Electrospun Nanofibrous Materials for Neural Tissue Engineering
Abstract
: The use of biomaterials processed by the electrospinning technique has gained considerable interest for neural tissue engineering applications. The tissue engineering strategy is to facilitate the regrowth of nerves by combining an appropriate cell type with the electrospun scaffold. Electrospinning can generate fibrous meshes having fiber diameter dimensions at the nanoscale and these fibers can be nonwoven or oriented to facilitate neurite extension via contact guidance. This article reviews studies evaluating the effect of the scaffold's architectural features such as fiber diameter and orientation on neural cell function and neurite extension. Electrospun meshes made of natural polymers, proteins and compositions having electrical activity in order to enhance neural cell function are also discussed.1. Introduction
Central and peripheral nervous system injuries may benefit from the use of neural tissue engineering strategies that use scaffolds to facilitate the regrowth of nerves. In neurodegenerative diseases, such as alzheimer's and parkinson's diseases, the common pathological onset is the accumulation of insoluble filamentous aggregates which underlie early axonal dysfunction and pathology, leading to potentially irreversible neuron degeneration [1-3]. Current therapeutic strategies focus on stabilizing symptoms and slowing the progression of the disease. Researchers are also focusing on pharmacological or cellular based treatments [4], wherein scaffolds could be utilized to direct neuronal growth and differentiation. Brain damage caused by traumatic brain injury (TBI) results in immediate and delayed cell death leading to cavity formation and glial scarring [5]. Providing neuroprotection to reduce inflammation and prevent secondary cell death is of interest as well as replacing damaged neurons, providing appropriate factors, and promoting neurite regeneration and growth to restore original neural structure [6]. Neural tissue engineering strategies are actively being sought for the latter. Similarly for spinal cord injury (SCI), the initial neurological damage provokes a series of cellular and biochemical responses leading to further secondary damage. This secondary injury further prohibits nerve regeneration and causes more cell death. The initial cell death creates a cavity at the injury site and glial scar around the lesion [7]. Therapeutic strategies for spinal cord repair are to regrow the damaged axon, promote neurite or axonal growth across the lesion site, and to direct neurite or axonal elongation and reinnervation of the axon to the appropriate target [7]. The most severe peripheral nervous system (PNS) injury is complete transection of the nerve fiber. After the injury, protease activity increases at the site of injury initiating a series of degradation events at the distal ends of the injury. New axonal formation is usually initiated from the unmyelinated region of the nodes of Ranvier. In humans, the axon regeneration rate has been reported to be approximately 2 to 5 mm per day [8]. For large nerve defects, the recovery rate is delayed. Autologous nerve grafts are commonly used in these cases [9,10], but the disadvantage is the loss of function at the donor site. Neural tissue engineering strategies may be a promising approach to bridge these defects.
In a tissue engineering approach, an engineered scaffold loaded with a specific cell type may promote functional restoration. Surface and bulk properties of a well-designed scaffold, similar to the environmental cues in the extracellular matrix, can provide appropriate signals for cell growth, differentiation and subsequent tissue formation. Surface physicochemical properties, such as topography, surface charge and protein adsorption/immobilization/release, have been shown to influence cell behavior. The electrospun nano-fibrous scaffolds have gained considerable interest because the architecture is similar to the naturally occurring protein fibrils in the extracellular environment [11,12]. Each individual nano-scale fiber has a high surface to volume and aspect ratio allowing for more surface area contact of the scaffold with the cell. The physical and biological properties of the scaffold are dependent on the material used for electrospinning and its properties such as surface wettability, mechanical properties and degradation. The properties of the scaffolds can be manipulated by copolymerization or polymer blending of various biodegradable, non-biodegradable, synthetic and/or natural materials.
The following sections provide an overview of the electrospinning apparatus and studies using electrospun fibers for neural tissue engineering applications. Investigations examining the effects of architectural characteristics of the fibers such as fiber diameter and alignment are discussed. Electrospun scaffolds that also incorporate proteins as well as electrical activity to enhance neural cell function are also reviewed.
2. Overview of the Electrospinning Process
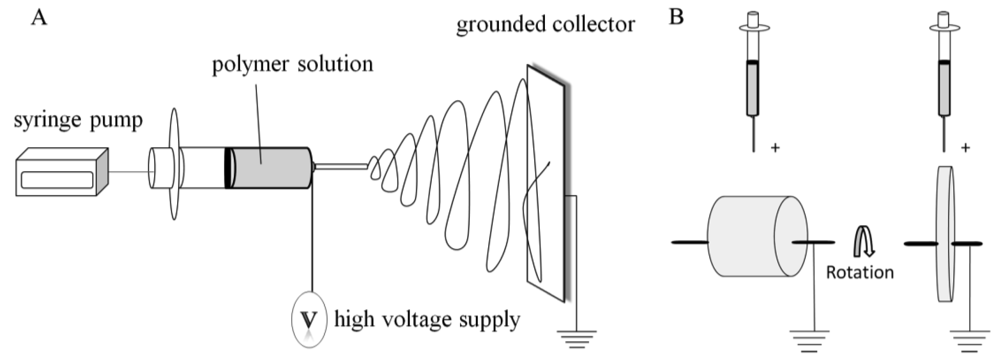
A general procedure can be described for fabricating nonwoven, electrospun fibrous scaffolds [13]. The electrospinning process consists of a high voltage power source, syringe pump, syringe, needle, and a grounded collection device (Figure 1(A)). A polymer solution is loaded into the syringe having an attached needle. A high voltage is applied to the polymer solution as it is ejected at a desired flow rate (controlled by the syringe pump). When a high voltage is injected into the polymer solution, an electrostatic force overcomes the surface tension of the polymer solution at the tip of the needle and a Taylor cone is formed, which is further elongated into a fluid jet. The charged fluid jet is collected on a grounded device due to the electrical potential difference between the polymer solution at the tip of the syringe and the grounded collecting device. The whipping motion of the polymer jet that takes place between the needle and the plate allows for the solvent to evaporate, which results in the collection of a polymer fiber mesh on the collection plate. The resulting mesh consists of nonwoven, random fibers that can vary in diameter from the nano to micron-scale depending upon the processing parameters used.
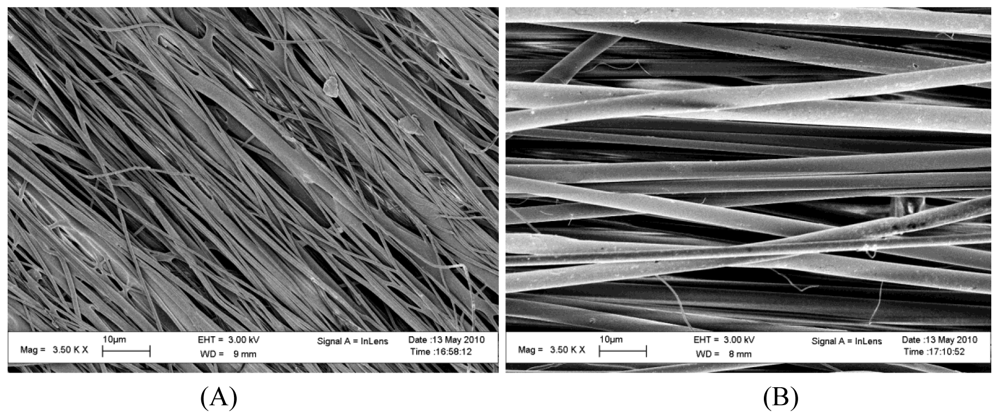
Aligned fibrous meshes (Figure 2) can be achieved by varying the collection method. The most common methods are collecting on a high speed rotating drum or disk [15] (Figure 1(B)). This allows for the fiber to collect along the direction of rotation. Small diameter tubes can also be fabricated by this method and have been used in vascular repair studies [16]. A high rotation speed produces increased fiber alignment as compared to lower rotation speed, but may cause fiber discontinuity [15,17].
3. Effect of Fiber Orientation and Size
A promising tissue engineering strategy for neural repair is to create aligned fibrous scaffolds to provide guidance for cell migration and directional axonal regeneration across the glial scar and lesion site in both central nervous system (CNS) and PNS injuries. Yang et al. first published electrospun aligned poly L-lactic acid (PLLA) scaffolds for neural application in 2005 [18]. Research interest has gradually increased in developing an electrospun based conduit for neural repair. The majority of these studies have focused on evaluating neural proliferation, differentiation, and neurite extension of various cell types on aligned fibrous scaffolds fabricated with different materials and fiber sizes.
Various synthetic and natural polymers have been investigated for fabricating scaffolds for neural applications. The polyesters, PLLA, poly glycolic acid (PGA), and poly ε-caprolactone (PCL), are the most commonly used synthetic, biodegradable, and biocompatible polymers for neural repair. Studies have evaluated these compositions as electrospun mats having aligned and/or random fiber arrangements. Mouse embryonic stem cell differentiation into neurons, astrocytes, and oligodendrocytes was enhanced when cultured on aligned and random PCL fibers [19]. In other studies, aligned PLLA nanofibrous scaffold also supported the growth and extension of dorsal root ganglion (DRGs) [20,21] and primary motor neurons [20]. Primary motor neurons developed neurites earlier on PLLA nanofibers when compared to PLLA films [22]. However, no differences in neurite number and length were detected between aligned and random PLLA fibers [22]. For human Schwann cells on PCL scaffolds, aligned fibers promoted cytoskeleton and nuclei alignment along the fiber. Random and aligned electrospun PCL scaffolds induced up-regulation of myelin-associated glycoprotein (MAG), an early myelination marker and down-regulation of NCAM-1 (immature Schwann cell marker) [23]. However, the increase in expression of P0, a myelin-specific gene, was only observed on the aligned PCL scaffolds suggesting promotion of Schwann cell maturation is more favored on aligned fibrous scaffolds as opposed to random mats [23]. A spiral construct incorporated with electrospun random and aligned PCL fibrous meshes improved rat Schwann cell attachment as compared to other porous PCL scaffolds without fibers in both static and dynamic culture conditions [24]. Cells showed maximum alignment and largest neurite outgrowth on aligned fibrous PLGA meshes in the presence of fluid shear stresses of 0.50 Pa and 0.25 Pa, respectively [25]. This finding suggested a combination of fiber alignment with low fluid shear stress is a promising method for neurite outgrowth stimulation. In general, studies conclude that the fibrous meshes facilitate the differentiation and that the aligned structure enhances directional neurite extension.
Fiber diameter can also influence cell adhesion, proliferation, migration, and differentiation. Yang et al. demonstrated electrospun nano-sized fibers (300 nm) of PLLA enhanced neural differentiation of neonatal mouse cerebellum C17.2 stem cells as compared to micron-sized (1.25 μm) fibers [26]. The cells elongated and oriented neurites along the direction of the aligned fibers. In addition, cells on nano-aligned fibers had the longest neurite extension as compared to micron-aligned fibrous matrices and all random mats. Rat hippocampal-derived neural stem cells were shown to differentiate into more oligodendrocytes and neurons on laminin-coated electrospun polyethersulfone fibrous scaffolds having 283 nm and 749 nm fiber diameters, respectively, as compared to tissue culture polystyrene [27]. Nano-sized fibers promoted higher proliferation and cell spreading and less cell aggregation in comparison to micron-sized fibers. Both stem cell studies indicate that contact guidance cues provided by the nanofiber plays an important role in regulating differentiation and proliferation. For neuronal cell phenotypes, fiber diameter appeared to play a lesser role. PC12 neurite extension on aligned PCL fibers with diameters of 3.7 ± 0.5 μm and 5 ± 0.9 μm were significantly longer than on non-aligned fibers with a diameter of 4.4 ± 0.5 μm and neurites extended parallel to the aligned fibers for all fiber diameters studied [28]. No differences in neurite extension were detected between the aligned fiber meshes having different fiber diameters. The authors of this study also developed a novel electrospinning technique to fabricate a nerve conduit with an outer layer of random and an inner layer of aligned PCL fibers with the goal of the inner layer providing structural guidance cues for nerve growth. Axon formation and length of rat embryonic hippocampal neurons has been shown to be independent of fiber diameter for poly-D-lysine coated PLGA mats [29]. However, neurite polarization was slightly greater on submicron fibers and aligned fibers suggesting the degree of fiber alignment to be the key factor in axon formation and elongation [29].
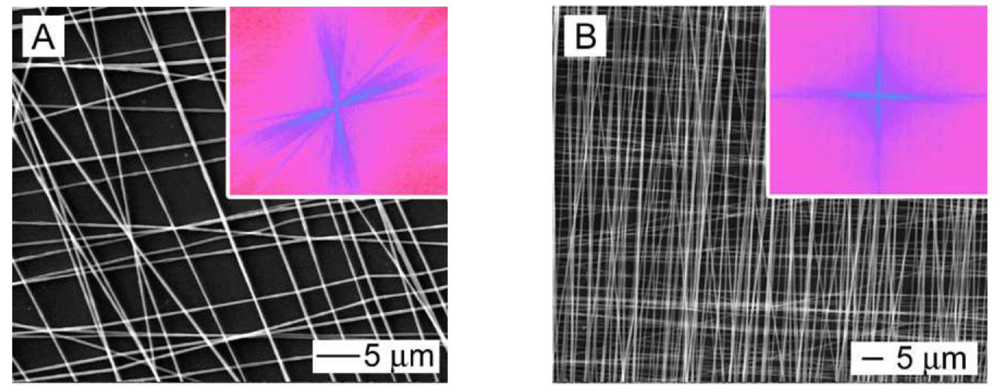
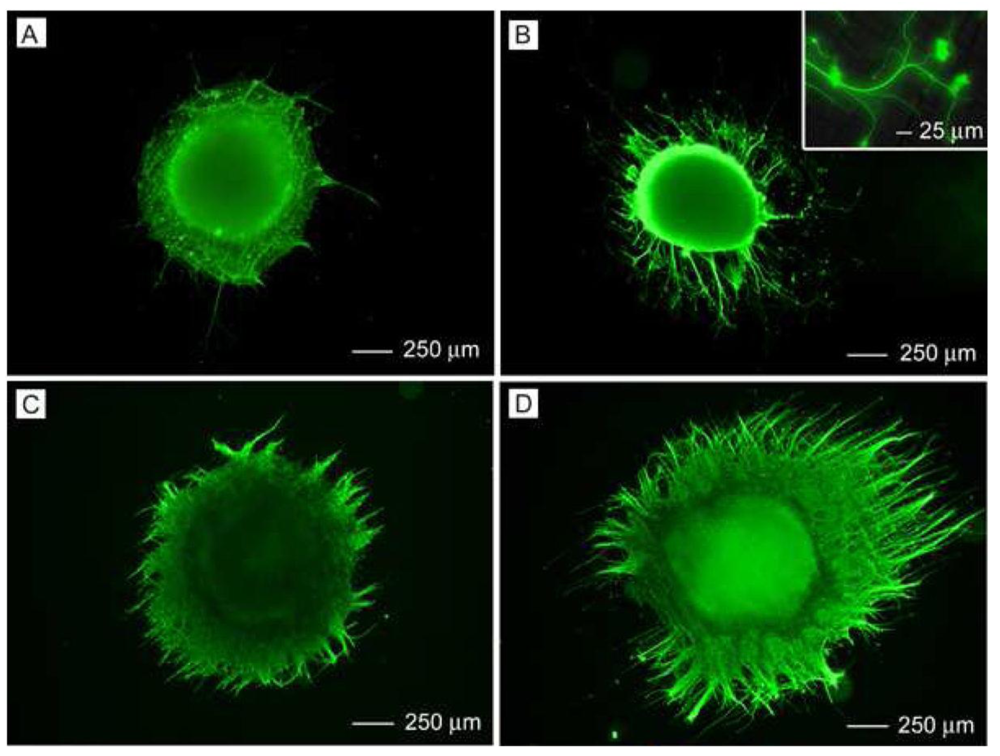
Three-dimensional configurations of aligned fibers have also been developed for CNS and PNS repair. Double layers of varying degrees of PLLA fiber alignment influenced DRG neurite extension where neurites were able to penetrate through the layers [30]. As shown in Figure 3, multilayered scaffolds with aligned fibers having different orientations in each layer were formed. DRG neurites extended along the long-axis of the laminin-coated fibers in the top layer and interestingly, would make a sharp turn to follow the long-axis of fibers in the underlying layer (Figure 4). The authors concluded that the electrospinning process could provide a more versatile scaffold for forming neural networks. In order to design a scaffold for spinal cord injury using a minimally invasive surgical technique, electrospun PCL/PLLA or collagen scaffolds embedded in a hyaluronan-methylcellulose (HAMC) hydrogel resulted in a injectable, fast gelling hydrogel [31]. Rat neural stem/progenitor cells (NSPCs) on a hydrogel containing collagen fibers had poor proliferation and differentiation [31]. Yet, NSPCs on hydrogels containing PCL/PLLA fibers had better viability and had differentiated into more oligodendrocytes [31], indicating the composition of the fibers plays a major role. A bilayered chitosan tube with an outer layer of chitosan film and an inner layer of aligned chitosan fibers promoted nerve regeneration in a rat sciatic nerve model [32]. Aligned electrospun PCL and ethyl-ethylene phosphate (PCLEEP) containing human glial cell-derived neurotrophic factor (GDNF) showed maximal electrophysiological recovery in rat sciatic nerve as compared with longitudinally or circumferentially oriented aligned PCLEEP [33]. Half-cylindrical PLLA and PLGA scaffolds with inner aligned and outer random layers were implanted into male athymic rat with T9 to T11 lateral hemisection.
The scaffolds were immobilized with or without rolipram. Rolipram is an anti-inflammatory drug that increases neuroprotection after SCI. The scaffolds with rolipram had the least glial scarring and an improved hindlimb locomotion as compared to untreated or scaffold alone [34]. Polysulfone nerve conduit (Koch Membrane Systems) filled with either (1) saline, (2) aligned, or (3) random poly(acrylonitrile-co-methylacrylate) (PAN-MA) fibrous mats were used as a rat tibial nerve bridge (Figure 5) [35]. The aligned construct improved functional outcome significantly in a 17 mm nerve defect as compared to the random construct suggesting the important role of topography in neural regeneration. Aligned and random electrospun PCL scaffolds were implanted into the caudate putamen of the adult rat brain [36]. The fiber alignment influenced the neurite infiltration and had a minimal inflammatory response. Therefore, studies using three-dimensional constructs containing aligned fibers are showing promise for various neural applications.
4. Protein Incorporation for Improving Cell Function
Many synthetic polymers have surface properties that may result in poor cell adhesion and proliferation of neural cell types. The scaffolds are often treated by chemical processes or extracellular matrix (ECM) proteins to improve cell attachment and neurite extension. Nisbet et al. hydrolyzed electrospun PLLA and PLGA creating scaffolds with varying surface tension. Scaffolds with lower surface tension induced cortical neuron-neurite extension and scaffolds having a large inter-fiber distance allowed guidance for neurite extension [37]. Collagen type I was immobilized on electrospun poly-methyl methacrylate (PMMA) and acrylic acid (AA) (PMMAAA) nano-fibrous scaffolds. The amount of carboxyl content of the nanofibers correlated directly with the amount of collagen immobilization [38]. Cell viability and neurite extension of the cortical neurons increased with increasing collagen immobilization as a result of the carboxyl content [38]. Electrospun PCL treated with ethylenediamine (ED) increased the hydrophilicity, thus, affecting cell attachment but did not affect the differentiation of rat brain-derived neural stem cells [39]. When in the presence of 10% fetal bovine serum, the neural stem cells on the PCL scaffolds would primarily differentiate into oligodendrocytes, indicating that these scaffolds could regulate differentiation. Laminin is a cell adhesion molecule that promotes neurite outgrowth through multiple adhesion sites [40]. Incorporating laminin by physical absorption, covalent coupling, or blending with the polymer solution for PLLA nanofibers was examined [41]. PC12 neurite extension was enhanced on blended laminin PLLA fibers, but not statistically significant over other laminin incorporating methods. Hence, all three are efficient functionalization techniques to incorporate ECM molecules or other components to enhance the properties of the scaffolds.
The use of synthetic polymers blended with natural materials in order to enhance cellular attachment and neurite extension has also gained interest. Aligned nanofibrous PCL/gelatin and PCL/collagen scaffolds promoted C17.2 (neonatal mouse cerebellum stem cell line) [42] and U373 (Human gliobalstoma-astrocytoma, epithelial-like cell ine) [43] proliferation and differentiation, respectively. U373 cells and human neural progenitor-astrocyte committed cells (hNPAcs) had similar astrocyte processes alignment and extension on PCL and PCL/collagen aligned nanofibers. The U373 cells demonstrated higher proliferation than the hNPAcs on both scaffolds. hNPAc adhesion and migration, however, was significantly improved on the collagen blend as compared to the PCL alone [43]. Human neuroblastoma cell line (SH-SY5Y)'s processes demonstrated poor adhesion to both PCL and PCL/collagen fibers but had long axonal growth up to 600 µm [43]. Schwann cell migration and process formation was also enhanced on collagen/PCL aligned scaffolds [44]. Incorporating ECM components such as collagen with electrospun PCL scaffolds improved process length of fibroblast and olfactory ensheathing cells [44]. Neurite outgrowth for DRGs, however, was favored on pure PCL scaffolds over collagen/PCL blends. Electrospun matrigel nanofibers improved neurite growth and branching of chick DRGs [45]. The matrigel nanofibers were designed to serve as a temporary layer for Schwann cell localization.
Growth factors incorporated into electrospun scaffolds in order to regulate proliferation and differentiation has also been of interest. However, the studies to date have been limited for neural applications. In order to prevent the degradation of the bioactive agents due to the harsh solvents and high voltages used in the electrospinning process, recent studies have examined immobilizing the growth factors on the surface of the fibers after processing. In a recent study, the number of cortical neural stem cells increased on PCL scaffolds with immobilized brain-derived neural factor (BDNF) as compared to PCL scaffolds combined with soluble BDNF [46]. Cells on the BDNF immobilized scaffolds also differentiated towards neuronal and oligodendrocyte phenotypes [46]. In another study, nerve growth factor (NGF) was immobilized onto amine-functionalized block copolymers [47]. An increase in expression of neuronal markers was detected for mesenchymal stem cells seeded onto NGF conjugated meshes. Alignment of the fibers also increased the expression of neuronal markers. Therefore, bioactive modified electrospun mats may be a promising approach for directing neural differentiation.
5. Electrically-Active Electrospun Mats
The use of electric fields in order to stimulate neurite growth and neuronal function has been explored for several decades. Electrospun fibers that incorporate electrical activity may provide both contact guidance due to the physical morphology of the fiber as well as electrical stimulation to enhance cell function. It has been demonstrated that electrical stimulation through a polypyrrole (PPy) film improved PC12 neurite extension significantly [48]. Electrospun aligned and random PLGA meshes were coated with PPy to provide electric stimulation to promote and guide neurite extension. Electrically stimulated PC12 cells had significantly longer neurites than cells on un-stimulated scaffolds [49]. Similarly, stimulated PC12 cells also had longer neurites on the stimulated aligned vs. random PPy-PLGA scaffolds. However, no differences in neurite elongation and the number of neurite bearing cells were detected for embryonic hippocampal neurons on electrically stimulated versus unstimulated scaffolds. In addition, fiber alignment did not increase axon establishment or elongation for the hippocampal neurons. Electrospun PCL and PLLA nanofibers were coated with PPy by in-situ polymerization to create a core-sheath fiber morphology [50]. Electrically stimulated random and aligned scaffolds enhanced the neurite length of DRGs by 83% and 47%, respectively, relative to unstimulated controls. Another electrically conductive polymer, polyaniline (PANI), has gained recent interest in tissue engineering applications [51-53]. Electrospinning a polymer blend of PANI with PCL/gelatin enhanced nerve stem cell proliferation and neurite outgrowth, when electrically stimulated [54]. Overall, the studies suggest that electrical stimuli in electrospun fibers may enhance neurite extension.
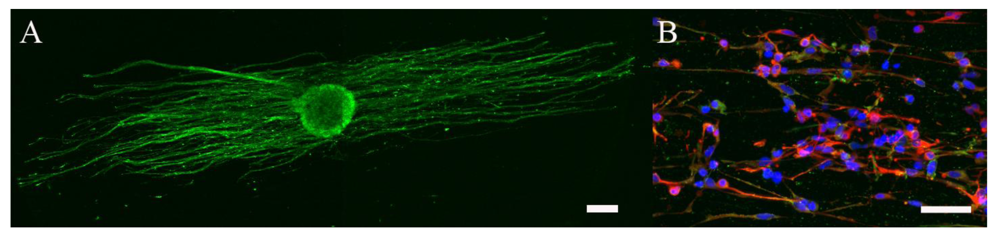
Piezoelectric polymers are also being sought for electrospun mats because they can induce a transient change in surface charge without requiring additional energy sources or electrodes and have been shown to yield a higher level of neuronal differentiation and neurite outgrowth of mouse neuroblastoma cells [55]. The piezoelectric phenomenon is closely related to the change of dipole crystal orientation when a force is applied. The dipole structures in a piezoelectric material are organized and no net charge is produced at rest. When a mechanical force is applied, the shifting or rotation of the dipole crystal results in the change of polarization density, hence, causing the transient change of electric charge. Upon the removal of mechanical force, the dipole crystal returns to its original space. Polyvinylidene fluoride (PVDF) and polyvinylidene fluoride- trifluoroethylene (PVDF-TrFE) are semi-crystalline polymers where the former becomes piezoelectric if it is in an all-trans oriented dipole configuration [56]. Electrospinning PVDF solutions induces the piezoelectric properties [57]. The piezoelectric properties of PVDF-TrFE are not altered by the electrospinning process [58]. DRGs and human NPSCs have been shown to extend neurites along aligned PVDF-TrFE scaffolds (Figure 6). The piezoelectric property may be induced in these fibers in vitro and in vivo via minute deformations of the fibers due to cell attachment and migration, which has been shown in other non-neural cell types on collagen fiber matrices [59]. Piezoelectric activity in these fibers may also be activated in vivo due to bulk deformations from the cerebrospinal fluid circulation or in the PNS, in response to changes in neighboring anatomic structures.
6. Conclusions and Future Directions
Electrospun fibrous meshes hold considerable promise for use in tissue engineering strategies for neural tissue repair. Studies have demonstrated that the composition and architecture of the electrospun mats can affect neural cell function, but this effect can vary depending upon the neural cell type used. For clinical application, recent studies have shown promise in configuring electrospun mats into three-dimensional structures to promote tissue formation for full restoration of function. Emphasis, however, on scaffold design given the greater level of complexity in forming functional three-dimensional neural tissues will be needed in the future. The major limitation with conventional electrospinning is the creation of membrane or sheet-like matrices due to the dense packing of fibers which limits cellular infiltration. Alternative methods, such as the use of sacrificial agents during the spinning process [60], the use of a combination of micro and nanoscaled fiber morphologies to increase porosity and pore size [61], and three-dimensional collectors instead of the traditional flat-plate [62], are being explored.
For brain and spinal cord repair, the challenge is not only in promoting neuronal survival, promoting regenerating axons across the injury site and/or restoring connections with the target of innervations, but also to control the inflammatory response which results in further damage. Electrospun scaffolds in the future may have several functions such as delivering neuroprotective drugs as well as bioactive agents/growth factors to promote the formation of neural tissue. In addition, multiple materials, growth factors, and drugs may need to be combined and incorporated into the electrospun mats to obtain the appropriate response. Similarly multiple cell types may need to be combined with the electrospun mats to obtain functional recovery. Nervous system injury remains a complex problem requiring innovative strategies for full functional repair.

Acknowledgments
The authors would like to thank financial support from the New Jersey Commission on Spinal Cord Research.
References
- Brandt, R. Cytoskeletal mechanisms of neuronal degeneration. Cell Tissue Res. 2001, 305, 255–265. [Google Scholar]
- Skovronsky, D.M.; Lee, V.M.; Trojanowski, J.Q. Neurodegenerative diseases: New concepts of pathogenesis and their therapeutic implications. Annu. Rev. Pathol. 2006, 1, 151–170. [Google Scholar]
- Vickers, J.C.; King, A.E.; Woodhouse, A.; Kirkcaldie, M.T.; Staal, J.A.; McCormack, G.H.; Blizzard, C.A.; Musgrove, R.E.; Mitew, S.; Liu, Y.; Chuckowree, J.A.; Bibari, O.; Dickson, T.C. Axonopathy and cytoskeletal disruption in degenerative diseases of the central nervous system. Brain Res. Bull. 2009, 80, 217–223. [Google Scholar]
- Park, D.H.; Eve, D.J.; Borlongan, C.V.; Klasko, S.K.; Cruz, L.E.; Sanberg, P.R. From the basics to application of cell therapy, a steppingstone to the conquest of neurodegeneration: A meeting report. Med. Sci. Monit. 2009, 15, RA23–RA31. [Google Scholar]
- Fitch, M.T.; Doller, C.; Combs, C.K.; Landreth, G.E.; Silver, J. Cellular and molecular mechanisms of glial scarring and progressive cavitation: In vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J. Neurosci. 1999, 19, 8182–8198. [Google Scholar]
- Orive, G.; Anitua, E.; Pedraz, J.L.; Emerich, D.F. Biomaterials for promoting brain protection, repair and regeneration. Nat. Rev. Neurosci. 2009, 10, 682–692. [Google Scholar]
- Thuret, S.; Moon, L.D.F.; Gage, F.H. Therapeutic interventions after spinal cord injury. Nat. Rev. Neurosci. 2006, 7, 628–643. [Google Scholar]
- Jacobson, S.; Guth, L. An electrophysiological study of the early stages of peripheral nerve regeneration. Experim. Neurol. 1965, 11, 48–60. [Google Scholar]
- Lundborg, G. Nerve Injury and Repair; Churchill Livingstone: Oxford, UK, 1988. [Google Scholar]
- Mackinnon, S.E.; Dellon, A.L. Surgery of the Peripheral Nerve; Thieme Med. Pub.: New York, NY, USA, 1988. [Google Scholar]
- Li, W.J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar]
- Ma, Z.; Kotaki, M.; Inai, R.; Ramakrishna, S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005, 11, 101–109. [Google Scholar]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. Engl. 2007, 46, 5670–5703. [Google Scholar]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar]
- Wen, X.; Tresco, P.A. Effect of filament diameter and extracellular matrix molecule precoating on neurite outgrowth and Schwann cell behavior on multifilament entubulation bridging device in vitro. J. Biomed. Mater. Res. A 2006, 76, 626–637. [Google Scholar]
- Wang, H.B.; Mullins, M.E.; Cregg, J.M.; Hurtado, A.; Oudega, M.; Trombley, M.T.; Gilbert, R.J. Creation of highly aligned electrospun poly-L-lactic acid fibers for nerve regeneration applications. J. Neural. Eng. 2009, 6, 016001. [Google Scholar]
- Lee, Y.-S.; Arinzeh, T.L. Electrospun Nanofibers for neural applications. In Nanotechnology in Tissue Engineering and Regenerative Medicine; Popat, K.C., Ed.; CRC Press/Taylor and Francis: New York, NY, USA, 2010. [Google Scholar]
- Yang, F. Eletcrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar]
- Xie, J.; Willerth, S.M.; Li, X.; Macewan, M.R.; Rader, A.; Sakiyama-Elbert, S.E.; Xia, Y. The differentiation of embryonic stem cells seeded on electrospun nanofibers into neural lineages. Biomaterials 2009, 30, 354–362. [Google Scholar]
- Corey, J.M.; Gertz, C.C.; Wang, B.S.; Birrell, L.K.; Johnson, S.L.; Martin, D.C.; Feldman, E.L. The design of electrospun PLLA nanofiber scaffolds compatible with serum-free growth of primary motor and sensory neurons. Acta Biomater. 2008, 4, 863–875. [Google Scholar]
- Corey, J.M.; Lin, D.Y.; Mycek, K.B.; Chen, Q.; Samuel, S.; Feldman, E.L.; Martin, D.C. Aligned electrospun nanofibers specify the direction of dorsal root ganglia neurite growth. J. Biomed. Mater. Res. A 2007, 83, 636–645. [Google Scholar]
- Gertz, C.C.; Leach, M.K.; Birrell, L.K.; Martin, D.C.; Feldman, E.L.; Corey, J.M. Accelerated neuritogenesis and maturation of primary spinal motor neurons in response to nanofibers. Dev. Neurobiol. 2010, 70, 589–603. [Google Scholar]
- Chew, S.Y.; Mi, R.; Hoke, A.; Leong, K.W. The effect of the alignment of electrospun fibrous scaffolds on Schwann cell maturation. Biomaterials 2008, 29, 653–661. [Google Scholar]
- Valmikinathan, C.M.; Hoffman, J.; Yu, X. Impact of scaffold micro and macro architecture on Schwann cell proliferation under dynamic conditions in a rotating wall vessel bioreactor. Mater. Sci. Eng. C 2010, 31, 22–29. [Google Scholar]
- Kim, I.A.; Park, S.A.; Kim, Y.J.; Kim, S.H.; Shin, H.J.; Lee, Y.J.; Kang, S.G.; Shin, J.W. Effects of mechanical stimuli and microfiber-based substrate on neurite outgrowth and guidance. J. Biosci. Bioeng. 2006, 101, 120–126. [Google Scholar]
- Yang, F.; Murugan, R.; Wang, S.; Ramakrishna, S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar]
- Christopherson, G.T.; Song, H.; Mao, H.Q. The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials 2009, 30, 556–564. [Google Scholar]
- Yao, L.; O'Brien, N.; Windebank, A.; Pandit, A. Orienting neurite growth in electrospun fibrous neural conduits. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 483–491. [Google Scholar]
- Lee, J.Y.; Bashur, C.A.; Gomez, N.; Goldstein, A.S.; Schmidt, C.E. Enhanced polarization of embryonic hippocampal neurons on micron scale electrospun fibers. J. Biomed. Mater. Res. A 2010, 92, 1398–1406. [Google Scholar]
- Xie, J.; MacEwan, M.R.; Li, X.; Sakiyama-Elbert, S.E.; Xia, Y. Neurite outgrowth on nanofiber scaffolds with different orders, structures, and surface properties. ACS Nano 2009, 3, 1151–1159. [Google Scholar]
- Hsieh, A.; Zahir, T.; Lapitsky, Y.; Amsden, B.; Wan, W.; Shoichet, M.S. Hydrogel/electrospun fiber composites influence neural stem/progenitor cell fate. Soft Matter 2010, 6, 2227–2237. [Google Scholar]
- Ahmed, I.; Liu, H.Y.; Mamiya, P.C.; Ponery, A.S.; Babu, A.N.; Weik, T.; Schindler, M.; Meiners, S. Three-dimensional nanofibrillar surfaces covalently modified with tenascin-C-derived peptides enhance neuronal growth in vitro. J. Biomed. Mater. Res. A 2006, 76, 851–860. [Google Scholar]
- Chew, S.Y.; Mi, R.; Hoke, A.; Leong, K.W. Aligned protein-polymer composite fibers enhance nerve regeneration: A potential tissue-engineering platform. Adv. Funct. Mater. 2007, 17, 1288–1296. [Google Scholar]
- Zhu, Y.; Wang, A.; Shen, W.; Patel, S.; Zhang, R.; Young, W.L.; Li, S. Nanofibrous patches for spinal cord regeneration. Adv. Funct. Mater. 2010, 20, 1433–1440. [Google Scholar]
- Kim, Y.T.; Haftel, V.K.; Kumar, S.; Bellamkonda, R.V. The role of aligned polymer fiber-based constructs in the bridging of long peripheral nerve gaps. Biomaterials 2008, 29, 3117–3127. [Google Scholar]
- Nisbet, D.R.; Rodda, A.E.; Horne, M.K.; Forsythe, J.S.; Finkelstein, D.I. Neurite infiltration and cellular response to electrospun polycaprolactone scaffolds implanted into the brain. Biomaterials 2009, 30, 4573–4580. [Google Scholar]
- Nisbet, D.R.; Pattanawong, S.; Ritchie, N.E.; Shen, W.; Finkelstein, D.I.; Horne, M.K.; Forsythe, J.S. Interaction of embryonic cortical neurons on nanofibrous scaffolds for neural tissue engineering. J. Neural Eng. 2007, 4, 35–41. [Google Scholar]
- Li, W.; Guo, Y.; Wang, H.; Shi, D.; Liang, C.; Ye, Z.; Qing, F.; Gong, J. Electrospun nanofibers immobilized with collagen for neural stem cells culture. J. Mater. Sci. Mater. Med. 2008, 19, 847–854. [Google Scholar]
- Nisbet, D.R.; Yu, L.M.Y.; Zahir, T.; Forsythe, J.S.; Shoichet, M.S. Characterization of neural stem cells on electrospun poly(Îμ- caprolactone) submicron scaffolds: Evaluating their potential in neural tissue engineering. J. Biomater. Sci. Polym. Ed. 2008, 19, 623–634. [Google Scholar]
- Powell, S.K.; Kleinman, H.K. Neuronal laminins and their cellular receptors. Int. J. Biochem. Cell Biol. 1997, 29, 401–414. [Google Scholar]
- Koh, H.S.; Yong, T.; Chan, C.K.; Ramakrishna, S. Enhancement of neurite outgrowth using nano-structured scaffolds coupled with laminin. Biomaterials 2008, 29, 3574–3582. [Google Scholar]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrospun poly(epsilon-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar]
- Gerardo-Nava, J.; Fuhrmann, T.; Klinkhammer, K.; Seiler, N.; Mey, J.; Klee, D.; Moller, M.; Dalton, P.D.; Brook, G.A. Human neural cell interactions with orientated electrospun nanofibers in vitro. Nanomedicine (London, England) 2009, 4, 11–30. [Google Scholar]
- Schnell, E.; Klinkhammer, K.; Balzer, S.; Brook, G.; Klee, D.; Dalton, P.; Mey, J. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials 2007, 28, 3012–3025. [Google Scholar]
- de Guzman, R.C.; Loeb, J.A.; VandeVord, P.J. Electrospinning of matrigel to deposit a basal lamina-like nanofiber surface. J. Biomater. Sci. Polym. Ed. 2010, 21, 1081–1101. [Google Scholar]
- Horne, M.K.; Nisbet, D.R.; Forsythe, J.S.; Parish, C.L. Three-dimensional nanofibrous scaffolds incorporating immobilized BDNF promote proliferation and differentiation of cortical neural stem cells. Stem Cells Dev. 2010, 19, 843–852. [Google Scholar]
- Cho, Y.I.; Choi, J.S.; Jeong, S.Y.; Yoo, H.S. Nerve growth factor (NGF)-conjugated electrospun nanostructures with topographical cues for neuronal differentiation of mesenchymal stem cells. Acta Biomater. 2010, 6, 4725–4733. [Google Scholar]
- Schmidt, C.E.; Shastri, V.R.; Vacanti, J.P.; Langer, R. Stimulation of neurite outgrowth using an electrically conducting polymer. Proc. Natl. Acad. Sci. USA 1997, 94, 8948–8953. [Google Scholar]
- Lee, J.Y.; Bashur, C.A.; Goldstein, A.S.; Schmidt, C.E. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials 2009, 30, 4325–4335. [Google Scholar]
- Xie, J.; MacEwcm, M.R.; Willerth, S.M.; Li, X.; Moran, D.W.; Sakiyama-Elbert, S.E.; Xia, Y. Conductive core-sheath nanofibers and their potential application in neural tissue engineering. Adv. Funct. Mater. 2009, 19, 2312–2318. [Google Scholar]
- Bidez, P.R.; Li, S.; Macdiarmid, A.G.; Venancio, E.C.; Wei, Y.; Lelkes, P.I. Polyaniline, an electroactive polymer, supports adhesion and proliferation of cardiac myoblasts. J. Biomater. Sci. Polym. Ed. 2006, 17, 199–212. [Google Scholar]
- Guimard, N.K.; Gomez, N.; Schmidt, C.E. Conducting polymers in biomedical engineering. Progr. Polym. Sci. (Oxford) 2007, 32, 876–921. [Google Scholar]
- Li, M.; Guo, Y.; Wei, Y.; MacDiarmid, A.G.; Lelkes, P.I. Electrospinning polyaniline-contained gelatin nanofibers for tissue engineering applications. Biomaterials 2006, 27, 2705–2715. [Google Scholar]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrical Stimulation of nerve cells using conductive nanofibrous scaffolds for nerve tissue engineering. Tissue Eng. A 2009, 15, 3605–3619. [Google Scholar]
- Valentini, R. Electrically charged polymeric sybstrates enchance nerve-fiber outgrowth in vitro. Biomaterials 1992, 13, 183–190. [Google Scholar]
- Lovinger, A.J. Ferroelectric Polymers. Science 1983, 220, 1115–1121. [Google Scholar]
- Zhao, Z.; Li, J.; Yuan, X.; Li, X.; Zhang, Y.; Sheng, J. Preparation and properties of electrospun poly(vinylidene fluoride) membranes. J. Appl. Polym. Sci. 2005, 97, 466–474. [Google Scholar]
- Weber, N.; Lee, Y.S.; Shanmugasundaram, S.; Jaffe, M.; Arinzeh, T.L. Characterization and in vitro cytocompatibility of piezoelectric electrospun scaffolds. Acta Biomater. 2010, 6, 3550–3556. [Google Scholar]
- Friedrichs, J.; Taubenberger, A.; Franz, C.M.; Muller, D.J. Cellular Remodelling of Individual Collagen Fibrils Visualized by Time-lapse AFM. J. Mol. Biol. 2007, 372, 594–607. [Google Scholar]
- Baker, B.M.; Gee, A.O.; Metter, R.B.; Nathan, A.S.; Marklein, R.A.; Burdick, J.A.; Mauck, R.L. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials 2008, 29, 2348–2358. [Google Scholar]
- Patlolla, A.; Collins, G.; Arinzeh, T.L. Solvent-dependent properties of electrospun fibrous composites for bone tissue regeneration. Acta Biomater. 2010, 6, 90–101. [Google Scholar]
- Blakeney, B.A.; Tambralli, A.; Anderson, J.M.; Andukuri, A.; Lim, D.J.; Dean, D.R.; Jun, H.W. Cell infiltration and growth in a low density, uncompressed three-dimensional electrospun nanofibrous scaffold. Biomaterials 2011, 32, 1583–1590. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, Y.-S.; Livingston Arinzeh, T. Electrospun Nanofibrous Materials for Neural Tissue Engineering. Polymers 2011, 3, 413-426. https://doi.org/10.3390/polym3010413
Lee Y-S, Livingston Arinzeh T. Electrospun Nanofibrous Materials for Neural Tissue Engineering. Polymers. 2011; 3(1):413-426. https://doi.org/10.3390/polym3010413
Chicago/Turabian StyleLee, Yee-Shuan, and Treena Livingston Arinzeh. 2011. "Electrospun Nanofibrous Materials for Neural Tissue Engineering" Polymers 3, no. 1: 413-426. https://doi.org/10.3390/polym3010413



