Observation of Long-Range Vicinal Effect in Chiral Cu(II)-Cr(VI) or Cu(II)-W(VI) Bimetallic Coordination Polymers
Abstract
: We have prepared some diastereomers of [CuL2][M2O7] (L is 1,2-diaminocyclohexane and its derivatives; M = Cr and W) bimetallic coordination polymers and confirmed their structural similarity and inner electronic states by means of XRD and XAS, respectively. For the first time, we have successfully observed distant vicinal effect of which the chiral source is only chiral organic ligands of [CuL2]2+ moieties (acting as ligand complex), while probe bands for solid state CD spectra are charge transfer (CT) bands of [M2O7]2− moieties (achiral complex) with the d0 electronic configuration. The new concept (interpretation) of this observation will be important for supramolecular chirality of coordination polymers built by ligand complexes.1. Introduction
Recently we have proposed a new concept of “supramolecular vicinal effect”, which is long-range induced chiroptical observation of an achiral unit from non-bonded (supramolecular assemblies) or weakly surrounded (host-guest co-crystal) chiral sources [1]. It is different to the normal vicinal effect (observation of CD bands in d-d region from chiral ligands through coordination bonds) [2,3] or conventionally induced CD of many supramolecular assemblies [4,5]. As for chiral coordination polymers, thermally-accessible lattice strain and local pseudo Jahn-Teller distortion of [CuL2]3[M(CN)6]2·4H2O (L = trans-cyclohexane-(1R, 2R)-diamine; M = Cr, Co, and Fe) [6] have been reported. In its crystal packing of co-crystals of one-dimensional cyanide-bridged Cu(II)-Cr(III), and Cu(II)-Co(III) bimetallic assemblies and mononuclear Cu(II) complexes, (pseudo) Jahn-Teller effect play an important role in flexible distortion of crystal structures especially in the Cu(II)coordination environment. Moreover, we prepared their H/D isotope derivatives to confirm Jahn-Teller effect [7]. Though solid-state CD spectra were also measured, supramolecular co-crystals of chiral components makes their chiroptical properties difficult to understand.
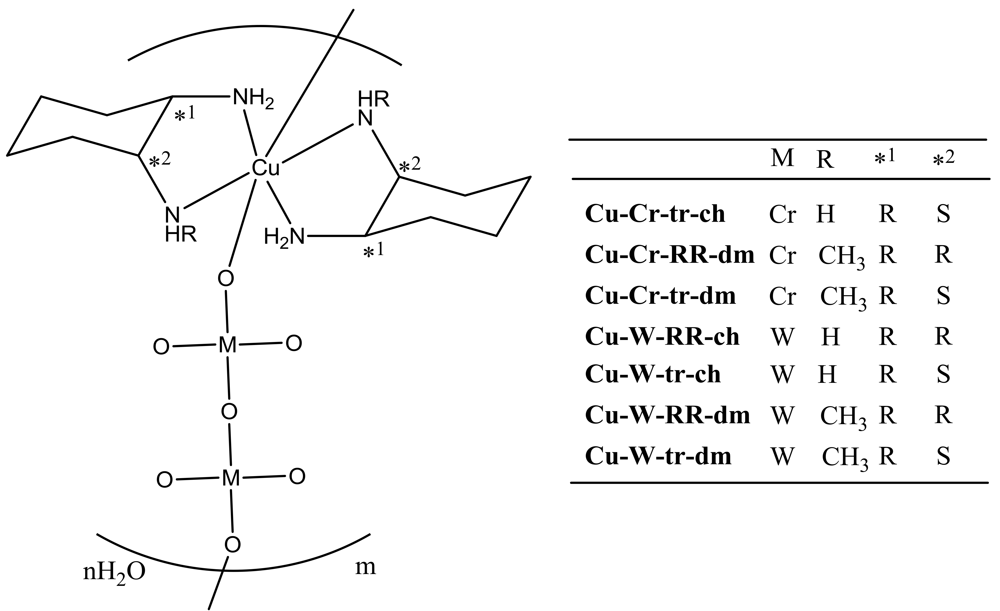
Herein, we have prepared some diastereomers of [CuL2][M2O7] (L is 1,2-diaminocyclohexane and its derivatives; M = Cr and W) bimetallic coordination polymers (Figure 1). For the solid state CD bands, charge transfer of [M2O7]2− moieties result from long-range vicinal effect by chiral organic ligands of [CuL2]2+ moieties (acting as ligand complex). In addition, there are only a few examples, [Cu(ligands)][Cr2O7] [8], structurally characterized.
2. Results and Discussion
2.1. Solid State CD and Electronic Spectra
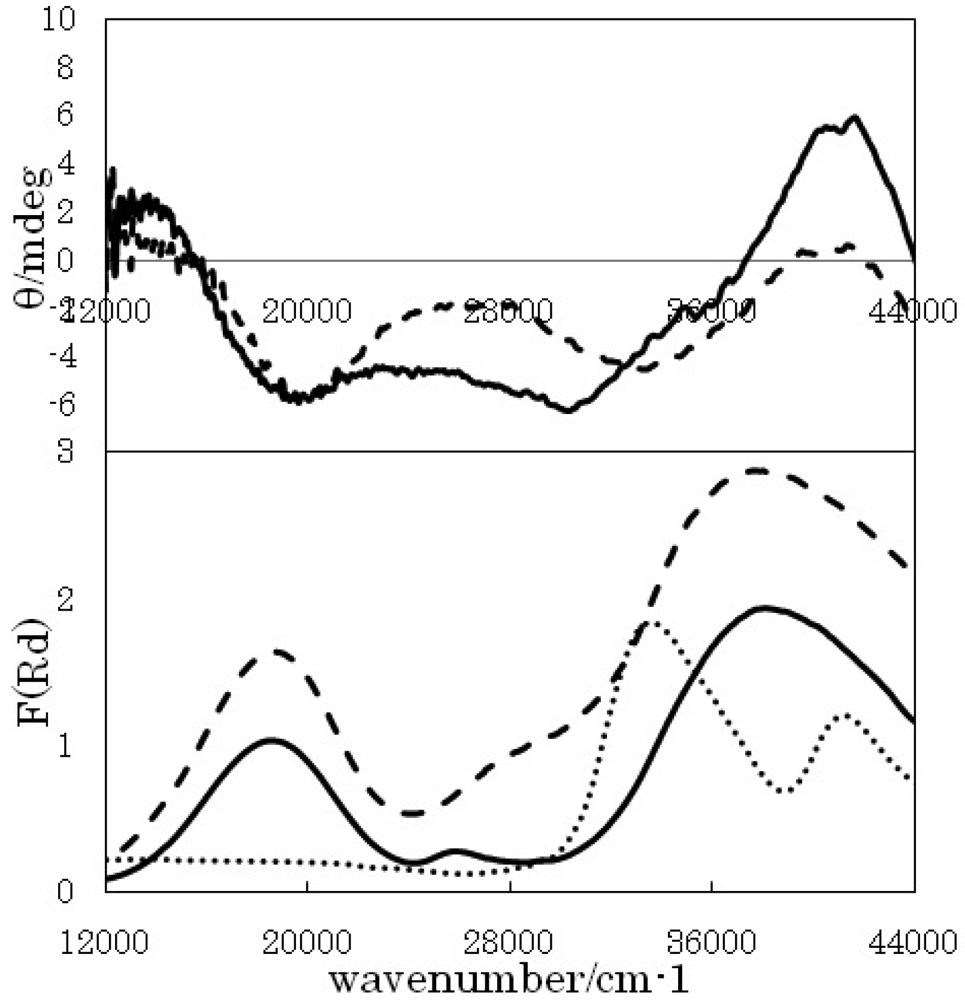
Figures 2, 3, and 4 show solid state CD (as KBr pellets) and diffuse reflectance electronic spectra for Cu-Cr-RR-dm, Cu-W-RR-ch, and Cu-W-RR-ch, respectively. The CD spectrum of Cu-Cr-RR-dm shows a negative peak at 18,000 cm−1, a negative peak at 30,000 cm− 1, and a positive peak at 37,000 cm−1. The corresponding peaks in diffuse reflectance electronic spectra are 20,000 cm−1, and a rather broad band in the 3,00,400 cm−1 region. While the diffuse reflectance electronic spectra (not shown) exhibit peaks or shoulders at 17,000, 21,000, 26,000, 27,000, and 38,000 cm−1 for Cu-Cr-tr-ch and 17,000, 21,000, 25,000, 33,000, and 38,000 cm−1 for Cu-Cr-tr-dm.
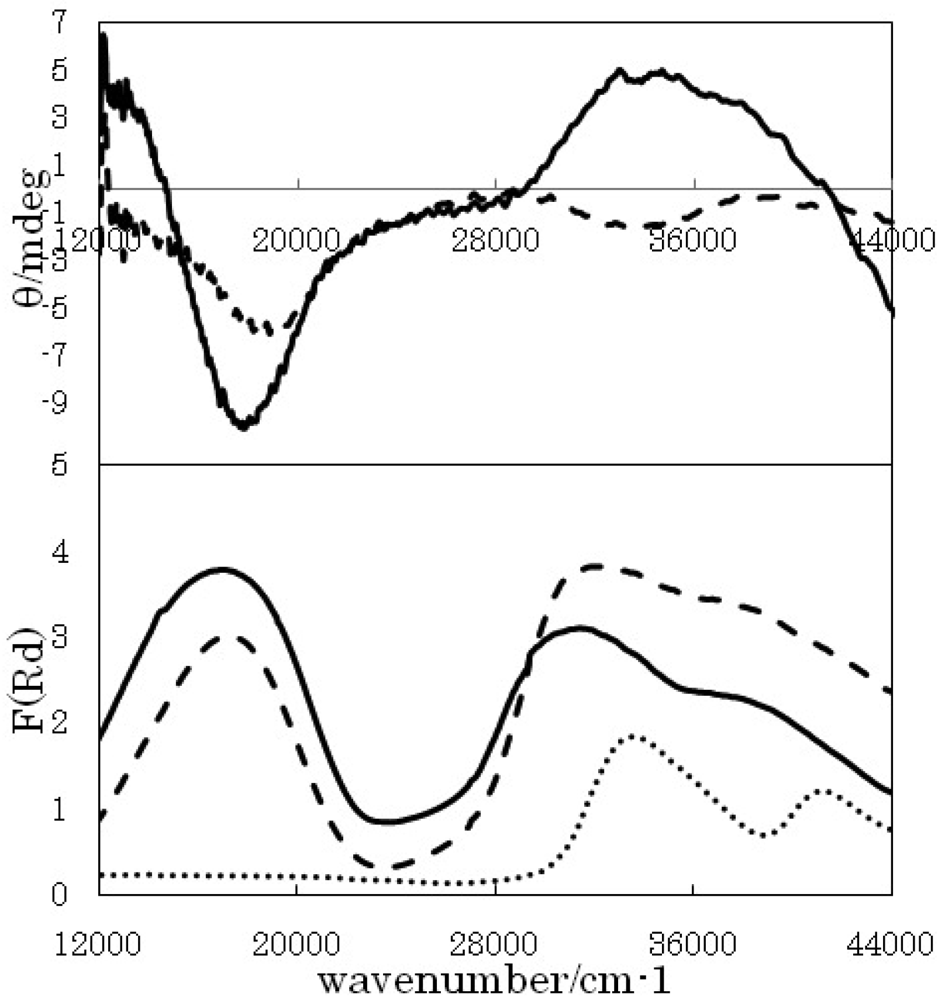
On the other hand, the CD spectrum of Cu-W-RR-ch shows a negative peak at 18,000 cm−1, which is the sole obvious peak. The corresponding peaks in diffuse reflectance electronic spectra are 18,000 and 37,000 cm−1. The CD spectrum of Cu-W-RR-dm shows a negative peak at 17,000 cm−1 and a positive peak at 34,000 cm−1. The corresponding peaks in diffuse reflectance electronic spectra are 17,000, 31,000, and 38,000 cm−1. While the diffuse reflectance electronic spectra (not shown) exhibit peaks or shoulders at 18,000 and 37,000 cm−1 for Cu-W-tr-ch and 17,000, 31,000, and 38,000 cm−1 for Cu-W-tr-dm.
Systematic comparison of the related spectra and peaks of Cu-precursor at 17,000 (d-d) and 32,000 (CT) cm −1, Cr-precursor at 20,000 (CT) cm−1, and W-precursor at 32,000 and 40,000 (CT) cm−1 should be helpful for establishing assignment experimentally. It should be noted that CD bands in d-d region of Cu-precursor are attributed to vicinal effect by chiral organic ligand, while CD bands in CT region of CT band of bimetallic coordination polymers are attributed to vicinal effect by chiral complex ligand (namely the Cu-precursor). For example, a negative peak around 30,000 cm−1 for Cu-Cr-RR-dm (Figure 2) is a typical CD band of long-range vicinal effect classified as a novel case.
2.2. Powder XRD Patterns
As mentioned above, the corresponding metal-substituted coordination polymers with identical ligands, indicate similar XRD patterns at room temperature except for the effect of metal ion size. Selected predominant peaks are also described in the experimental section. In the course work, we have investigated negative or positive thermal expansion of lattice and local bond compression or elongation about Jahn-Teller distortion of axial bonds, which are usually flexible. The aim of temperature dependence XRD measurement is to check there are no abnormal structural changes with changing temperatures.
For example, we measured powder XRD patterns (not shown) for Cu-W-RR-ch measured at 110– 300 K with an interval of 10 K. The predominant peaks appeared at 2θ = 7.685, 12.905, 14.964, 16.936, 21.286, 24.853, 27.202, 27.840, 28.362, 29.377, 30.189, 31.117, 32.770, 33.959, 41.789, 43.500, and 44.950 degree at 110 K. On heating up to 300 K, the corresponding peaks appeared at 2θ = 7.656, 12.847, 14.906, 16.878, 21.228, 24.737, 27.057, 27.724, 28.246, 29.232, 30.073, 31.001, 32.625, 33.814, 41.615, 43.326, and 44.776 degrees at 300 K, i.e., diffraction peaks shift to low-angles. Thermal shift features with detailed values for the most intense peaks are listed in Table 1. Therefore, the crystal lattice normally exhibits positive thermal expansion depending on the temperature.
2.3. XAS Spectra
Figure 5 shows temperature dependence of soft X-ray absorption spectra (XAS) for Cu-W-RR-ch measured at 28, 50, 100, 150, 200, and 250 K. At each temperature, the Cu2p1/2 and Cu2p3/2 peaks appeared at about 952 and 932 eV, respectively. Absence of weak peaks between them suggests that valence state is not copper(I) nor mixed-valence of copper(I) and copper(II) but clearly copper(II). The bridging [Cr2O7]2− moieties did not contribute to delocalization of charges. In addition, little difference between temperature changes indicates that the electronic states of inner shells are stable.
3. Experimental Section
Materials
All the reagents (Aldrich, TCI, and Wako) were commercially available and were used as received without further purification. The precursor mononuclear copper(II) complexes were prepared according to procedures in the literature (e.g., [4]) with corresponding starting compounds.
Preparation of Cu-Cr-tr-ch
Slow diffusion of an aqueous solution (45 mL) of the precursor (0.0150 g, 0.0423 mmol) onto an aqueous solution (5 mL) of Na2CrO4 (0.0107 g, 0.0926 mmol) at 298 K gave rise to brown precipitates after several days. Yield 0.0032 g (14.0%). IR (KBr, cm−1): 938, 877 (Cr-O). XRD (λ = 1.54184 Å, 2θ/degree): 7.946, 17.342, 19.082, 19.198, 20.909, 23.838, 29.638, 43.471.
Preparation of Cu-Cr-RR-dm
Yield 0.0041 g (17.6%). IR (KBr, cm−1): 936, 873 (Cr-O). XRD (λ = 1.54184 Å, 2θ/degree): 9.135, 15.283, 17.835, 18.183, 19.865, 21.779, 23.954, 25.172, 27.202, 30.943.
Preparation of Cu-Cr-tr-dm
Yield 0.0048 g (20.0%). IR (KBr, cm−1): 935, 873 (Cr-O). XRD (λ = 1.54184 Å, 2θ/degree): 6.989, 7.540, 8.207, 8.903, 10.324, 12.180, 12.992, 13.804, 14.500, 23.693, 28.449, 29.174.
Preparation of Cu-W-RR-ch
Yield 0.0251 g (70.2%). IR (KBr, cm−1): 852, 819 (W-O). XRD (λ = 1.54184 Å, 2θ/degree): 16.878, 23.432, 27.724, 29.551, 32.654, 43.355.
Preparation of Cu-W-tr-ch
Yield 0.0218 g (61.0%). IR (KBr, cm−1): 852, 819 (W-O). XRD (λ = 1.54184 Å, 2θ/degree): 7.279, 14.587, 16.878, 27.724, 29.522, 32.625, 39.208, 43.355.
Preparation of Cu-W-RR-dm
Yield 0.0141 g (40.0%). IR (KBr, cm−1): 852, 819 (W-O). XRD (λ = 1.54184 Å, 2θ/degree): 16.878, 23.432, 27.724, 29.551, 32.654, 43.355.
Preparation of Cu-W-tr-dm
Yield 0.0113 g (32.0%). IR (KBr, cm −1): 852, 820 (W-O). XRD (λ = 1.54184 Å, 2θ/degree): 12.847, 14.906, 16.820, 21.054, 21.199, 25.839, 27.057, 28.246, 29.232, 30.044, 31.001, 33.553, 33.814, 41.615, 44.747.
Physical Measurements
Infrared spectra were recorded as KBr pellets on a JASCO FT-IR 4200 plus spectrophotometer equipped with polarizer in the range of 4,000–400 cm−1 at 298 K. Diffuse reflectance electronic spectra were measured on a JASCO V-570 UV/VIS/NIR spectrophotometer equipped with an integrating sphere in the range of 800–200 nm at 298 K. Circular dichroism (CD) spectra were measured as KBr pellets on a JASCO J-820 spectropolarimeter in the range of 800–200 nm at 298 K. Powder XRD patterns were also measured by using synchrotron radiation beamline at KEK-PF BL-8B (2010G511) with 8 keV (λ = 1.54184 Å) with a RIGAKU imaging plate. The Cu2p3/2 and Cu2p1/2 peaks of XAS (soft X-ray absorption spectra) were measured at KEK PF BL-19B (2010G510) under variable temperature. The spectra were corrected by the standard Au sample.
4. Conclusions
In summary, we have prepared some diastereomers of [CuL2][M2O7] (L is 1,2-diaminocyclohexane and its derivatives; M = Cr and W) bimetallic coordination polymers and confirmed their structural similarity and inner electronic states for selected compounds. We have successfully observed distant vicinal effect in the chiral Cu(II)-Cr(VI) or Cu(II)-W(VI) bimetallic coordination polymers. The only chiral source is chiral organic ligands of [CuL2]2+ moieties, while probe bands for solid state CD spectra are charge transfer bands of [M2O7]2− moieties with d0 electronic configuration. Furthermore a detailed study of an analogous complex (Cu-Cr-RR-ch), discussed as a single crystal, will be reported in a separate paper.


| T/K | 2θ/degree | Intensity | Half width/degree |
|---|---|---|---|
| 110 | 27.84 | 4,862 | 0.435 |
| 120 | 27.84 | 4,860 | 0.406 |
| 130 | 27.81 | 4,850 | 0.435 |
| 140 | 27.81 | 4,935 | 0.435 |
| 150 | 27.81 | 4,888 | 0.435 |
| 160 | 27.78 | 4,831 | 0.435 |
| 170 | 27.78 | 4,868 | 0.435 |
| 180 | 27.78 | 4,876 | 0.435 |
| 190 | 27.78 | 4,881 | 0.435 |
| 200 | 27.78 | 4,807 | 0.435 |
| 210 | 27.78 | 4,864 | 0.406 |
| 220 | 27.75 | 4,713 | 0.435 |
| 230 | 27.75 | 4,731 | 0.406 |
| 240 | 27.75 | 4,724 | 0.435 |
| 250 | 27.75 | 4,696 | 0.435 |
| 260 | 27.72 | 4,669 | 0.435 |
| 270 | 27.75 | 4,651 | 0.435 |
| 280 | 27.72 | 4,682 | 0.435 |
| 290 | 27.72 | 4,673 | 0.435 |
| 300 | 27.72 | 4,637 | 0.435 |
Acknowledgments
This work with synchrotron radiation was carried out at KEK-PF BL-8B (2010G511) and BL-19B (2010G510).
References
- Akitsu, T. Syntheses, structure, and electronic properties of assemblies of diastereomers of nickel(II) complexes with tetracyanometalates [M(CN)4]2− (M = Ni, Pd, and Pt). In Stereochemistry Research Trends; Monika, M.A., Golob, J.H., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2008; Chapter 4; pp. 107–128. [Google Scholar]
- Mason, S.F. Molecular Optical Activity & the Chiral Discriminations; Cambridge University Press: Cambridge, UK, 1982. [Google Scholar]
- Amouri, H.; Gruselle, M. Chirality in Transiton Metal Chemistry; Wiley: Chichester, UK, 2008. [Google Scholar]
- Koslowski, A.; Seerama, N.; Woody, R.W. Theoretical approach to electronic optical activity. In Circular Dichroism Principle and Applications, 2nd ed.; Berova, N., Nakanishi, K., Woody, R.W., Eds.; Wiley-VCH: New York, NY, USA, 2000; pp. 55–96. [Google Scholar]
- Akitsu, T.; Einaga, Y.; Yoza, K. Thermally-accessible lattice strain and local pseudo Jahn-Teller distortion in various dimensional CuII-MIII bimetallic cyanide-bridged assemblies. Open Inorg. Chem. J. 2008, 1, 1–10. [Google Scholar]
- Akitsu, T.; Sonoki, S. Influence of water molecules on properties of binuclear or bridged structures for chiral CuII-NiII, CuII-PdII, and CuII-PtII tetracyano-bimetallic assemblies. Open Cryst. J. 2011, 4, 8–15. [Google Scholar]
- Jameson, G.B.; Seferiadis, N.; Oswald, H.R. μ-Dichromato(VI)-O,O′-bis(ethylenediamine)copper(II): Structure, thermoehemistry and magnetic susceptibility. Acta Crystallographica 1986, C42, 984–987. [Google Scholar]
©2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hayashi, N.; Akitsu, T. Observation of Long-Range Vicinal Effect in Chiral Cu(II)-Cr(VI) or Cu(II)-W(VI) Bimetallic Coordination Polymers. Polymers 2011, 3, 1029-1035. https://doi.org/10.3390/polym3031029
Hayashi N, Akitsu T. Observation of Long-Range Vicinal Effect in Chiral Cu(II)-Cr(VI) or Cu(II)-W(VI) Bimetallic Coordination Polymers. Polymers. 2011; 3(3):1029-1035. https://doi.org/10.3390/polym3031029
Chicago/Turabian StyleHayashi, Naoshi, and Takashiro Akitsu. 2011. "Observation of Long-Range Vicinal Effect in Chiral Cu(II)-Cr(VI) or Cu(II)-W(VI) Bimetallic Coordination Polymers" Polymers 3, no. 3: 1029-1035. https://doi.org/10.3390/polym3031029




