Solution Properties of Water-Soluble “Smart” Poly(N-acryloyl-N′-ethyl piperazine-co-methyl methacrylate)
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of N-Acryloyl-N′-ethyl piperazine (AcrNEP)
2.3. Synthesis of Copolymers
| Copolymer | XFeed | XCopolymer | Mw × 10−5 (g∙mol−1) | pKap | ||
| AcrNEP | MMA | AcrNEP | MMA | |||
| BCP-1 | 0.5200 | 0.4799 | 0.4412 | 0.5588 | 0.95 | 4.65 |
| BCP-2 | 0.6236 | 0.3764 | 0.5502 | 0.4498 | 0.91 | 4.66 |
| BCP-3 | 0.6982 | 0.3018 | 0.6017 | 0.3983 | 0.89 | 4.66 |
| BCP-4 | 0.7618 | 0.2382 | 0.6627 | 0.3374 | 0.97 | 4.66 |

2.4. Characterization
 (1)
(1) (2)
(2) (3)
(3)3. Results and Discussion
3.1. Synthesis of Copolymers and Solubility
3.2. FTIR Spectroscopy and Copolymer Composition
 (4)
(4)
3.3. Lower Critical Solution Temperature (LCST) in Water and Buffer Solution

 (5)
(5) (6)
(6)
3.4. Effect of Simple Salts and Cationic Surfactants on the LCST

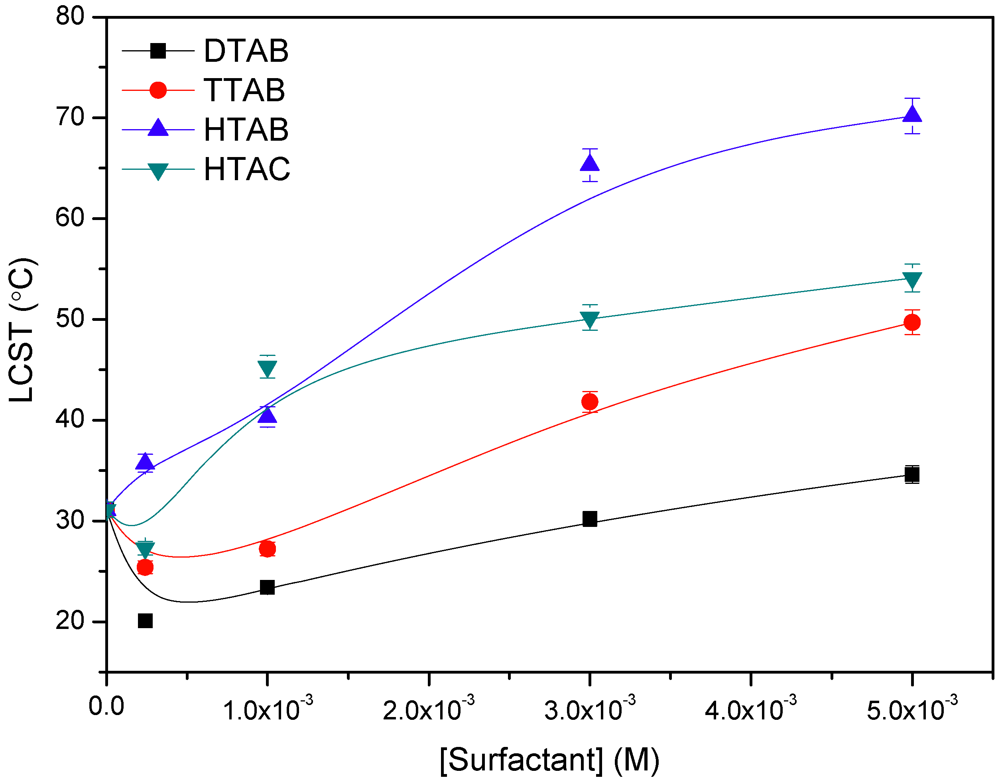
3.5. Microcalorimetric Study
| Copolymer | LCST Water (°C) | ΔH (J∙g−1) | Cp (J∙g−1∙K−1) | Tm (°C) |
| BCP-1 | 33.3 | 24.35 | 2.89 | 32.10 |
| BCP-4 | 61.2 | 24.40 | 3.08 | 28.02 |
3.6. Viscosity Measurements
 (7)
(7) (8)
(8)
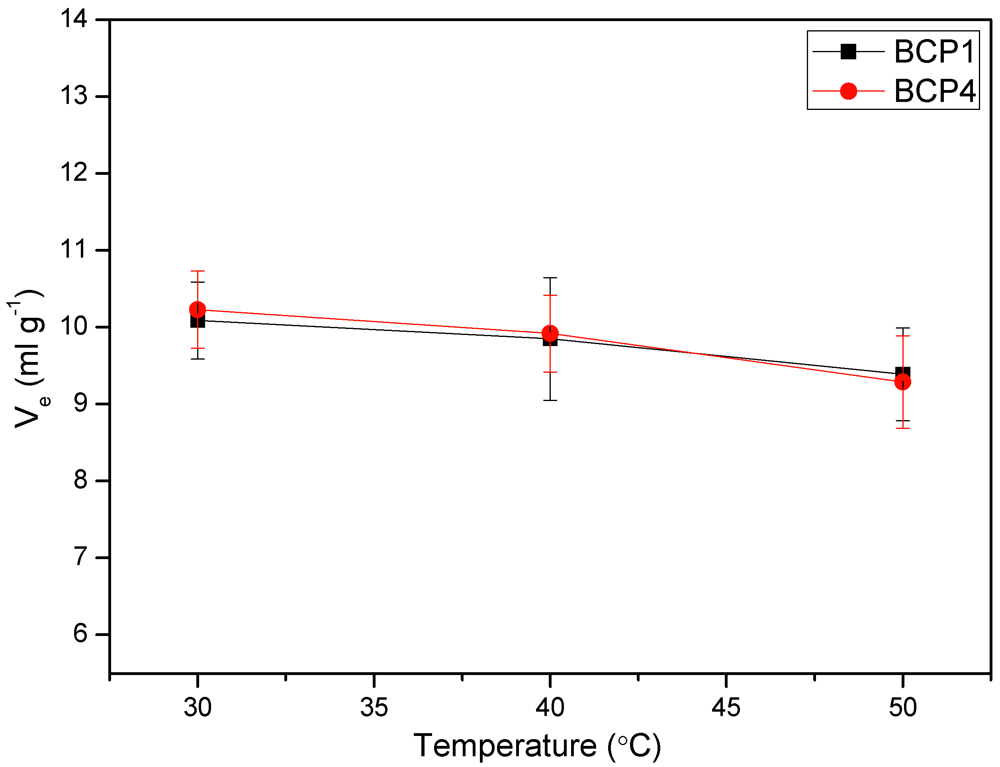

3.7. Light Scattering Measurements
| Copolymer | Mw (10−5 g∙mol−1) | Rh (nm) | A2 (10−4 mol∙cm3∙g−2) |
| BCP-1 | 0.92 | 20.28 | 1.79 |
| BCP-2 | 0.96 | 20.86 | 1.88 |
| BCP-3 | 0.87 | 21.32 | 1.93 |
| BCP-4 | 0.98 | 22.01 | 1.98 |
3.8. Glass Transition Temperature
| Copolymer | Tg (°C) |
| BCP-1 | 114.12 |
| BCP-2 | 111.20 |
| BCP-3 | 109.36 |
| BCP-4 | 109.00 |
| PAcrNEP | 108.32 |
| PMMA | 114.70 |
4. Conclusions
Acknowledgements
References
- Okano, T.; Bae, Y.H.; Jacobs, H.; Kim, S.W. Thermally on-off switching polymers for drug permeation and release. J. Control. Release 1990, 11, 255–265. [Google Scholar] [CrossRef]
- Okuzaki, H.; Osada, Y. Electro-driven polyelectrolyte gel with biomimetic motility. Electrochim. Acta 1995, 40, 2229–2232. [Google Scholar] [CrossRef]
- Hoffman, A.S. The origins and evolution of ‘controlled’ drug delivery systems. J. Control. Release 2008, 132, 153–156. [Google Scholar] [CrossRef]
- Freitas, R.F.S.; Cussler, E.L. Temperature sensitive gels as extraction solvents. Chem. Eng. Sci. 1987, 42, 97–103. [Google Scholar] [CrossRef]
- Miyata, T.; Uragami, T.; Nakamae, K. Biomolecule-sensitive hydrogels. Adv. Drug Delivery Rev. 2002, 54, 79–98. [Google Scholar] [CrossRef]
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Vaidya, A.A.; Lele, B.S.; Kulkarni, M.G.; Mashelkar, R.A. Thermoprecipitation and lysozyme from egg white using copolymers of N-isopropylacrylamide and acidic monomers. J. Biotechnol. 2001, 87, 95–107. [Google Scholar]
- Alli, A.; Hazer, B. Poly(N-isoproplyacrylamide) thermoresponsive cross-linked conjugates containing polymeric soyabean oil and/or polypropylene glycol. Eur. Polym. J. 2008, 44, 1701–1713. [Google Scholar] [CrossRef]
- Zhang, C.; Easteal, A.J. Study of free-radical copolymerization of N-isopropylacrylamide with 2-acrylamido-2-methyl-1-propanesulphonic acid. J. Appl. Polym. Sci. 2003, 88, 2563–2569. [Google Scholar] [CrossRef]
- Gürdağ, G.; Kurtuluş, B. Synthesis and characterization of novel poly(N-isopropylacrylamide-co-N,N′-dimethylaminoethyl methacrylate sulphate) hydrogels. Ind. Eng. Chem. Res. 2010, 49, 12675–12684. [Google Scholar]
- González, N.; Elvira, C.; San Román, J. Novel dual-stimuli-responsive polymers derived from ethylpyrrolidine. Macromolecules 2005, 38, 9298–9303. [Google Scholar] [CrossRef]
- Mujumdar, S.K.; Siegel, R.A. Introduction of pH-sensitivity into mechanically strong nanoclay composite hydrogels based on N-isopropylacrylamide. J. Polym. Sci. Part A: Polym. Chem. 2008, 46, 6630–6640. [Google Scholar] [CrossRef]
- Yuk, S.H.; Cho, S.H.; Lee, S.H. pH/temperature-responsive polymer composed of poly(N,N-dimethylamino ethyl methacrylate-co-ethylacrylamide). Macromolecules 1997, 30, 6856–6859. [Google Scholar] [CrossRef]
- Uzgören, A.; Rzaev, Z.M.O.; Okay, G. Bioengineering functional copolymers: Synthesis and characterization of poly(N-isopropylacrylamide-co-3,4-2H-dihydropyran)s. J. Polym. Res. 2007, 14, 329–338. [Google Scholar] [CrossRef]
- Roshan Deen, G.; Gan, L.H. Determination of reactivity ratios and swelling characteristics of ‘stimuli’responsive copolymers of N-acryloyl-N’-ethyl piperazine and methyl methacrylate. Polymer 2006, 47, 5025–5034. [Google Scholar]
- Roshan Deen, G. Studies of Piperazine-Based Polymers and Hydrogels. Ph.D. dissertation, Nanyang Technological University, Singapore, 2000. [Google Scholar]
- Asaduzamman, A.K.M.; Rakshit, A.K.; Devi, S. Solution properties of methyl methacrylate and acrylonitrile. J. Appl. Polym. Sci. 1993, 47, 1813–1819. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Deen, G.R. Solution Properties of Water-Soluble “Smart” Poly(N-acryloyl-N′-ethyl piperazine-co-methyl methacrylate). Polymers 2012, 4, 32-45. https://doi.org/10.3390/polym4010032
Deen GR. Solution Properties of Water-Soluble “Smart” Poly(N-acryloyl-N′-ethyl piperazine-co-methyl methacrylate). Polymers. 2012; 4(1):32-45. https://doi.org/10.3390/polym4010032
Chicago/Turabian StyleDeen, G. Roshan. 2012. "Solution Properties of Water-Soluble “Smart” Poly(N-acryloyl-N′-ethyl piperazine-co-methyl methacrylate)" Polymers 4, no. 1: 32-45. https://doi.org/10.3390/polym4010032




