Global and Local Aging in Differently Stabilized Polypropylenes Exposed to Hot Chlorinated Water with and without Superimposed Mechanical-Environmental Loads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Specimens
2.3. Monitoring of Global Aging with Micro-Sized Specimens
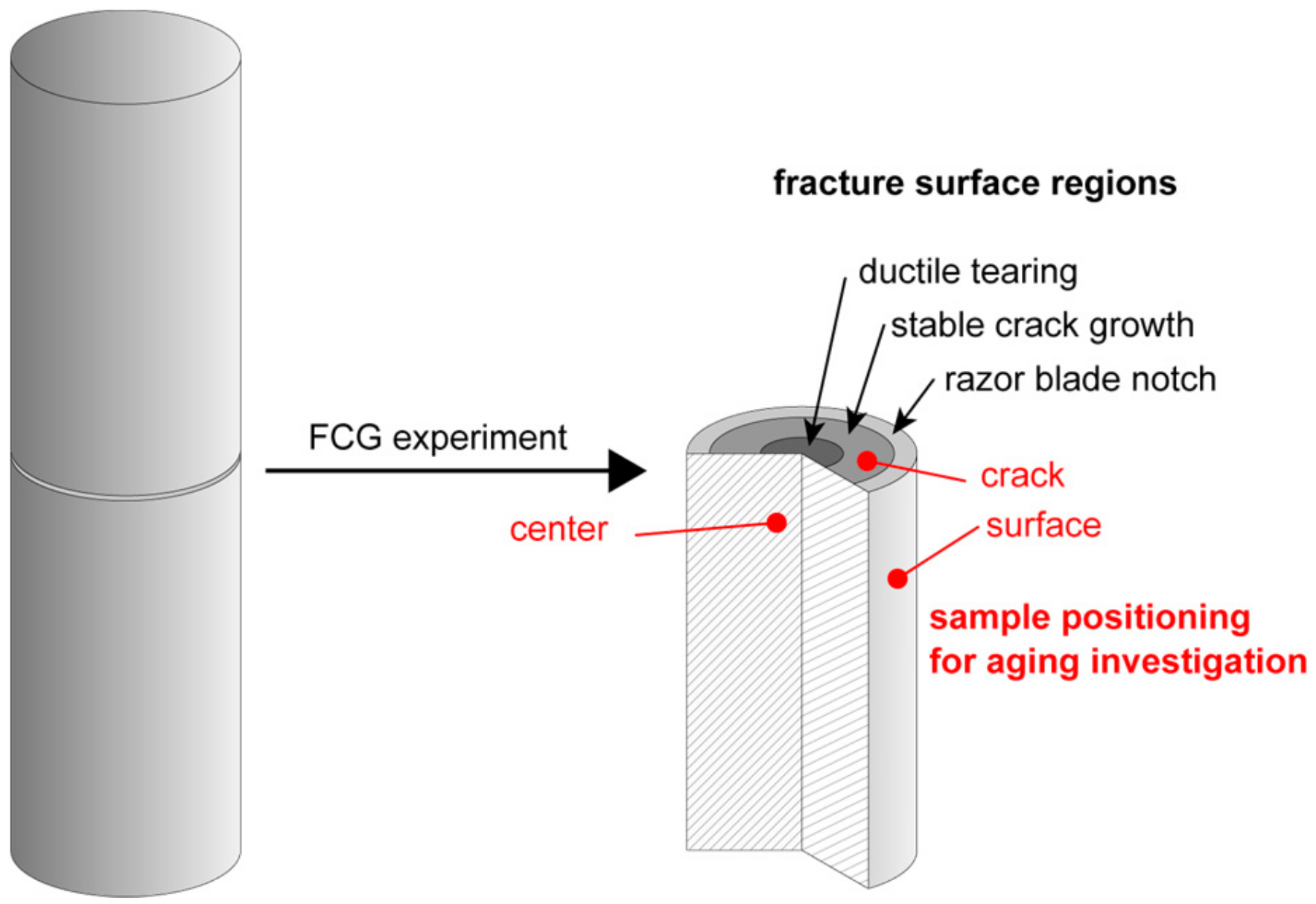
2.4. Fatigue Crack Growth Behavior and Monitoring of Local Aging under Superimposed Mechanical-Environmental Conditions Tested with Cracked Round Bar (CRB) Specimens
3. Results
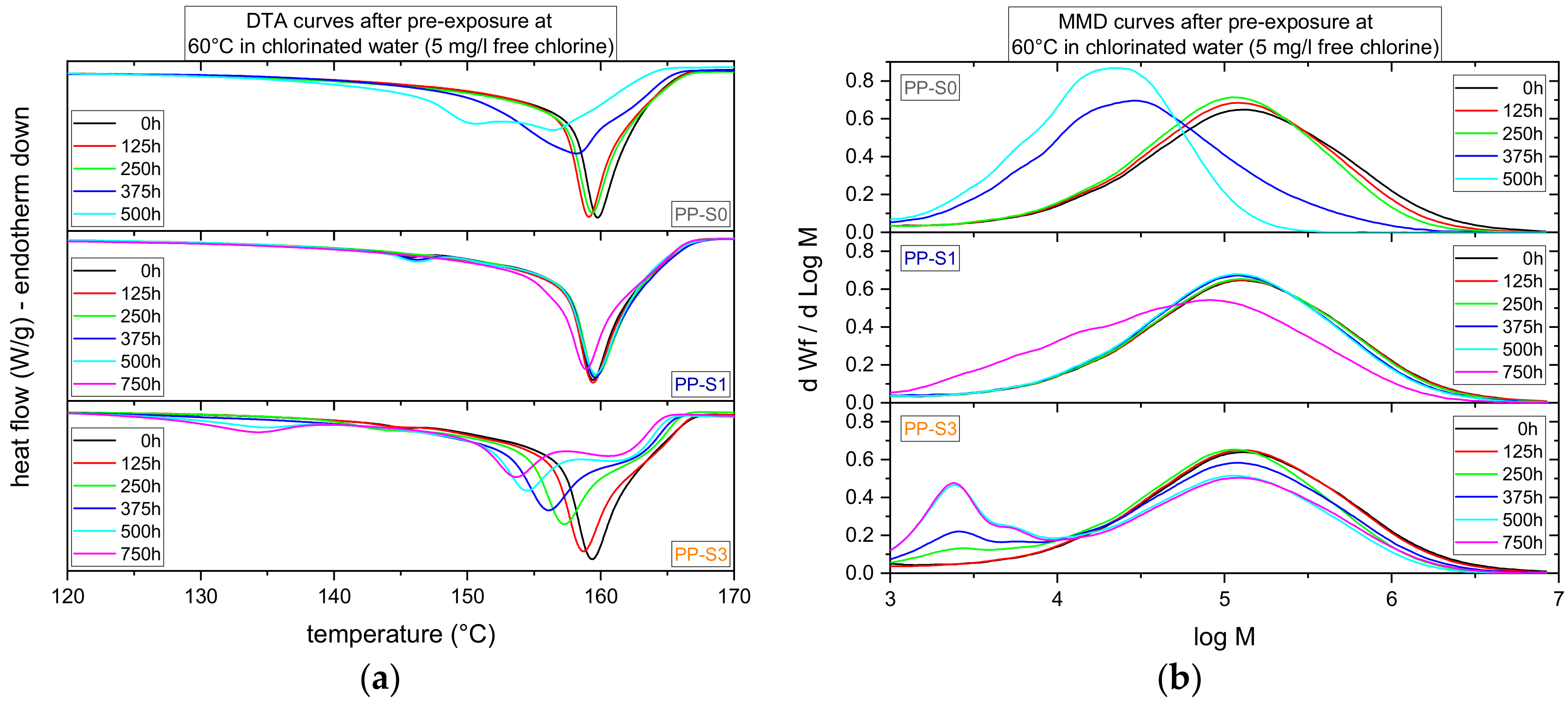
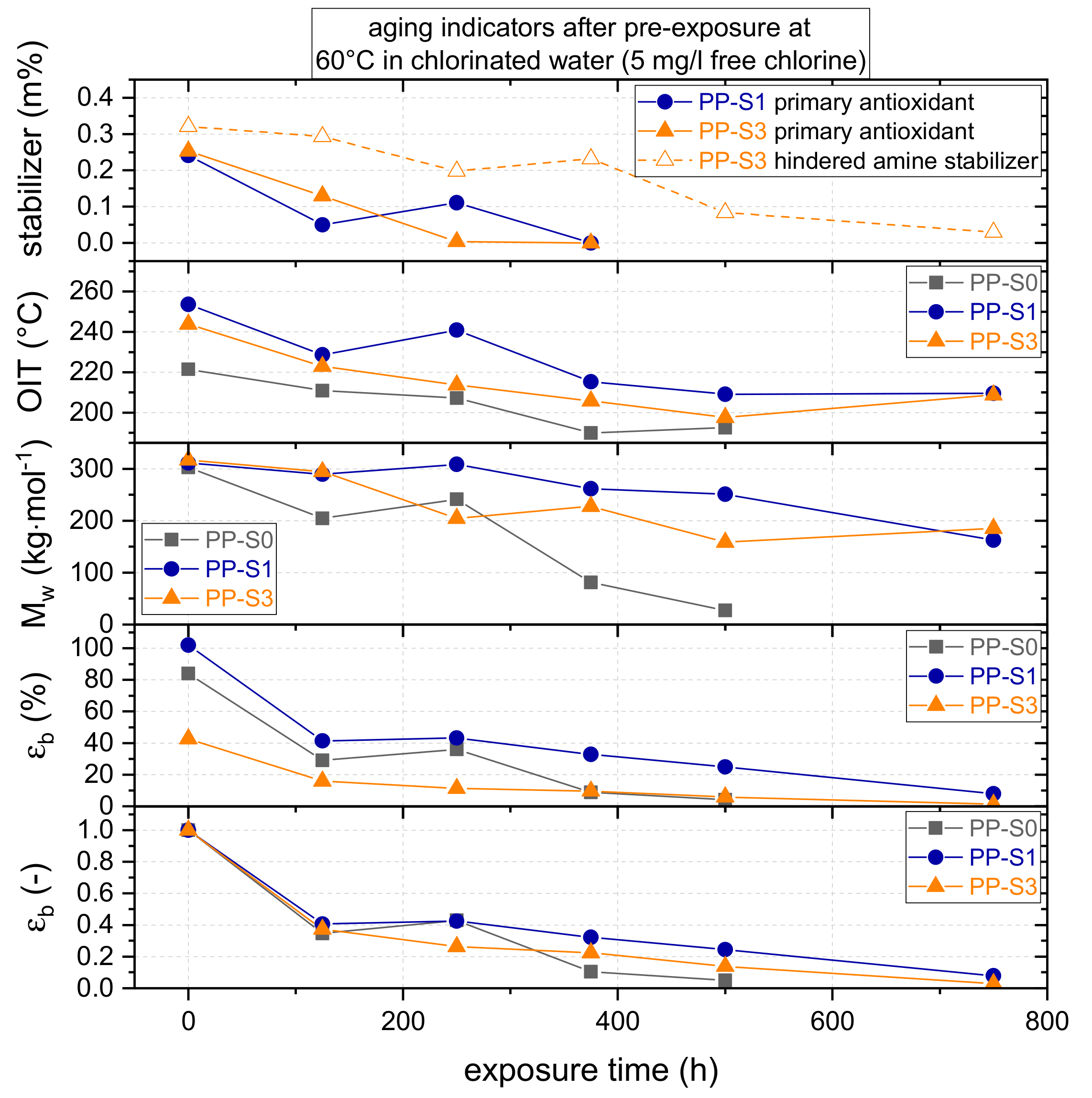
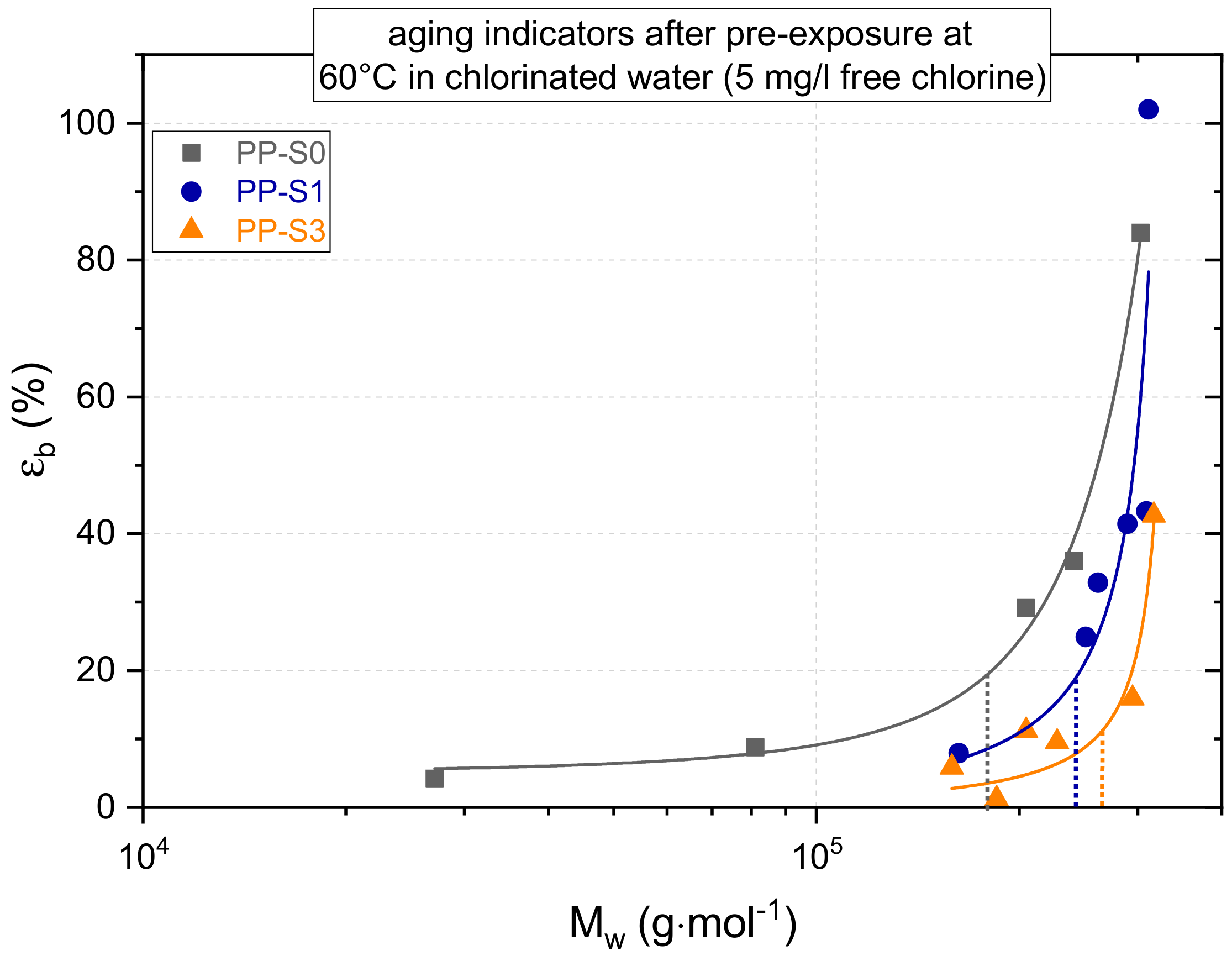
3.1. Effect of Chlorinated Water on Global Aging Behavior of Micro-Sized Specimens
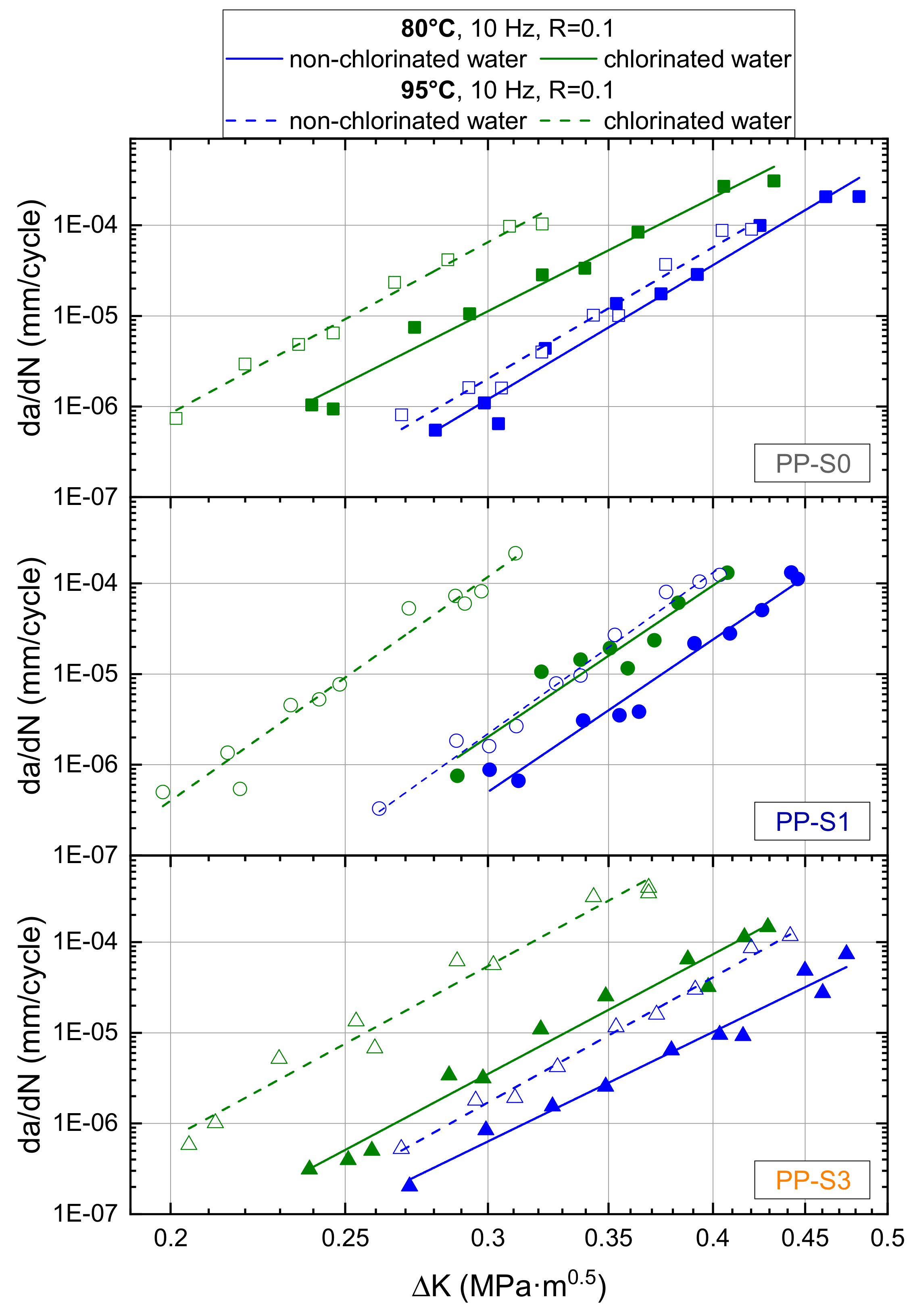
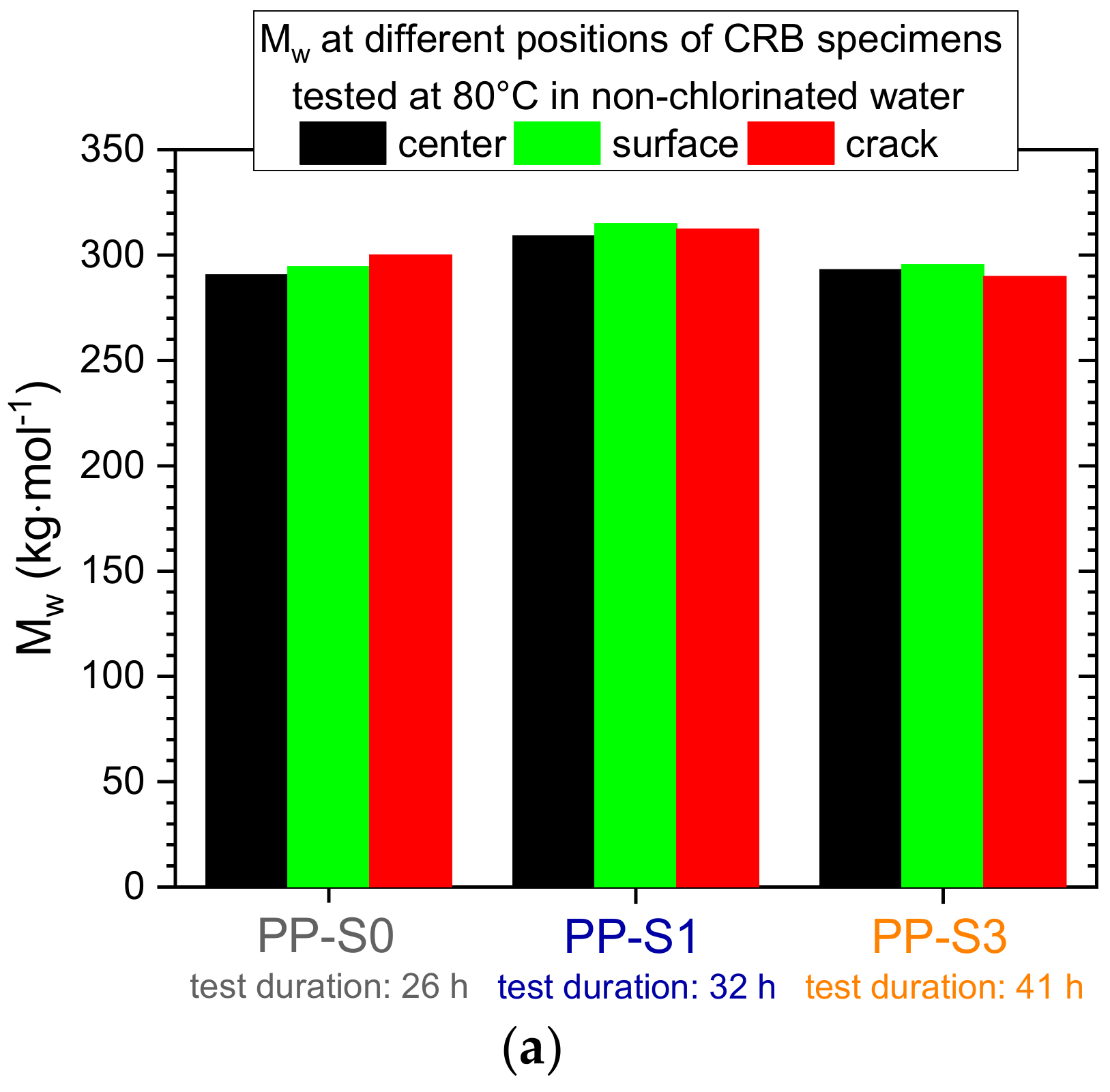
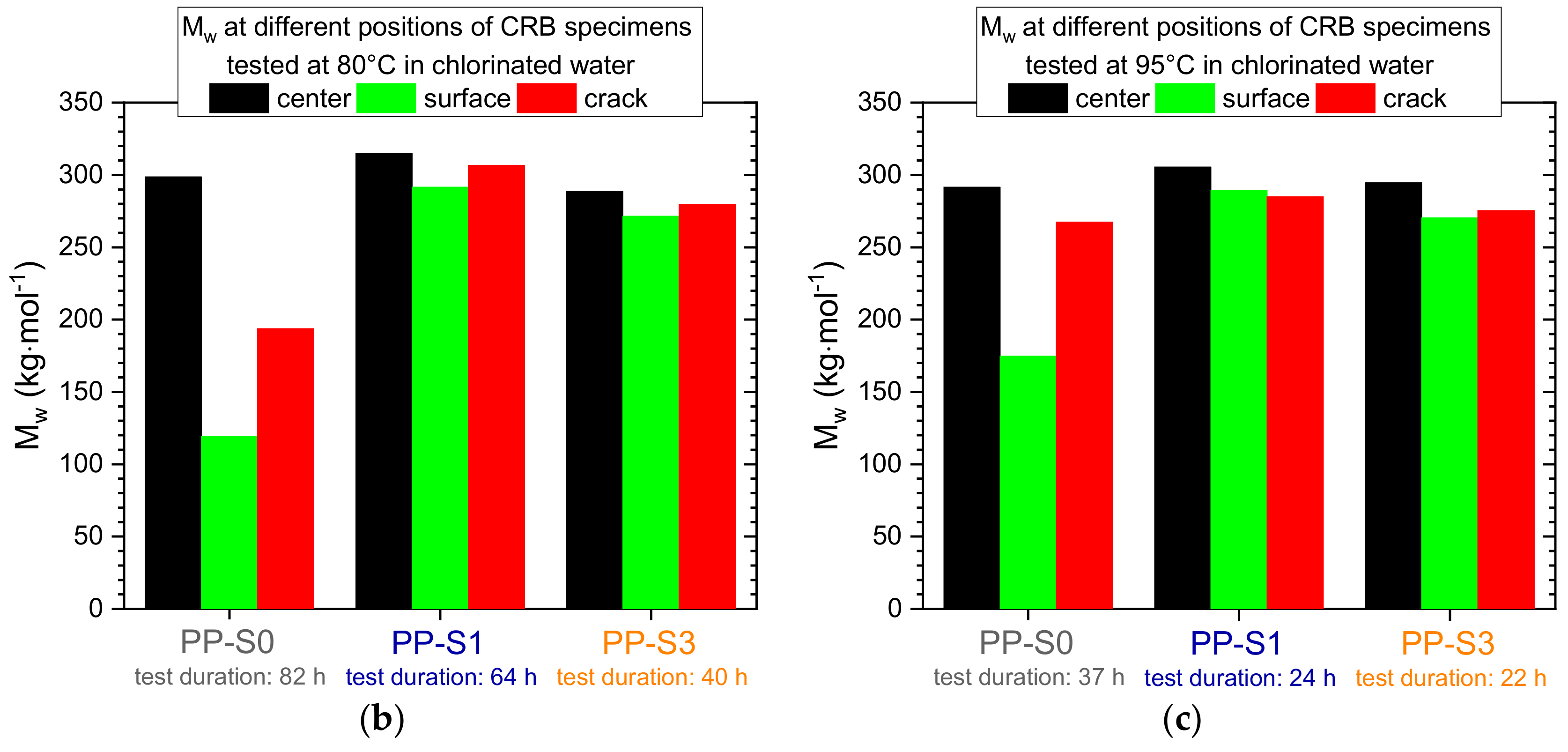
3.2. Effect of Chlorinated Water on the Fatigue Crack Growth Resistance and Local Aging Behavior in Cracked Round Bar (CRB) Specimens
4. Discussion
4.1. Effect of Chlorinated Water on Global Aging Behavior of Micro-Sized Specimens
4.2. Effect of Chlorinated Water on the Fatigue Crack Growth Resistance and Local Aging Behavior in CRB Specimens
5. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- ISO. ISO/TC 138/SC 2 Plastics pipes and fittings for water supplies. In ISO 15874-1:2013 Plastics Piping Systems for Hot and Cold Water Installations—Polypropylene (PP)—Part 1: General, 2nd ed.; 23.040.20; 91.140.60; ISO: Geneva, Switzerland, 2013. [Google Scholar]
- F17 Committee. Specification for Pressure-Rated Polypropylene (PP) Piping Systems; ASTM International: West Conshohocken, PA, USA, 2007. [Google Scholar]
- Gahleitner, M.; Paulik, C. Polypropylene and Other Polyolefins. In Brydson’s Plastics Materials; Elsevier: Amsterdam, The Netherlands, 2017; pp. 279–309. ISBN 9780323358248. [Google Scholar]
- Grabmann, M.K.; Wallner, G.M.; Grabmayer, K.; Nitsche, D.; Lang, R.W. Aging behavior and lifetime assessment of polyolefin liner materials for seasonal heat storage using micro-specimen. Sol. Energy 2018, 170, 988–990. [Google Scholar] [CrossRef]
- Grabmann, M.K.; Wallner, G.M.; Ramschak, T.; Buchinger, R.; Lang, R.W. Global Aging and Lifetime Prediction of Polymeric Materials for Solar Thermal Systems-Part 1: Polypropylene Absorbers for Pumped Systems. In EuroSun2016, Proceedings of the EuroSun2016, Palma de Mallorca, Spain, 11–14 October 2016; Martínez, V., González, J., Eds.; International Solar Energy Society: Freiburg, Germany, 2016; pp. 1–6. ISBN 978-3-9814659-6-9. [Google Scholar]
- Grabmann, M.K.; Wallner, G.M.; Ramschak, T.; Ziegler, G.; Lang, R.W. Global Aging and Lifetime Prediction of Polymeric Materials for Solar Thermal Systems-Part 2: Polyamide 66 Glass Fiber Reinforced Absorbers for Integrated Storage Collectors. In EuroSun2016, Proceedings of the EuroSun2016, Palma de Mallorca, Spain, 11–14 October 2016; Martínez, V., González, J., Eds.; International Solar Energy Society: Freiburg, Germany, 2016; pp. 1–6. ISBN 978-3-9814659-6-9. [Google Scholar]
- Wallner, G.M.; Povacz, M.; Hausner, R.; Lang, R.W. Lifetime modeling of polypropylene absorber materials for overheating protected hot water collectors. Sol. Energy 2016, 125, 324–331. [Google Scholar] [CrossRef]
- Deborde, M.; Von Gunten, U.R.S. Reactions of chlorine with inorganic and organic compounds during water treatment-Kinetics and mechanisms: A critical review. Water Res. 2008, 42, 13–51. [Google Scholar] [CrossRef] [PubMed]
- World Chlorine Council. Drinking Water Chlorination: World Chlorine Council Position Paper 2008. Available online: www.worldchlorine.org (accessed on 13 March 2019).
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; ISBN 9241548150. [Google Scholar]
- Bartram, J.; Chartier, Y.; Lee, J.V.; Pond, K.; Surman-Lee, S. Legionella and the Prevention of Legionellosis; World Health Organization: Geneva, Switzerland, 2007; ISBN 92-4-156297-8. [Google Scholar]
- Fischer, J.; Freudenthaler, P.J.; Lang, R.W.; Buchberger, W.; Mantell, S.C. Chlorinated Water Induced Aging of Pipe Grade Polypropylene Random Copolymers. Polymers 2019, 11, 996. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Mantell, S.C.; Bradler, P.R.; Wallner, G.M.; Lang, R.W. Effect of aging in hot chlorinated water on the mechanical behavior of polypropylene grades differing in their stabilizer systems. Mater. Today Proc. 2019, 10, 385–392. [Google Scholar] [CrossRef]
- Fischer, J.; Bradler, P.R.; Lang, R.W. Fatigue crack growth testing in chlorinated water at elevated temperatures-test equipment. In Proceedings of the Plastic Pipes XIX, Las Vegas, NV, USA, 24–26 September 2018. [Google Scholar]
- Fischer, J.; Bradler, P.R.; Lang, R.W. Test equipment for fatigue crack growth testing of polymeric materials in chlorinated water at different temperatures. Eng. Fract. Mech. 2018, 203, 44–53. [Google Scholar] [CrossRef]
- Fischer, J.; Eckerstorfer, M.; Bradler, P.R.; Wallner, G.M.; Lang, R.W. Investigation of the effect of stabilizer system, medium and temperature on the fatigue crack growth resistance of polypropylene for a proper material selection. In Proceedings of the ANTEC 2018, Orlando, FL, USA, 9 May 2018. [Google Scholar]
- Fischer, J.; Freudenthaler, P.J.; Bradler, P.R.; Lang, R.W.; Mantell, S.C. Effect of beta-nucleation on aging and crack growth resistance of polypropylene exposed to chlorinated water. In Proceedings of the Plastic Pipes XIX, Las Vegas, NV, USA, 30 May 2018. [Google Scholar]
- Fischer, J.; Bradler, P.R.; Akhras, M.H.; Wallner, G.M.; Lang, R.W. Influence of Hot Chlorinated Water and Stabilizer Package on the Fatigue Crack Growth Resistance of Glass Fiber Reinforced Polyamide. Polymers 2018, 10, 829. [Google Scholar] [CrossRef]
- Fischer, J.; Bradler, P.R.; Lang, R.W.; Wallner, G.M. Fatigue crack growth resistance of polypropylene in chlorinated water at different temperatures. In Proceedings of the Plastic Pipes XVIII, Berlin, Germany, 14 September 2016. [Google Scholar]
- Fischer, J.; Mantell, S.C.; Bradler, P.R.; Wallner, G.M.; Lang, R.W. Effect of Aging in Hot Chlorinated Water on the Mechanical Behavior of Polypropylene for Solar-Thermal Applications. In SWC2017/SHC2017, Proceedings of the ISES Solar World Conference 2017 and the IEA SHC Solar Heating and Cooling Conference for Buildings and Industry 2017, Abu Dhabi, United Arab Emirates, 29 October–2 November 2017; Romero, M., Mugnier, D., Renné, D., Guthrie, K., Griffiths, S., Eds.; International Solar Energy Society: Freiburg, Germany, 2017; pp. 1–6. ISBN 978-3-9814659-7-6. [Google Scholar]
- Hassinen, J.; Lundbaeck, M.; Ifwarson, M.; Gedde, U. Deterioration of polyethylene pipes exposed to chlorinated water. Polym. Degrad. Stab. 2004, 84, 261–267. [Google Scholar] [CrossRef]
- Majewski, K.; Cosgriff, E.; Mantell, S.; Bhattacharya, M. Fracture Properties of HDPE Exposed to Chlorinated Water. In Proceedings of the ANTEC 2018, Orlando, FL, USA, 9 May 2018. [Google Scholar]
- F17 Committee. Test Method for Evaluating the Oxidative Resistance of Polyethylene (PE) Pipe to Chlorinated Water; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- F17 Committee. Test Method for Evaluating the Oxidative Resistance of Crosslinked Polyethylene (PEX) Tubing and Systems to Hot Chlorinated Water; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Yu, W.; Reitberger, T.; Hjertberg, T.; Oderkerk, J.; Costa, F.R.; Gedde, U.W. Antioxidant consumption in squalane and polyethylene exposed to chlorinated aqueous media. Polym. Degrad. Stab. 2012, 97, 2370–2377. [Google Scholar] [CrossRef]
- Castillo Montes, J.; Cadoux, D.; Creus, J.; Touzain, S.; Gaudichet-Maurin, E.; Correc, O. Ageing of polyethylene at raised temperature in contact with chlorinated sanitary hot water. Part I—Chemical aspects. Polym. Degrad. Stab. 2012, 97, 149–157. [Google Scholar] [CrossRef]
- Vibien, P.; Couch, J.; Oliphant, K.; Zhou, W.; Zhang, B.; Chudnovsky, A. Assessing material perfomance in chlorinated potable water applications. In Proceedings of the Plastic Pipes XI, Munich, Germany, 3–6 September 2001. [Google Scholar]
- Colin, X.; Audouin, L.; Verdu, J.; Rozental-Evesque, M.; Rabaud, B.; Martin, F.; Bourgine, F. Aging of polyethylene pipes transporting drinking water disinfected by chlorine dioxide. I. Chemical aspects. Polym. Eng. Sci. 2009, 49, 1429–1437. [Google Scholar] [CrossRef]
- Colin, X.; Audouin, L.; Verdu, J.; Rozental-Evesque, M.; Rabaud, B.; Martin, F.; Bourgine, F. Aging of polyethylene pipes transporting drinking water disinfected by chlorine dioxide. Part II-Lifetime prediction. Polym. Eng. Sci. 2009, 49, 1642–1652. [Google Scholar] [CrossRef]
- Damodaran, S.; Schuster, T.; Rode, K.; Sanoria, A.; Brüll, R.; Wenzel, M.; Bastian, M. Monitoring the effect of chlorine on the ageing of polypropylene pipes by infrared microscopy. Polym. Degrad. Stab. 2015, 111, 7–19. [Google Scholar] [CrossRef]
- Seidler, D. Aus Schaden klug werden. Kunststoffe 2012, 7, 70–71. [Google Scholar]
- Lang, R.W.; Stern, A.; Doerner, G. Applicability and limitations of current lifetime prediction models for thermoplastics pipes under internal pressure. Angew. Makromol. Chemie Appl. Macromol. Chem. Phys. 1997, 247, 131–145. [Google Scholar] [CrossRef]
- Lang, R.W.; Pinter, G.; Balika, W. Konzept zur Nachweisführung für Nutzungsdauer und Sicherheit von PE-Druckrohren bei beliebiger Einbausituation. 3R International 2005, 44, 32–41. [Google Scholar]
- Pinter, G.; Haager, M.; Wolf, C.; Lang, R.W. Thermo-Oxidative Degradation during Creep Crack Growth of PE-HD Grades as Assessed by FT-IR Spectroscopy. Macromol. Symp. 2004, 217, 307–316. [Google Scholar] [CrossRef]
- Pinter, G.; Duretek, I.; Aust, N.; Lang, R.W. Characterisation of the thermo-oxidative degradation of polyethylene pipes by chromatographical, rheological and thermo-analytical methods. Macromol. Symp. 2002, 181, 213–224. [Google Scholar] [CrossRef]
- Pinter, G.; Lang, R.W. Effect of stabilization on creep crack growth in high-density polyethylene. J. Appl. Polym. Sci. 2003, 90, 3191–3207. [Google Scholar] [CrossRef]
- Zweifel, H. Plastics Additives Handbook; Hanser Publishers: Munich, Germany, 1993; ISBN 9783446195790. [Google Scholar]
- Yu, W.; Reitberger, T.; Hjertberg, T.; Oderkerk, J.; Costa, F.R.; Englund, V.; Gedde, U.W. Chlorine dioxide resistance of different phenolic antioxidants in polyethylene. Polym. Degrad. Stab. 2015, 111, 1–6. [Google Scholar] [CrossRef]
- Grabmayer, K.; Beißmann, S.; Wallner, G.M.; Nitsche, D.; Schnetzinger, K.; Buchberger, W.; Schobermayr, H.; Lang, R.W. Characterization of the influence of specimen thickness on the aging behavior of a polypropylene based model compound. Polym. Degrad. Stab. 2015, 111, 185–193. [Google Scholar] [CrossRef]
- Grabmann, M.; Wallner, G.; Grabmayer, K.; Buchberger, W.; Nitsche, D. Effect of thickness and temperature on the global aging behavior of polypropylene random copolymers for seasonal thermal energy storages. Sol. Energy 2018, 172, 152–157. [Google Scholar] [CrossRef]
- Wallner, G.M.; Grabmann, M.K.; Klocker, C.; Buchberger, W.; Nitsche, D. Effect of carbon nanotubes on the global aging behavior of β-nucleated polypropylene random copolymers for absorbers of solar-thermal collectors. Sol. Energy 2018, 172, 141–145. [Google Scholar] [CrossRef]
- Grabmann, M.K.; Wallner, G.M.; Maringer, L.; Buchberger, W.; Nitsche, D. Hot air aging behavior of polypropylene random copolymers. J. Appl. Polym. Sci. 2019, 136, 47350. [Google Scholar] [CrossRef]
- Maringer, L.; Grabmann, M.; Muik, M.; Nitsche, D.; Romanin, C.; Wallner, G.; Buchberger, W. Investigations on the distribution of polymer additives in polypropylene using confocal fluorescence microscopy. Int. J. Polym. Anal. Charact. 2017, 22, 692–698. [Google Scholar] [CrossRef]
- Grabmayer, K. Polyolefin-Based Lining Materials for Hot Water Heat Storages Development of Accelerated Aging Characterization Methods and Screening of Novel Compounds. Ph.D. Dissertation, Johannes Kepler University, Linz, Austria, 2014. [Google Scholar]
- ISO. ISO/TC 138/SC 5 General properties of pipes, fittings and valves of plastic materials and their accessories—methods and basic specifications. In ISO 18489:2015 Polyethylene (PE) Materials for Piping Systems—Determination of Resistance to Slow Crack Growth under Cyclic Loading—Cracked Round Bar Test Method, 1st ed.; 23.040.20; 23.040.45; ISO: Geneva, Switzerland, 2015. [Google Scholar]
- Cosgriff, E.; Mantell, S. Method for degrading polyethylene sheet samples in an oxidative environment. In Proceedings of the ANTEC 2017, Anaheim, CA, USA, 1 January 2017; pp. 1228–1233. [Google Scholar]
- ISO. ISO/TC 61/SC 5 Physical-chemical properties. ISO 11357-3:2018 Plastics—Differential Scanning Calorimetry (DSC)—Part 3: Determination of Temperature and Enthalpy of Melting and Crystallization, 3rd ed.; 83.080.01; ISO: Geneva, Switzerland, 2018. [Google Scholar]
- Beißmann, S.; Reisinger, M.; Grabmayer, K.; Wallner, G.; Nitsche, D.; Buchberger, W. Analytical evaluation of the performance of stabilization systems for polyolefinic materials. Part I: Interactions between hindered amine light stabilizers and phenolic antioxidants. Polym. Degrad. Stab. 2014, 110, 498–508. [Google Scholar] [CrossRef]
- Ohkatsu, Y.; Fujiwara, T. Interaction between Nitroxide of Hindered Amine Light Stabilizers and Phenol. J. Jpn. Petrol. Inst. 2007, 50, 87–93. [Google Scholar] [CrossRef]
- Ohkatsu, Y. Search for Unified Action Mechanism of Hindered Amine Light Stabilizers. J. Jpn. Petrol. Inst. 2008, 51, 191–204. [Google Scholar] [CrossRef] [Green Version]
- Fayolle, B.; Audouin, L.; Verdu, J. A critical molar mass separating the ductile and brittle regimes as revealed by thermal oxidation in polypropylene. Polymer 2004, 45, 4323–4330. [Google Scholar] [CrossRef]
- Donald, A.M.; Kramer, E.J. The competition between shear deformation and crazing in glassy polymers. J. Mater. Sci. 1982, 17, 1871–1879. [Google Scholar] [CrossRef]
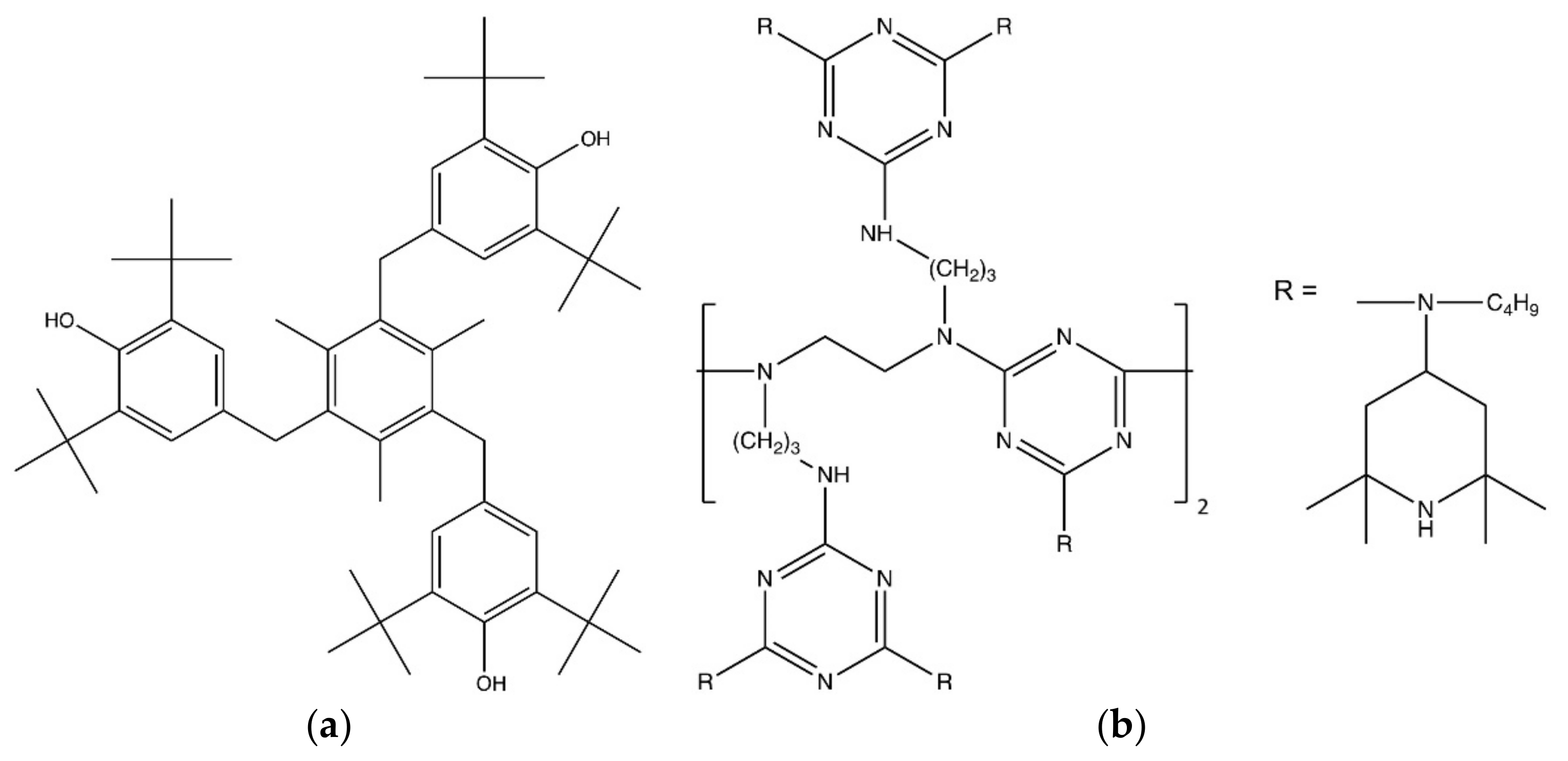
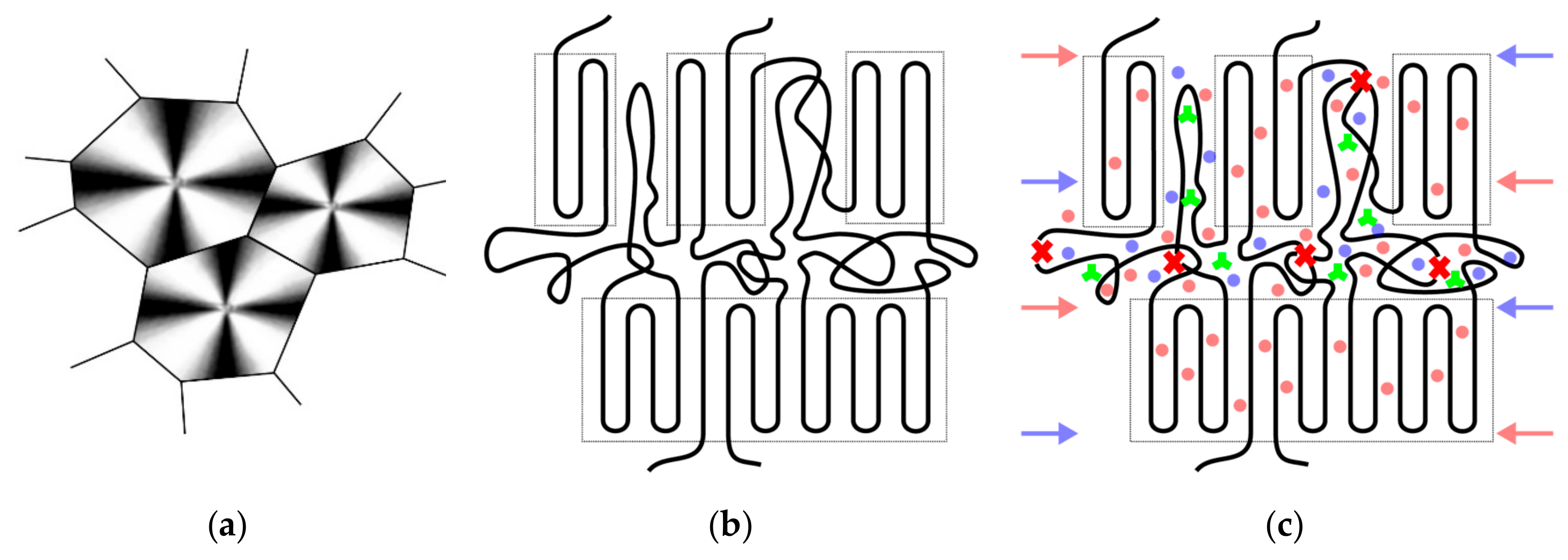
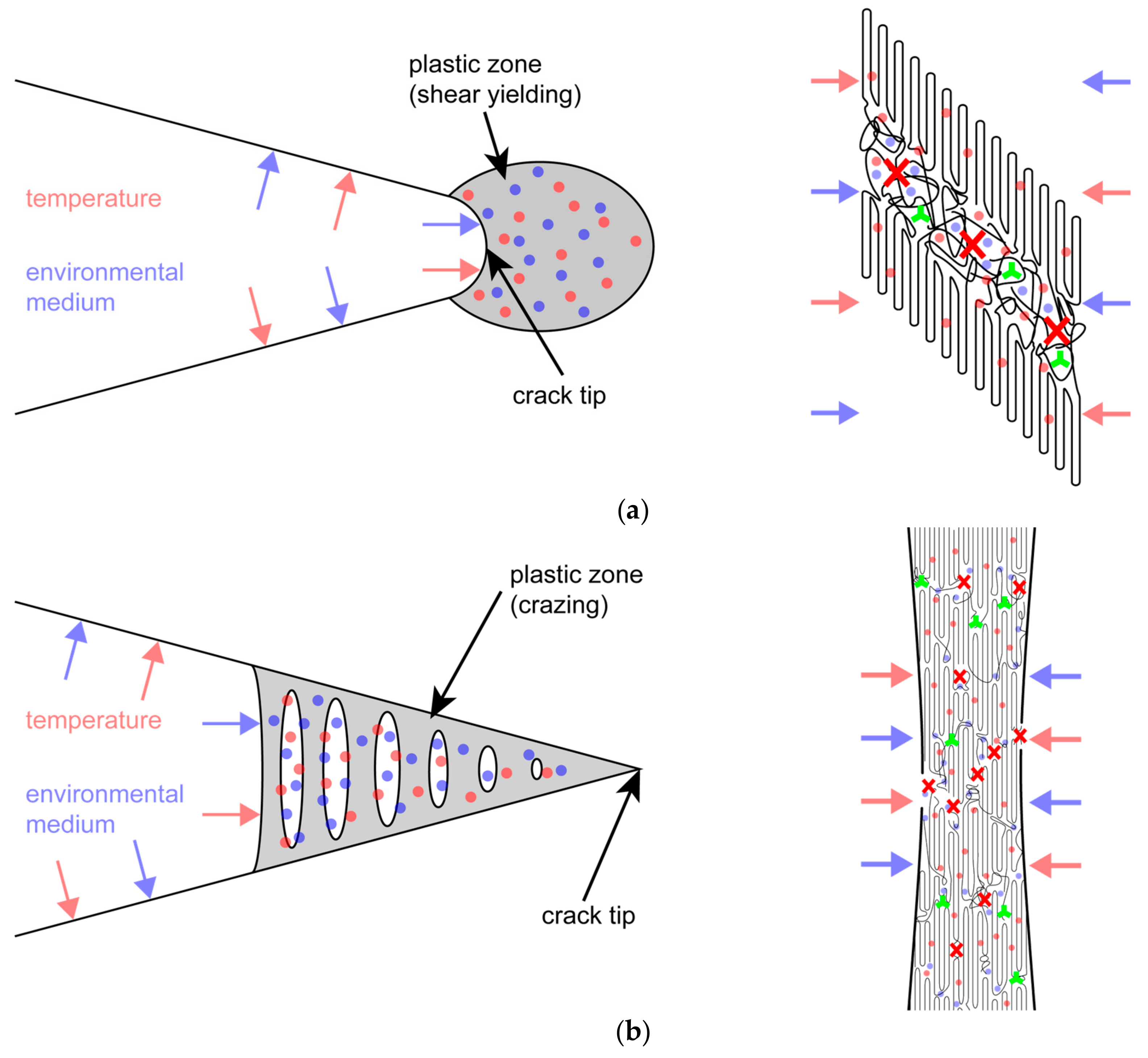
| Material Designation | Primary Phenolic Antioxidant | Hindered Amine Stabilizer |
|---|---|---|
| PP-S0 | - | - |
| PP-S1 | 0.3 m% | - |
| PP-S3 | 0.3 m% | 0.3 m% |
| Material | 80 °C | 95 °C | ||
|---|---|---|---|---|
| Non-Chlorinated Water | Chlorinated Water | Non-Chlorinated Water | Chlorinated Water | |
| PP-S0 | 1 | 9 | 1 | 32 |
| PP-S1 | 1 | 4 | 1 | 54 |
| PP-S3 | 1 | 6 | 1 | 32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, J.; Lang, R.W.; Bradler, P.R.; Freudenthaler, P.J.; Buchberger, W.; Mantell, S.C. Global and Local Aging in Differently Stabilized Polypropylenes Exposed to Hot Chlorinated Water with and without Superimposed Mechanical-Environmental Loads. Polymers 2019, 11, 1165. https://doi.org/10.3390/polym11071165
Fischer J, Lang RW, Bradler PR, Freudenthaler PJ, Buchberger W, Mantell SC. Global and Local Aging in Differently Stabilized Polypropylenes Exposed to Hot Chlorinated Water with and without Superimposed Mechanical-Environmental Loads. Polymers. 2019; 11(7):1165. https://doi.org/10.3390/polym11071165
Chicago/Turabian StyleFischer, Joerg, Reinhold W. Lang, Patrick R. Bradler, Paul J. Freudenthaler, Wolfgang Buchberger, and Susan C. Mantell. 2019. "Global and Local Aging in Differently Stabilized Polypropylenes Exposed to Hot Chlorinated Water with and without Superimposed Mechanical-Environmental Loads" Polymers 11, no. 7: 1165. https://doi.org/10.3390/polym11071165





