Synthesis and Characterization of a Lignin-Styrene-Butyl Acrylate Based Composite
Abstract
:1. Introduction
2. Experimental
2.1. Materials
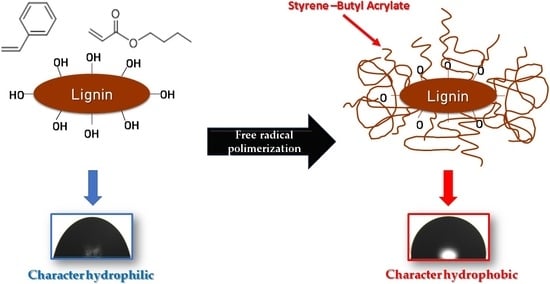
2.2. Composite Synthesis
2.3. Residual Styrene Analysis by HPLC
2.4. Gel Permeation Chromatography Analysis
2.5. Infrared Spectroscopy Analysis
2.6. Differential Scanning Calorimetry Analysis
2.7. X-ray Diffraction Analysis
2.8. Nuclear Magnetic Resonance Analysis
2.9. Morphological Analysis
2.10. Determination of Hardness Shore D
2.11. Contact Angle Determination
3. Results and Discussion
3.1. GPC Analysis
3.2. FTIR Analysis
3.3. DSC Analysis
3.4. X-ray Diffraction Anlysis
3.5. 1H-NMR Analysis
3.6. Morphological Analysis
3.7. Determination of Hardness
3.8. Contact Angle
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Le Digabel, F.; Avérous, L. Effects of lignin content on the properties of lignocellulose-based biocomposites. Carbohydr. Polym. 2006, 66, 537–545. [Google Scholar] [CrossRef]
- Nanou, P.; Huijgen, W.J.J.; Carbo, M.C.; Kiel, J.H.A. The role of lignin in the densification of torrefied wood in relation to the final product properties. Biomass Bioenergy 2018, 111, 248–262. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, L.; Zhang, T.; Qiu, F.; Yue, X.; Yang, D. Sustainable, Flexible, and Superhydrophobic Functionalized Cellulose Aerogel for Selective and Versatile Oil/Water Separation. ACS Sustain. Chem. Eng. 2019, 7, 9984–9994. [Google Scholar] [CrossRef]
- Wood, B.M.; Coles, S.R.; Maggs, S.; Meredith, J.; Kirwan, K. Use of lignin as a compatibiliser in hemp/epoxy composites. Compos. Sci. Technol. 2011, 71, 1804–1810. [Google Scholar] [CrossRef]
- Menon, M.P.; Selvakumar, R.; Suresh kumar, P.; Ramakrishna, S. Extraction and modification of cellulose nanofibers derived from biomass for environmental application. R. Soc. Chem. 2017, 7, 42750–42773. [Google Scholar] [Green Version]
- Feng, Y.; Lan, J.; Ma, P.; Dong, X.; Qu, J.; He, H. Chemical structure and thermal properties of lignin modified with polyethylene glycol during steam explosion. Wood Sci. Technol. 2016, 51, 135–150. [Google Scholar] [CrossRef]
- Luo, S.; Cao, J.; Mcdonald, A.G. Esterification of industrial lignin and its effect on the resulting poly(3-hydroxybutyrate-co-3-hydroxyvalerate ) or polypropylene blends. Ind. Crop. Prod. 2017, 97, 281–291. [Google Scholar] [CrossRef]
- Stewart, D. Lignin as a base material for materials applications: Chemistry, application and economics. Ind. Crops Prod. 2008, 27, 202–207. [Google Scholar] [CrossRef]
- Xie, S.; Li, Q.; Karki, P.; Zhou, F.; Yuan, J.S. Lignin as Renewable and Superior Asphalt Binder Modifier. ACS Sustain. Chem. Eng. 2017, 5, 2817–2823. [Google Scholar] [CrossRef]
- Iyer, K.A.; Torkelson, J.M. Sustainable Green Hybrids of Polyolefins and Lignin Yield Major Improvements in Mechanical Properties When Prepared via Solid-State Shear Pulverization. ACS Sustain. Chem. Eng. 2015, 3, 959–968. [Google Scholar] [CrossRef]
- Saito, T.; Brown, R.H.; Hunt, M.A.; Pickel, D.L.; Pickel, J.M.; Messman, J.M.; Baker, F.S.; Keller, M.; Naskar, A.K. Turning renewable resources into value-added polymer: Development of lignin-based thermoplastic. Green Chem. 2012, 14, 3295. [Google Scholar] [CrossRef]
- Şimşek, S.; Ulusoy, H.İ. Synthesis of a Useful and Economic Polymeric Material for Effective Removal of Bisphenol A. J. Polym. Environ. 2018, 26, 1605–1612. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Iqbal, H.M.N.; Hu, H.; Zhang, X. Biotransformation of lignocellulosic materials into value-added products—A review. Int. J. Biol. Macromol. 2017, 98, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, D.; Lu, S.; Qiu, X.; Qian, Y.; Li, P. Encapsulating TiO2 in Lignin-Based Colloidal Spheres for High Sunscreen Performance and Weak Photocatalytic Activity. ACS Sustain. Chem. Eng. 2019, 7, 6234–6242. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, J.; Erdocia, X.; Sánchez, C.; González Alriols, M.; Labidi, J. Lignin depolymerization for phenolic monomers production by sustainable processes. J. Energy Chem. 2017, 26, 622–631. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Hong, C.; Jeong, H.; Lee, S.; Weon, J.; Choi, I.; Kashif, M.; Sarkar, B.; Zeb, H.; Yi, M.; et al. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. J. Environ. Radioact. 2017, 121, 47–56. [Google Scholar]
- Chung, Y.L.; Olsson, J.V.; Li, R.J.; Frank, C.W.; Waymouth, R.M.; Billington, S.L.; Sattely, E.S. A renewable lignin-lactide copolymer and application in biobased composites. ACS Sustain. Chem. Eng. 2013, 1, 1231–1238. [Google Scholar] [CrossRef]
- Tran, C.D.; Chen, J.; Keum, J.K.; Naskar, A.K. A New Class of Renewable Thermoplastics with Extraordinary Performance from Nanostructured Lignin-Elastomers. Adv. Funct. Mater. 2016, 26, 2677–2685. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, L.; Tang, C. Sustainable Elastomers from Renewable Biomass. Acc. Chem. Res. 2017, 50, 1762–1773. [Google Scholar] [CrossRef]
- Jairam, S.; Tong, Z.; Wang, L.; Welt, B. Encapsulation of a biobased lignin-saponite nanohybrid into polystyrene co-butyl acrylate (PSBA) latex via miniemulsion polymerization. ACS Sustain. Chem. Eng. 2013, 1, 1630–1637. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, Y.; Yang, R.; Wang, C. Alkaline lignin extracted from furfural residues for pH-responsive Pickering emulsions and their recyclable polymerization. Green Chem. 2012, 14, 3230–3236. [Google Scholar] [CrossRef]
- Naseem, A.; Tabasum, S.; Zia, K.M.; Zuber, M.; Ali, M.; Noreen, A. Lignin-derivatives based polymers, blends and composites: A review. Int. J. Biol. Macromol. 2016, 93, 296–313. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Peng, C.; Wang, W.; Shi, K.; Liu, Z.; Ji, X. Preparation and absorption behavior to organic pollutants of macroporous hydrophobic polyvinyl alcohol–formaldehyde sponges. RSC Adv. 2014, 4, 35620–35628. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, Y.; Wang, J.; Zhang, X. Ultrasonic-assisted fabrication of montmorillonite-lignin hybrid hydrogel: Highly efficient swelling behaviors and super-sorbent for dye removal from wastewater. Colloids Surf. A Phys. Eng. Asp. 2017, 520, 903–913. [Google Scholar] [CrossRef]
- Feldman, D. Lignin nanocomposites. J. Macromol. Sci. Part A Pure Appl. Chem. 2016, 53, 382–387. [Google Scholar] [CrossRef]
- Espinoza Acosta, J.L.; Torres Chávez, P.I.; Olmedo Martínez, J.L.; Vega Rios, A.; Flores Gallardo, S.; Zaragoza Contreras, E.A. Lignin in storage and renewable energy applications: A review. J. Energy Chem. 2018, 27, 1422–1438. [Google Scholar] [CrossRef]
- Ye, D.; Jiang, L.; Ma, C.; Zhang, M.; Zhang, X. The graft polymers from different species of lignin and acrylic acid: Synthesis and mechanism study. Int. J. Biol. Macromol. 2014, 63, 43–48. [Google Scholar] [CrossRef]
- Campos, C.H.; Urbano, B.F.; Rivas, B.L. Synthesis and characterization of organic-inorganic hybrid composites from poly(acrylic acid)-[3-(trimethoxysilyl)propyl methacrylate]-Al2O3. Compos. Part B Eng. 2014, 57, 1–7. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Yu, J.; Li, S.; Wang, J.; Wang, C.; Chu, F. Integration of lignin and acrylic monomers towards grafted copolymers by free radical polymerization. Int. J. Biol. Macromol. 2014, 67, 483–489. [Google Scholar] [CrossRef]
- Ferdosian, F.; Yuan, Z.; Anderson, M.; Xu, C. (Charles) Synthesis and characterization of hydrolysis lignin-based epoxy resins. Ind. Crop. Prod. 2016, 91, 295–301. [Google Scholar] [CrossRef]
- Nair, S.S.; Kuo, P.; Chen, H.; Yan, N. Investigating the effect of lignin on the mechanical, thermal, and barrier properties of cellulose nanofibril reinforced epoxy composite. Ind. Crop. Prod. 2017, 100, 208–217. [Google Scholar] [CrossRef]
- Yeo, J.S.; Lee, J.H.; Hwang, S.H. Effects of lignin on the volume shrinkage and mechanical properties of a styrene/unsaturated polyester/lignin ternary composite system. Compos. Part B Eng. 2017, 130, 167–173. [Google Scholar] [CrossRef]
- Gregorova, A.; Kosikova, B.; Osvald, A. The study of lignin influence on properties of polypropylene composites. Wood Res. 2005, 50, 41–48. [Google Scholar]
- Kadla, J.F.; Kubo, S.; Venditti, R.A.; Gilbert, R.D.; Compere, A.L.; Griffith, W. Lignin-based carbon fibers for composite fiber applications. Carbon 2002, 40, 2913–2920. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, J.; Wu, T.; Wang, X.; Huang, G.; Liu, Y.; Qiu, H.; Li, Y.; Wang, W.; Gao, J. Cost-Effective Reduced Graphene Oxide-Coated Polyurethane Sponge As a Highly Efficient and Reusable Oil-Absorbent. ACS Appl. Mater. Interfaces 2013, 5, 10018–10026. [Google Scholar] [CrossRef] [PubMed]
- Chaochanchaikul, K.; Jayaraman, K.; Rosarpitak, V.; Sombatsompop, N. Influence of lignin content on photodegradation in wood/HDPE composites under UV weathering. BioResources 2012, 7, 38–55. [Google Scholar]
- Graichen, F.H.M.; Grigsby, W.J.; Hill, S.J.; Raymond, L.G.; Sanglard, M.; Smith, D.A.; Thorlby, G.J.; Torr, K.M.; Warnes, J.M. Yes, we can make money out of lignin and other bio-based resources. Ind. Crops Prod. 2017, 106, 74–85. [Google Scholar] [CrossRef]
- Nordström, Y.; Norberg, I.; Sjöholm, E.; Drougge, R. A new softening agent for melt spinning of softwood kraft lignin. J. Appl. Polym. Sci. 2013, 129, 1274–1279. [Google Scholar] [CrossRef]
- Marklund, E.; Eitzenberger, J.; Varna, J. Nonlinear viscoelastic viscoplastic material model including stiffness degradation for hemp/lignin composites. Compos. Sci. Technol. 2008, 68, 2156–2162. [Google Scholar] [CrossRef] [Green Version]
- Yeo, J.S.; Seong, D.W.; Hwang, S.H. Chemical surface modification of lignin particle and its application as filler in the polypropylene composites. J. Ind. Eng. Chem. 2015, 31, 80–85. [Google Scholar] [CrossRef]
- Sen, S.; Patil, S.; Argyropoulos, D.S. Thermal properties of lignin in copolymers, blends, and composites: A review. Green Chem. 2015, 17, 4862–4887. [Google Scholar] [CrossRef]
- Darestani, N.G.; Tikka, A.; Fatehi, P. Sulfonated lignin-g-styrene polymer: Production and characterization. Polymers 2018, 10, 928. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Cao, J.; Lu, Y. The electrospinning of the copolymer of styrene and butyl acrylate for its application as oil absorbent. Springerplus 2016, 5, 1383. [Google Scholar] [CrossRef] [PubMed]
- Konduri, M.K.; Kong, F.; Fatehi, P. Production of carboxymethylated lignin and its application as a dispersant. Eur. Polym. J. 2015, 70, 371–383. [Google Scholar] [CrossRef]
- Victor, P.A.; Gonçalves, S.B.; Machado, F. Styrene/Lignin-Based Polymeric Composites Obtained Through a Sequential Mass-Suspension Polymerization Process. J. Polym. Environ. 2017, 26, 1–20. [Google Scholar] [CrossRef]
- Han, Y.; Yuan, L.; Li, G.; Huang, L.; Qin, T.; Chu, F.; Tang, C. Renewable polymers from lignin via copper-free thermal click chemistry. Polymer 2016, 83, 92–100. [Google Scholar] [CrossRef]
- Nesvadba, P. Radical Polymerization in Industry. Encycl. Radic. Chem. Biol. Mater. 2012, 36. [Google Scholar] [CrossRef]
- Cheah, P.; Bhikha, C.N.; Haver, J.H.O.; Smith, A.E. Effect of Oxygen and Initiator Solubility on Admicellar Polymerization of Styrene on Silica Surfaces. Int. J. Polym. Sci. 2017, 2017, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Georges, M.K.; Veregin, R.P.N.; Kazmaier, P.M.; Hamer, G.K. Narrow Polydispersity Polystyrene by a Free-Radical Polymerization Process-Rate Enhancement. Macromolecules 1994, 27, 7228–7229. [Google Scholar] [CrossRef]
- Upton, B.M.; Kasko, A.M. Strategies for the conversion of lignin to high-value polymeric materials: Review and perspective. Chem. Rev. 2016, 116, 2275–2306. [Google Scholar] [CrossRef]
- Derkacheva, O.; Sukhov, D. Investigation of lignins by FTIR spectroscopy. Macromol. Symp. 2008, 265, 61–68. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, X.; Luo, X.; Zhang, C.; Zhu, H. A modified lignin adsorbent for the removal of 2,4,6-trinitrotoluene. Chem. Eng. J. 2011, 168, 1055–1063. [Google Scholar] [CrossRef]
- Jairam, S.; Bucklin, R.; Correll, M.; Sakthivel, T.S.; Seal, S.; Truett, J.; Tong, Z. UV resistance of polystyrene co-butyl acrylate (PSBA) encapsulated lignin-saponite nanohybrid composite film. Mater. Des. 2016, 90, 151–156. [Google Scholar] [CrossRef]
- Atz Dick, T.; Couve, J.; Gimello, O.; Mas, A.; Robin, J.J. Chemical modification and plasma-induced grafting of pyrolitic lignin. Evaluation of the reinforcing effect on lignin/poly(L-lactide) composites. Polymer 2017, 118, 280–296. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Yu, J.; Zhang, M.; Wang, C.; Xu, Y.; Chu, F. Preparation and characterization of lignin based macromonomer and its copolymers with butyl methacrylate. Int. J. Biol. Macromol. 2013, 60, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Misra, M.; Mohanty, A.K. Enhanced properties of lignin-based biodegradable polymer composites using injection moulding process. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1710–1718. [Google Scholar] [CrossRef]
- Gordobil, O.; Egüés, I.; Llano-Ponte, R.; Labidi, J. Physicochemical properties of PLA lignin blends. Polym. Degrad. Stab. 2014, 108, 330–338. [Google Scholar] [CrossRef]
- Ayoub, A.; Venditti, R.A.; Jameel, H.; Chang, H.M. Effect of irradiation on the composition and thermal properties of softwood kraft lignin and styrene grafted lignin. J. Appl. Polym. Sci. 2014, 131, 1–10. [Google Scholar] [CrossRef]
- Ning, L.Q.; Xu, N.K.; Wang, R.; Liu, Y. Fibrous membranes electrospun from the suspension polymerization product of styrene and butyl acrylate for oil-water separation. RSC. Adv. 2015, 5, 57101–57113. [Google Scholar] [CrossRef]
- David, A.; Meimoun, J.; Delaunay, T.; Wiatz, V.; Parcq, J.; Descamps, N. Structural characterization and mechanical properties of dextrin-graft-poly(butyl acrylate-co-styrene ) copolymers. Express Polym. Lett. 2019, 13, 235–247. [Google Scholar] [CrossRef]
- Sun, J.X.; Sun, X.F.; Sun, R.C.; Fowler, P.; Baird, M.S. Inhomogeneities in the Chemical Structure of Sugarcane Bagasse Lignin. J. Agric. Food Chem. 2003, 51, 6719–6725. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Huang, Z.; Zhang, Y.; Yang, M.; Chen, D.; Huang, K.; Hu, H.; Huang, A.; Qin, X.; Feng, Z. Efficient solid-phase synthesis of acetylated lignin and a comparison of the properties of different modified lignins. J. Appl. Polym. Sci. 2017, 134, 1–13. [Google Scholar] [CrossRef]
- Tobimatsu, Y.; Schuetz, M. Lignin polymerization: How do plants manage the chemistry so well? Curr. Opin. Biotechnol. 2019, 56, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Rallini, M.; Wang, D.Y.; Gao, D.; Dominici, F.; Torre, L.; Kenny, J.M.; Puglia, D. Role of lignin nanoparticles in UV resistance, thermal and mechanical performance of PMMA nanocomposites prepared by a combined free-radical graft polymerization/masterbatch procedure. Compos. Part A Appl. Sci. Manuf. 2018, 107, 61–69. [Google Scholar] [CrossRef]
- Marmur, A. Soft contact: Measurement and interpretation of contact angles. Soft Matter 2006, 2, 12–17. [Google Scholar] [CrossRef]
- Diraki, A.; Mackey, H.; Mckay, G.; Abdala, A.A. Chemical Engineering Research and Design Removal of oil from oil–water emulsions using thermally reduced graphene and graphene nanoplatelets. Chem. Eng. Res. Des. 2018, 137, 47–59. [Google Scholar] [CrossRef]
- Bogdanova, Y.G.; Kostina, J.V.; Dolzhikova, V.D.; Chernikova, E.V.; Plutalova, A.V. Surface properties of poly(styrene-co-n-butyl acrylate) binary copolymers: Effect of chain microstructure and composition. Russ. J. Phys. Chem. A 2015, 89, 2466–2472. [Google Scholar] [CrossRef]
- Santos, O.S.H.; Coelho da Silva, M.; Silva, V.R.; Mussel, W.N.; Yoshida, M.I. Polyurethane foam impregnated with lignin as a filler for the removal of crude oil from contaminated water. J. Hazard. Mater. 2017, 324, 406–413. [Google Scholar] [CrossRef]
- Sousa Junior, R.R.d.; Gouveia, J.R.; Nacas, A.M.; Tavares, L.B.; Ito, N.M.; Moura, E.N.d.; Gaia, F.A.; Pereira, R.F.; Santos, D.J.d. Improvement of Polypropylene Adhesion by Kraft Lignin Incorporation. Mater. Res. 2019, 22, 1–8. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, J.; Wu, L.; Fang, G.; Guo, Z. Enhanced anti-ultraviolet, anti-fouling and anti-bacterial polyelectrolyte membrane of polystyrene grafted with trimethyl quaternary ammonium salt modified lignin. Polymer 2017, 114, 113–121. [Google Scholar] [CrossRef]











| Composite | Lignin [wt.%] | Styrene [wt.%] | BA/St ratio |
|---|---|---|---|
| EBA | 0 | 86 | 0.16 |
| LEBA5 | 5 | 81 | 0.17 |
| LEBA10 | 10 | 76 | 0.18 |
| LEBA15 | 15 | 71 | 0.20 |
| LEBA20 | 20 | 66 | 0.21 |
| Atmosphere | Mn | Mw | I |
|---|---|---|---|
| Air | 15,404 | 83,986 | 5.45 |
| Nitrogen | 18,339 | 87,678 | 4.78 |
| Polymer | Type lignin | Application | Water Contact Angle, ° |
|---|---|---|---|
| Polyurethane (PU) [68] | Lignin waste (5.0 to 20.0 wt.%) | Removal oil in water | 123.0° for PU to 90.0° for PU whit 20 wt.% of lignin |
| Polypropylene (PP) [69] | Kraft lignin acid (5.0 wt.%) | Adhesion properties | 107.2° for PP and 92.46° for PP whit Kraft lignin (acid) |
| Polystyrene (PS) [70] | Alkali lignin (6.5 wt.%) modified whit trimethyl quaternary ammonium salt | Antibacterial activity | 55.9° for PS and 10.5° for PS whit lignin |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López Serna, D.; Elizondo Martínez, P.; Reyes González, M.Á.; Zaldívar Cadena, A.A.; Zaragoza Contreras, E.A.; Sánchez Anguiano, M.G. Synthesis and Characterization of a Lignin-Styrene-Butyl Acrylate Based Composite. Polymers 2019, 11, 1080. https://doi.org/10.3390/polym11061080
López Serna D, Elizondo Martínez P, Reyes González MÁ, Zaldívar Cadena AA, Zaragoza Contreras EA, Sánchez Anguiano MG. Synthesis and Characterization of a Lignin-Styrene-Butyl Acrylate Based Composite. Polymers. 2019; 11(6):1080. https://doi.org/10.3390/polym11061080
Chicago/Turabian StyleLópez Serna, Daniel, Perla Elizondo Martínez, Miguel Ángel Reyes González, Antonio Alberto Zaldívar Cadena, Erasto Armando Zaragoza Contreras, and María Guadalupe Sánchez Anguiano. 2019. "Synthesis and Characterization of a Lignin-Styrene-Butyl Acrylate Based Composite" Polymers 11, no. 6: 1080. https://doi.org/10.3390/polym11061080