenon of the assembled iron complexes with 1,2-bis(4-pyridyl)ethane as bridging ligand studied by Mössbauer spectroscopy. Hyperfine Interact. 2009, 188, 107–111. [Google Scholar]
- Cointe, M.B-L.; Ould-Moussa, N.; Trzop, E.; Moréac, A.; Molnar, G.; Toupet, L.; Bousseksou, A.; Létard, J.F.; Matouzenko, G.S. Symmetry breaking and light-induced spin-state trapping in a mononuclear FeII complex with two-step thermal conversion. Phys. Rev. B 2010, 82, 214106–214111. [Google Scholar]
- Neville, S.M.; Leita, B.A.; Halder, G.J.; Kepert, C.J.; Moubaraki, B.; Létard, J-F.; Murray, K.S. Understanding the two-step spin-transition phenomenon in iron(II) 1D chain materials. Chem. Eur. J. 2008, 14, 10123–10133. [Google Scholar]
- Garcia, Y.; Kahn, O.; Rabardel, L.; Chansou, B.; Salmon, L.; Tuchagues, J.P. Two-step spin conversion for the three-dimensional compound tris(4,4’-bis-1,2,4-triazole)iron(II) diperchlorate. Inorg. Chem. 1999, 38, 4663–4670. [Google Scholar]
- Niel, V.; Muñoz, M.C.; Gasper, A.B.; Galet, A.; Levchenko, G.; Real, J.A. Thermal-, pressure-, and light-induced spin transition in novel cyanide-bridged FeII-AgI bimetallic compounds with three-dimensional interpenetrating double structures {FeIILx[Ag(CN)2]2}·G. Chem. Eur J. 2002, 8, 2446–2453. [Google Scholar] [CrossRef]
- Genre, C.; Jeanneau, E.; Bousseksou, A.; Luneau, D.; Borschch, S.A.; Matouzenko, G.S. First dicyanamide-bridged spin-crossover coordination polymer: synthesis, structural, magnetic, and spectroscopic studies. Chem. Eur. J. 2008, 14, 697–705. [Google Scholar]
- Cheryshov, D.; Hostettler, M.; Törnroos, K.W.; Bürgi, H.B. Ordering phenomena and phase transitions in a spin-crossover compound—uncovering the nature of the intermediate phase of [Fe(2-pic)3]Cl2·EtOH. Angew. Chem. Int. Ed. 2003, 42, 3825–3830. [Google Scholar]
- Reger, D.L.; Little, C.A.; Young, V.G.; Maren, P. Variable-temperature X-ray structural investigation of {Fe[HC(3,5-Me2pz)3]2}(BF4)2 (pz = pyrazolyl ring): Observation of a thermally induced spin state change from all high spin to an equal high spin-low spin mixture, concomitant with onset of nonmerohedral twinning. Inorg. Chem. 2001, 40, 2870–2874. [Google Scholar]
- Grunert, C.M.; Schweifer, J.; Weinberger, P.; Linert, W.; Mereiter, K.; Hilscher, G.; Muller, M.; Wiesinger, G.; van Koningsbruggen, P.J. Structure and physical properties of [μ-tris(1,4-bis(tetrazol-1-yl)butane-N4,N4’)iron(II)] bis(hexafluorophosphate), a new Fe(II) spin-crossover compound with a three-dimensional threefold interlocked crystal lattice. Inorg. Chem. 2004, 43, 155–165. [Google Scholar]
- Bauer, W.; Pfaffeneder, T.; Achterhold, K.; Weber, B. Complete two-step spin-transition in a 1D chain iron(II) complex with a 110-K wide intermediate plateau. Eur. J. Inorg. Chem. 2011, 3183–3192. [Google Scholar]
- Neville, S.M.; Leita, B.A.; Offermann, D.A.; Duriska, M.B.; Moubaraki, B.; Chapman, K.W.; Halder, G.J.; Murray, K.S. Spin-crossover studies on a series of 1D chain and dinuclear iron(II) triazine-dipyridylamine compounds. Eur. J. Inorg. Chem. 2007, 1073–1085. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nakashima, S.; Morita, T.; Inoue, K.; Hayami, S. Spiral Assembly of the 1D Chain Sheet of Fe(NCBH3)2(bpa)2·(biphenyl) (bpa = 1,2-bis(4-pyridyl)ethane) and its Stepwise Spin-Crossover Phenomenon. Polymers 2012, 4, 880-888. https://doi.org/10.3390/polym4010880
Nakashima S, Morita T, Inoue K, Hayami S. Spiral Assembly of the 1D Chain Sheet of Fe(NCBH3)2(bpa)2·(biphenyl) (bpa = 1,2-bis(4-pyridyl)ethane) and its Stepwise Spin-Crossover Phenomenon. Polymers. 2012; 4(1):880-888. https://doi.org/10.3390/polym4010880
Chicago/Turabian StyleNakashima, Satoru, Takaki Morita, Katsuya Inoue, and Shinya Hayami. 2012. "Spiral Assembly of the 1D Chain Sheet of Fe(NCBH3)2(bpa)2·(biphenyl) (bpa = 1,2-bis(4-pyridyl)ethane) and its Stepwise Spin-Crossover Phenomenon" Polymers 4, no. 1: 880-888. https://doi.org/10.3390/polym4010880




