Novel Biobased Polyamide 410/Polyamide 6/CNT Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
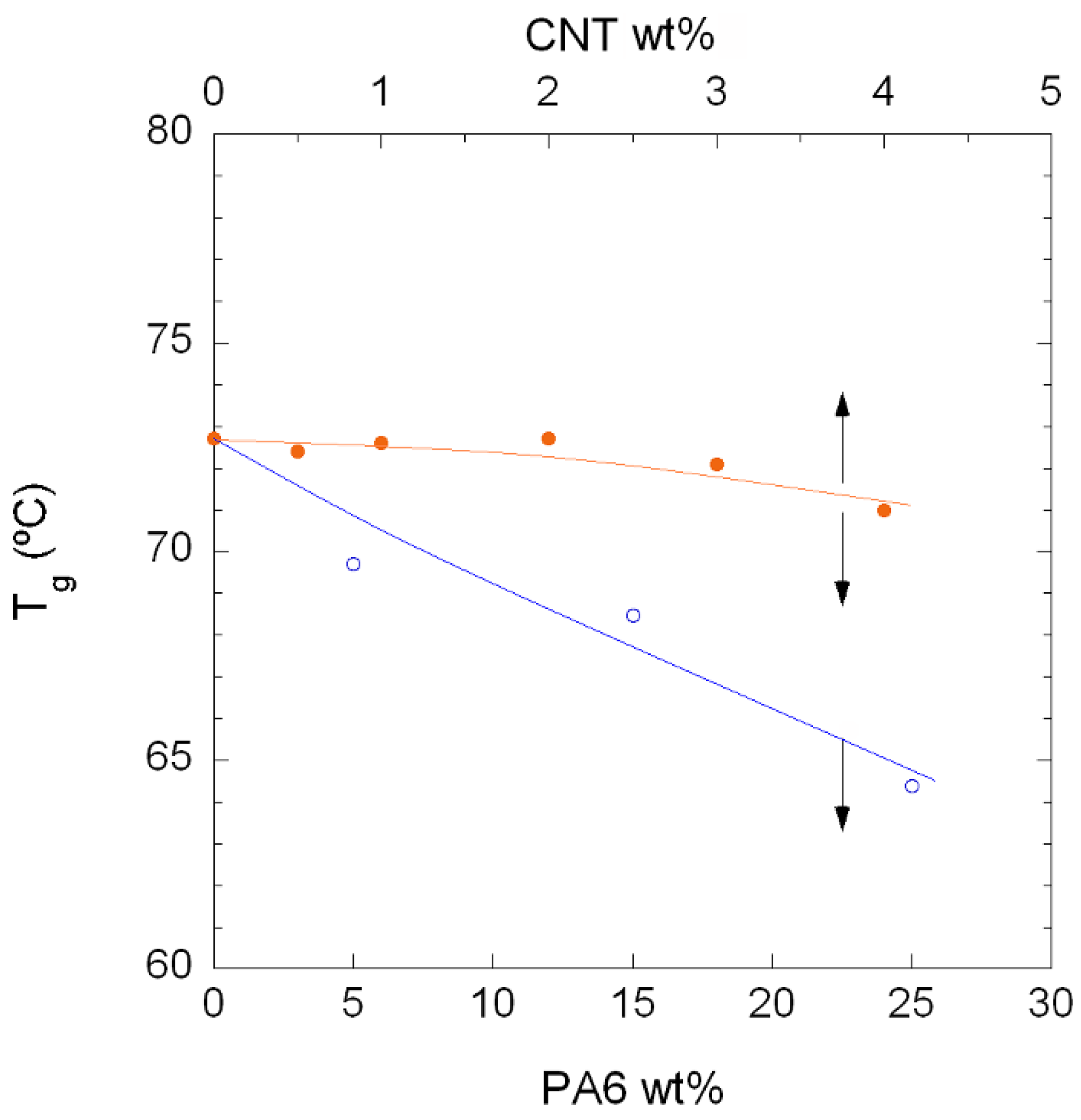
3.1. Phase Behaviour
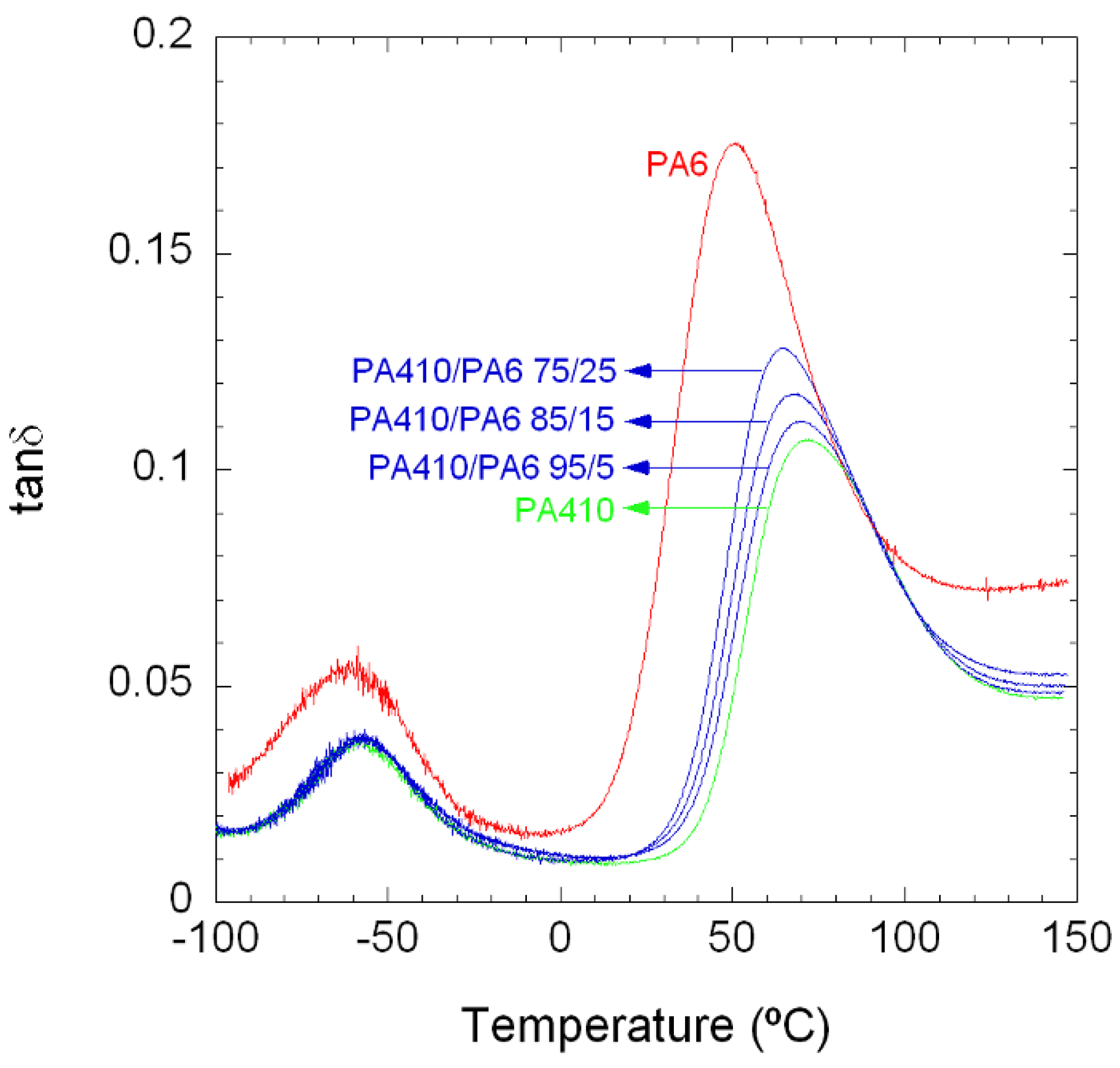
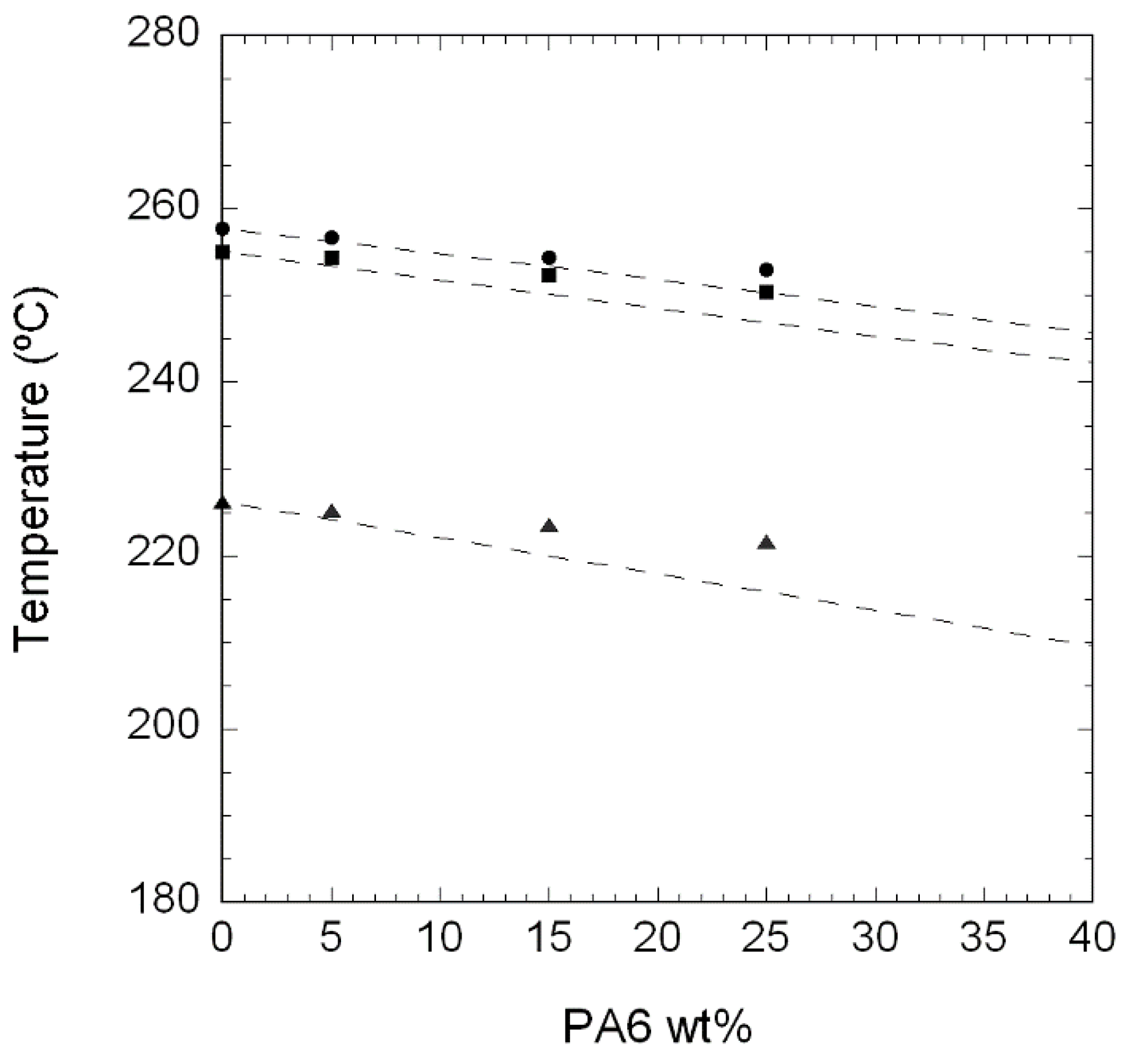
3.1.1. Unfilled PA410/PA6 Blends
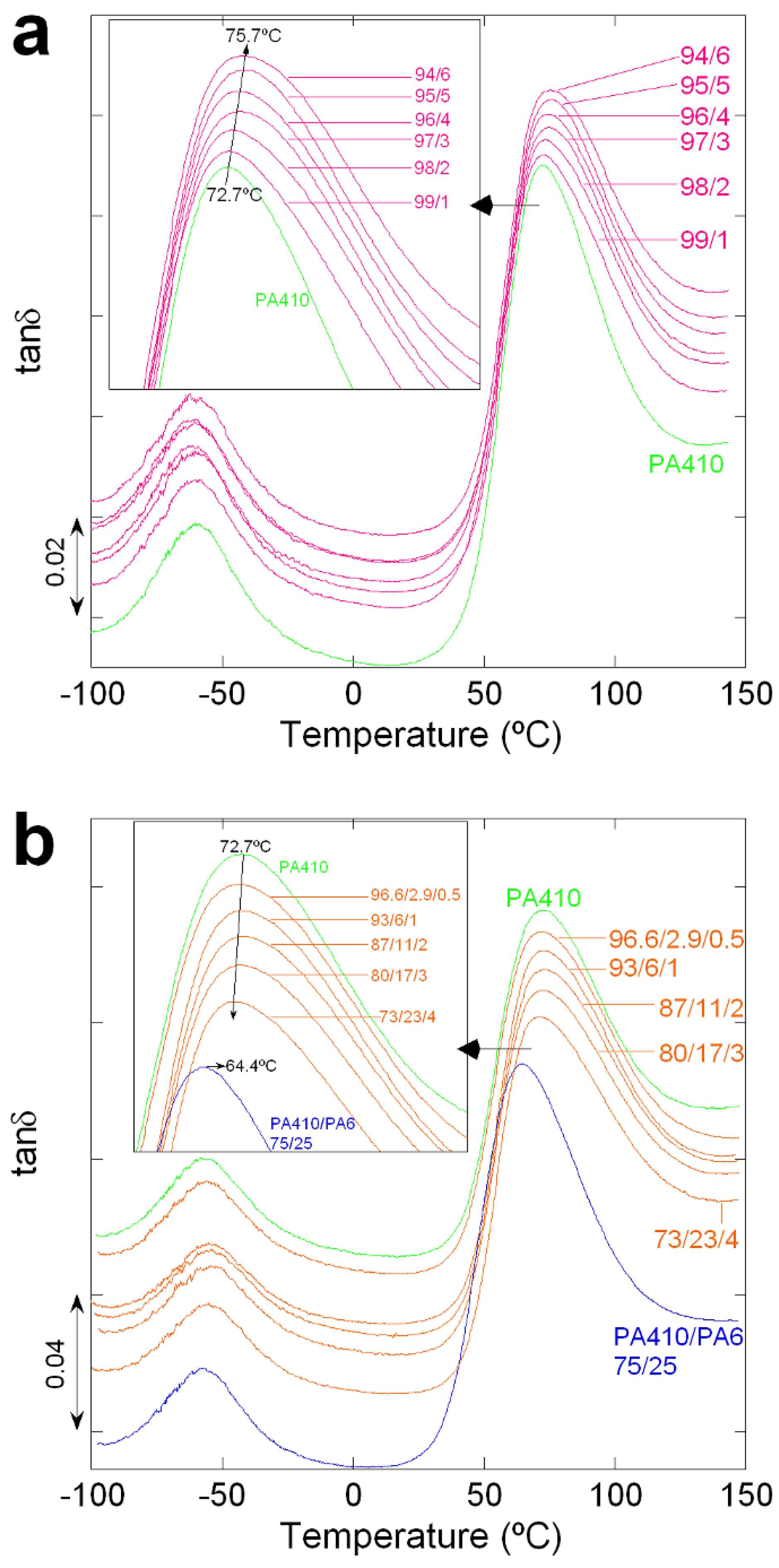
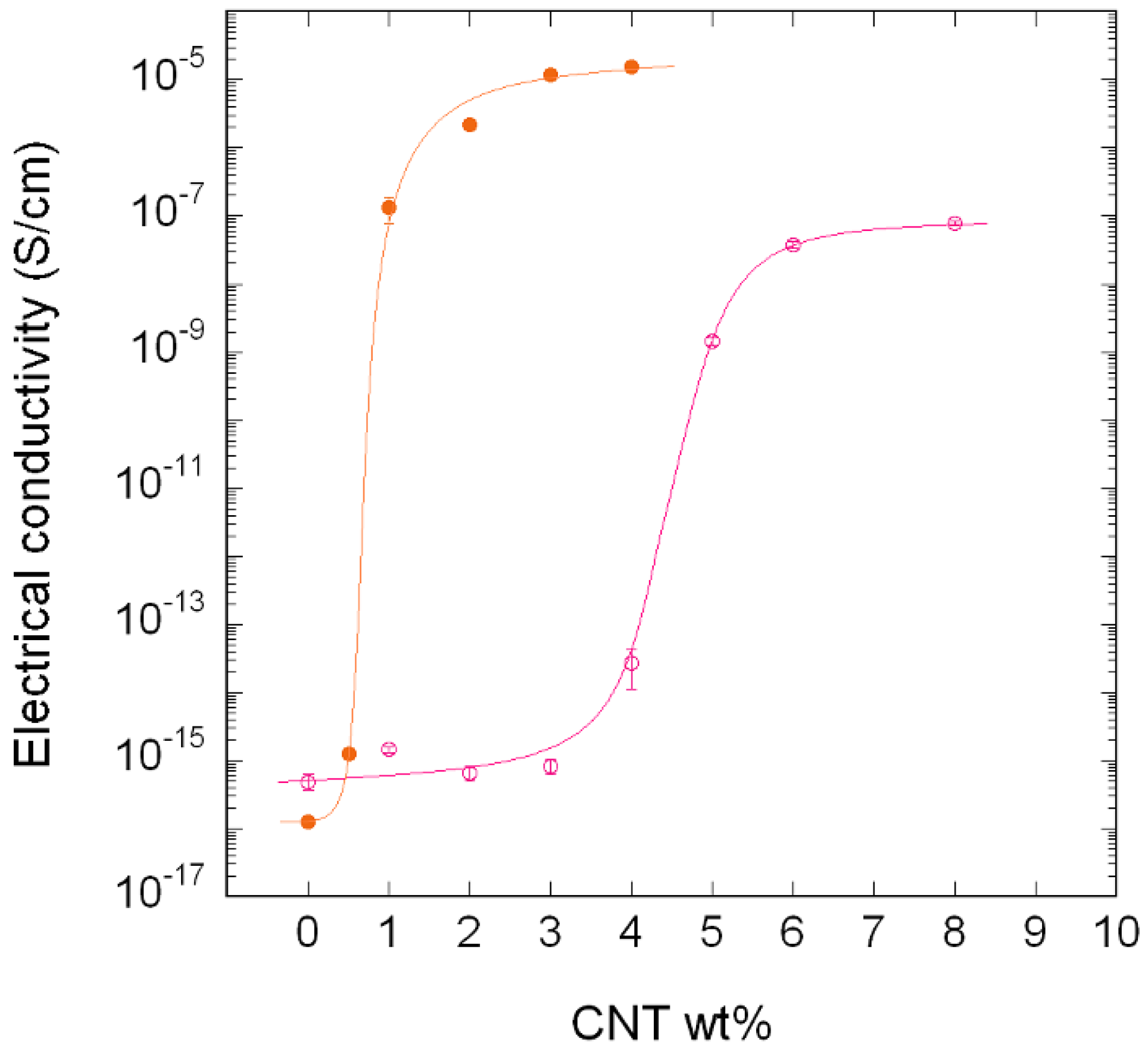
3.1.2. PA410/PA6/CNT NCs
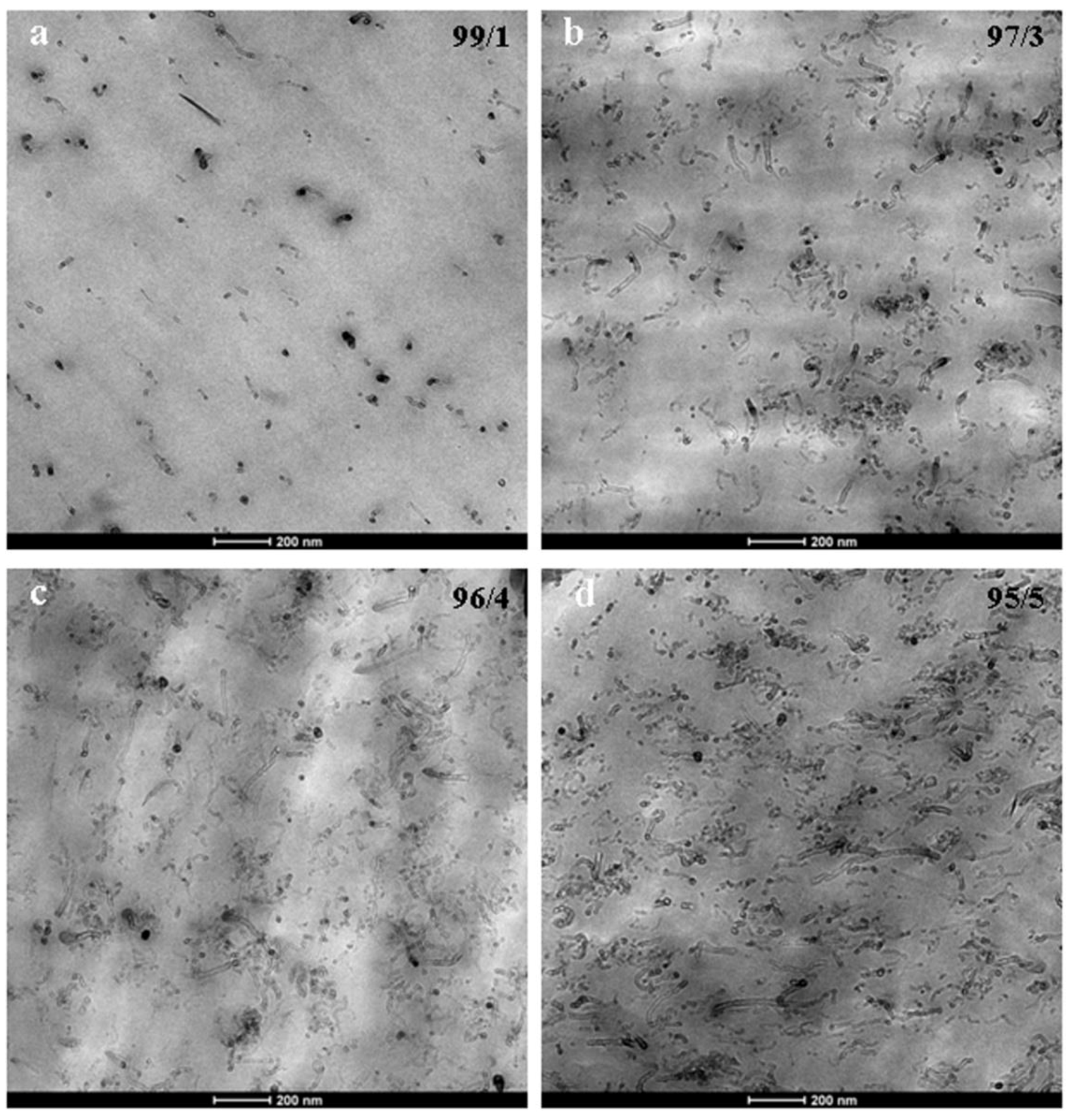
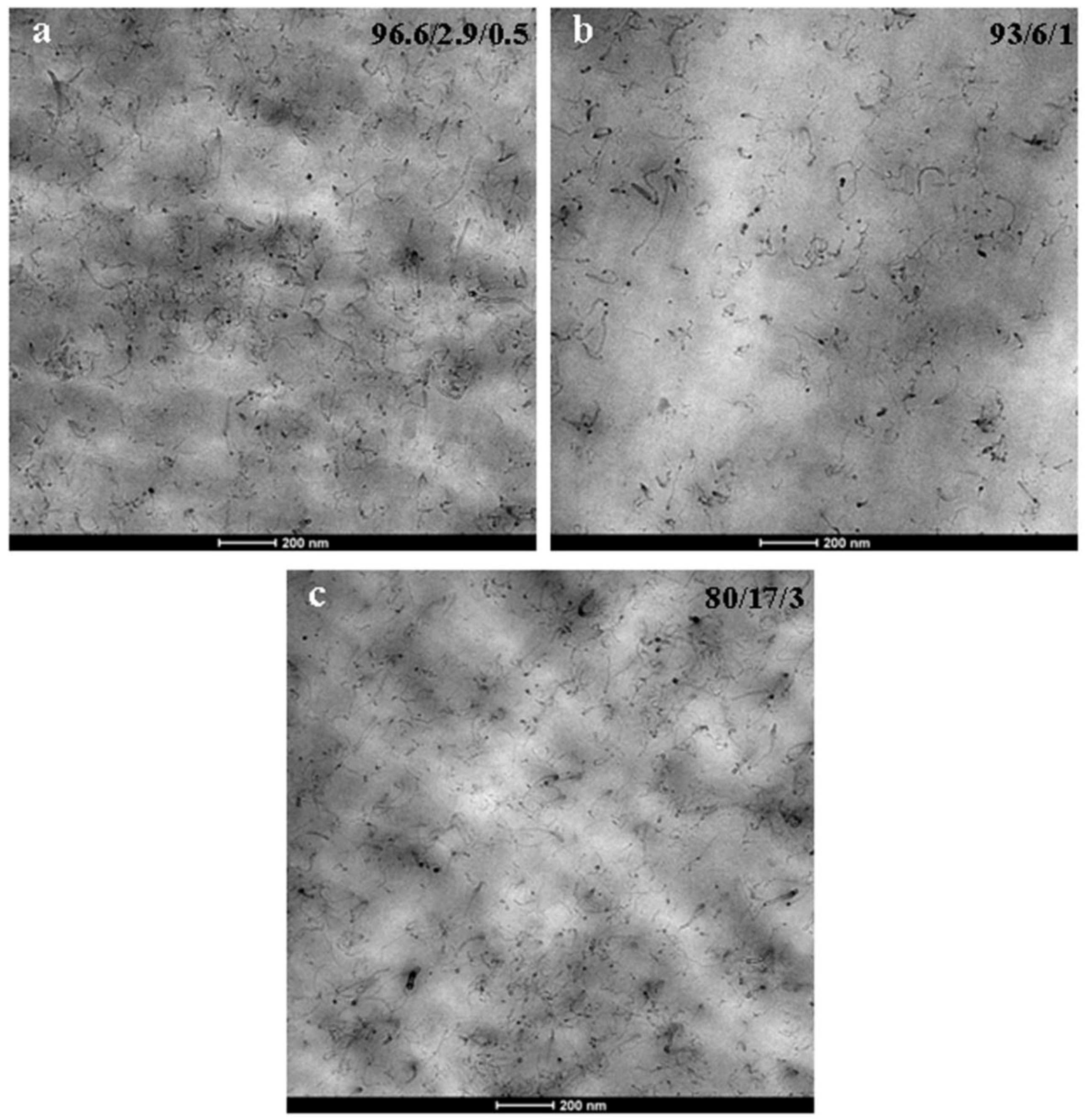
3.2. Nanostructure
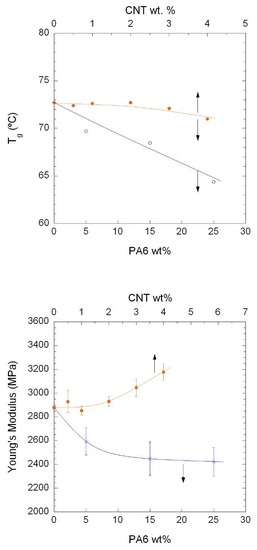
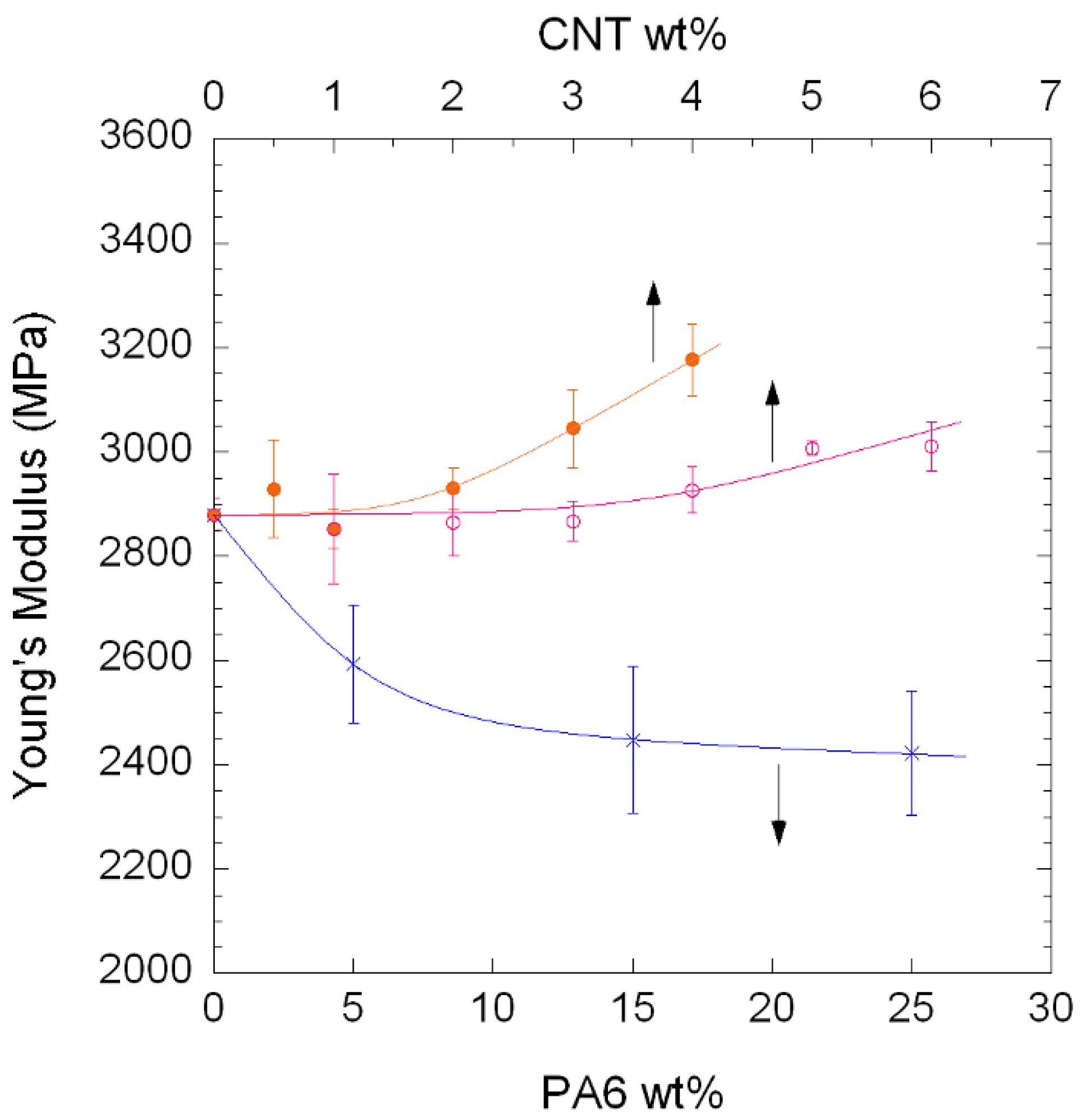
3.3. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moran, C.S.; Barthelon, A.; Pearsall, A.; Mittal, V.; Dorgan, J.R. Biorenewable blends of polyamide-4,10 and polyamide-6,10. J. Appl. Polym. Sci. 2016, 133, 43626. [Google Scholar] [CrossRef]
- Arboleda-Clemente, L.; Ares-Pernas, A.; Garcia, X.; Dopico, S.; Abad, M.J. Influence of polyamide ratio on the CNT dispersion in polyamide 66/6 blends by dilution of PA66 or PA6-MWCNT masterbatches. Synth. Met. 2016, 221, 134–141. [Google Scholar] [CrossRef]
- Niaounakis, M. Introduction to Biopolymers. In Biopolymers Reuse, Recycling, and Disposal; Niaounakis, M., Ed.; William Andrew Publishing: Oxford, UK, 2013; pp. 1–75. [Google Scholar]
- Tran, T.Q.; Headrick, R.J.; Amram Bengio, E.; Myint, S.M.; Khoshnevis, H.; Jamali, V.; Duong, H.M.; Pasquali, M. Purification and disolution of carbon nanotube fibers spun from floating catalyst method. ACS Appl. Mater. Interfaces 2017, 9, 37112–37119. [Google Scholar] [CrossRef] [PubMed]
- Khoshnevis, H.; Mint, S.M.; Yedinak, E.; Tran, T.Q.; Zadhoush, A.; Youseffi, M.; Pasquali, M.; Duong, H.M. Super high-rate fabrication of high-purity carbon nanotube aerogels from floating catalyst method for oil spill cleaning. Chem. Phys. Lett. 2018, 693, 146–151. [Google Scholar] [CrossRef]
- Alig, I.; Pötschke, P.; Lellinger, D.; Skipa, T.; Pegel, S.; Kasaliwal, G.R.; Villmow, T. Establishment, morphology and properties of carbon nanotube networks in polymer melts. Polymer 2012, 53, 4–28. [Google Scholar] [CrossRef]
- Meng, H.; Sui, G.X.; Fang, P.F.; Yang, R. Effects of acid- and diamine-modified MWNTs on the mechanical properties and crystallization behavior of polyamide 6. Polymer 2008, 49, 610–620. [Google Scholar] [CrossRef]
- Mahmood, N.; Islam, M.; Hameed, A.; Saeed, S.; Khan, A.N. Polyamide-6-based composites reinforced with pristine or functionalized multi-walled carbon nanotubes produced using melt extrusion technique. J. Compos. Mater. 2014, 48, 1197–1207. [Google Scholar] [CrossRef]
- Li, J.; Ke, C.; Fang, K.; Fan, X.; Guo, Z.; Fang, Z. Crystallization and rheological behaviors of amino-functionalized multiwalled carbon nanotubes filled polyamide 6 composites. J. Macromol. Sci. Part B Phys. 2010, 49, 405–418. [Google Scholar] [CrossRef]
- Qiu, L.; Chen, Y.; Yang, Y.; Xu, L.; Liu, X.A. study of surface modifications of carbon nanotubes on the properties of polyamide 66/multiwalled carbon nanotube composites. J. Nanomater. 2013, 252417. [Google Scholar] [CrossRef]
- Lin, S.Y.; Chen, E.C.; Liu, K.Y.; Wu, T.M. Isothermal crystallization behavior of polyamide 6,6/multiwalled carbon nanotube nanocomposites. Polym. Eng. Sci. 2009, 49, 2447–2453. [Google Scholar] [CrossRef]
- Chatterjee, S.; Nuesch, F.A.; Chu, B.T.T. Comparing carbon nanotubes and graphene nanoplatelets as reinforcements in polyamide 12 composites. Nanotechnology 2011, 22, 275714. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, D.; Zhang, Y.; Zhang, K.; Wu, G. Influences of carbon nanotube networking on the conductive, crystallization, and thermal expansion behaviors of pa610-based nanocomposites. J. Macromol. Sci. Part B Phys. 2013, 52, 910–923. [Google Scholar] [CrossRef]
- Wang, B.; Sun, G.; Liu, J.; He, X.; Li, J. Crystallization behavior of carbon nanotubes-filled polyamide 1010. J. Appl. Polym. Sci. 2006, 100, 3794–3800. [Google Scholar] [CrossRef]
- Huang, Y.; Tan, L.; Zheng, S.; Liu, Z.; Feng, J.; Yang, M.B. Enhanced dielectric properties of polyamide 11/multi-walled carbon nanotubes composites. J. Appl. Polym. Sci. 2015, 132, 42642. [Google Scholar] [CrossRef]
- Chiu, F.C.; Kao, G.F. Polyamide 46/multi-walled carbon nanotube nanocomposites with enhanced thermal, electrical, and mechanical properties. Compos. Part A 2012, 43, 208–218. [Google Scholar] [CrossRef]
- Sun, X.; Yuan, X.; Zhang, X.; Liu, W.; Zhou, Q. Crystal structure study of nylon1212/carbon nanotube composites under tension force. Qingdao Keji Daxue Xuebao Ziran Kexueban 2006, 27, 423–426. [Google Scholar]
- Zhang, W.D.; Shen, L.; Phang, I.Y.; Liu, T. Carbon Nanotubes reinforced nylon-6 composite prepared by simple melt-compounding. Macromolecules 2004, 37, 256–259. [Google Scholar] [CrossRef]
- Satapathy, B.K.; Weidisch, R.; Poetschke, P.; Janke, A. Crack toughness behaviour of multiwalled carbon nanotube (MWNT)/polycarbonate nanocomposites. Macromol. Rapid Commun. 2005, 26, 1246–1252. [Google Scholar] [CrossRef]
- Breuer, O.; Sundararaj, U. Big returns from small fibers: A review of polymer/carbon nanotube composites. Polym. Compos. 2004, 25, 630–645. [Google Scholar] [CrossRef]
- Meincke, O.; Kaempfer, D.; Weickmann, H.; Friedrich, C.; Vathauer, M.; Warth, H. Mechanical properties and electrical conductivity of carbon-nanotube filled polyamide-6 and its blends with acrylonitrile/butadiene/styrene. Polymer 2004, 45, 739–748. [Google Scholar] [CrossRef]
- Gonzalez, I.; Eguiazabal, J.I. Widely dispersed PEI-based nanocomposites with multi-wall carbon nanotubes by blending with a masterbatch. Compos. Part A 2013, 53, 176–181. [Google Scholar] [CrossRef]
- Isayev, A.I.; Kumar, R.; Lewis, T.M. Ultrasound assisted twin screw extrusion of polymer-nanocomposites containing carbon nanotubes. Polymer 2009, 50, 250–260. [Google Scholar] [CrossRef]
- Leszczynska, A.; Kicilinski, P.; Pielichowski, K. Biocomposites of polyamide 4,10 and surface modified microfibrillated cellulose (MFC): Influence of processing parameters on structure and thermomechanical properties. Cellulose 2015, 22, 2551–2569. [Google Scholar] [CrossRef]
- Pagacz, J.; Raftopoulos, K.N.; Leszczynska, A.; Pielichowski, K. Bio-polyamides based on renewable raw materials-glass transition and crystallinity studies. J. Therm. Anal. Calorim. 2016, 123, 1225–1237. [Google Scholar] [CrossRef]
- Kim, J.H.; Min, B.R.; Kang, Y.S. Thermodynamic model of the glass transition behavior for miscible polymer blends. Macromolecules 2006, 39, 1297–1299. [Google Scholar] [CrossRef]
- Mahfuz, H.; Adnan, A.; Rangari, V.K.; Hasan, M.M.; Jeelani, S.; Wright, W.J.; DeTeresa, S.J. Enhancement of strength and stiffness of Nylon 6 filaments through carbon nanotubes reinforcement. Appl. Phys. Lett. 2006, 88, 083119. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Arrigo, R.; Nasillo, G.; Caponetti, E.; La Mantia, F.P. Effect of the nanotube aspect ratio and surface functionalization on the morphology and properties of multiwalled carbon nanotube polyamide-based fibers. J. Appl. Polym. Sci. 2013, 129, 2479–2489. [Google Scholar] [CrossRef]
- Krause, B.; Potschke, P.; Haeussler, L. Influence of small scale melt mixing conditions on electrical resistivity of carbon nanotube-polyamide composites. Compos. Sci. Technol. 2009, 69, 1505–1515. [Google Scholar] [CrossRef]
- Gonzalez, I.; Eguiazabal, J.I.; Nazabal, J. Attaining high electrical conductivity and toughness in PA6 by combined addition of MWCNT and rubber. Compos. Part A 2012, 43, 1482–1489. [Google Scholar] [CrossRef]
- Weber, M.; Kamal, M.R. Estimation of the volume resistivity of electrically conductive composites. Polym. Compos. 1997, 18, 711–725. [Google Scholar] [CrossRef]
- Socher, R.; Krause, B.; Mueller, M.T.; Boldt, R.; Poetschke, P. The influence of matrix viscosity on MWCNT dispersion and electrical properties in different thermoplastic nanocomposites. Polymer 2012, 53, 495–504. [Google Scholar] [CrossRef]
- Gorrasi, G.; Bredeau, S.; Di Candia, C.; Patimo, G.; De Pasquale, S.; Dubois, P. Electroconductive polyamide 6/mwnt nanocomposites: effect of nanotube surface-coating by in situ catalyzed polymerization. Macromol. Mater. Eng. 2011, 296, 408–413. [Google Scholar] [CrossRef]
- Kodgire, P.V.; Bhattacharyya, A.R.; Bose, S.; Gupta, N.; Kulkarni, A.R.; Misra, A. Control of multiwall carbon nanotubes dispersion in polyamide 6 matrix: An assessment through electrical conductivity. Chem. Phys. Lett. 2006, 432, 480–485. [Google Scholar] [CrossRef]
- Aranburu, N.; Eguiazabal, J.I. Electrically conductive multi-walled carbon nanotube-reinforced amorphous polyamide nanocomposites. Polym. Compos. 2014, 35, 587–595. [Google Scholar] [CrossRef]
- Puch, F.; Hopmann, C. Morphology and tensile properties of unreinforced and short carbon fibre reinforced Nylon 6/multiwalled carbon nanotube-composites. Polymer 2014, 55, 3015–3025. [Google Scholar] [CrossRef]
- Puch, F.; Hopmann, C. Nylon 6/multiwalled carbon nanotube composites: Effect of the melt-compounding conditions and nanotube content on the morphology, mechanical properties, and rheology. J. Appl. Polym. Sci. 2014, 131, 40893. [Google Scholar] [CrossRef]
- Aranburu, N.; Eguiazabal, J.I. Compatible blends of polypropylene with an amorphous polyamide. J. Appl. Polym. Sci. 2013, 127, 5007–5013. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Peng, F.; Liu, M.; Zhao, Q.; Fu, P.F. Morphology of Nylon 1212 toughened with a maleated EPDM rubber. Polym. Int. 2009, 58, 190–197. [Google Scholar] [CrossRef]
- Gonzalez, I.; Eguiazabal, J.I.; Nazabal, J. Toughening and brittle-tough transition in blends of an amorphous polyamide with a modified styrene/ethylene-butylene/styrene triblock copolymer. Polym. Eng. Sci. 2009, 49, 1350–1356. [Google Scholar] [CrossRef]


| Nomenclature | PA410 (wt %) | PA6 (wt %) | CNT (wt %) | |
|---|---|---|---|---|
| PA410/ CNTbinary NCs | 100/0 | 100 | - | 0 |
| 99/1 | 99 | - | 1 | |
| 98/2 | 98 | - | 2 | |
| 97/3 | 97 | - | 3 | |
| 96/4 | 96 | - | 4 | |
| 96/5 | 95 | - | 5 | |
| 94/6 | 94 | - | 6 | |
| PA410/PA6/CNT ternary NCs | 96.6/2.9/0.5 | 96.6 | 2.9 | 0.5 |
| 93/6/1 | 93 | 6 | 1 | |
| 87/11/2 | 87 | 11 | 2 | |
| 80/17/3 | 80 | 17 | 3 | |
| 73/23/4 | 73 | 23 | 4 | |
| PA410/PA6 unfilled blends | 95/5 | 95 | 5 | 0 |
| 85/15 | 85 | 15 | 0 | |
| 75/25 | 75 | 25 | 0 |
| PA410/PA6 Composition | ||||||
|---|---|---|---|---|---|---|
| 100/0 | 257.7 | 58 | 226.3 | 64 | 255.1 | 64 |
| 95/5 | 256.7 | 63 | 225.3 | 65 | 254.4 | 65 |
| 85/15 | 254.4 | 57 | 223.3 | 62 | 252.4 | 63 |
| 75/25 | 253.1 | 58 | 221.6 | 54 | 250.4 | 55 |
| 0/100 | 227.4 | 59 | 184.6 | 65 | 223.1 | 68 |
| PA410/CNT Composition | χc (%) | ||||
|---|---|---|---|---|---|
| 100/0 | 256.7 | 59 | 22 | 224.3 | −39 |
| 99/1 | 257.1 | 61(62) | 23 | 233.6 | −46 |
| 98/2 | 256.7 | 59(60) | 22 | 234.9 | −49 |
| 97/3 | 255.4 | 59(61) | 23 | 235.9 | −51 |
| 96/4 | 257.4 | 57(59) | 22 | 235.3 | −53 |
| 95/5 | 255.7 | 60(63) | 23 | 235.3 | −55 |
| 94/6 | 253.7 | 55(58) | 22 | 235.6 | −55 |
| PA410/PA6/CNT Composition | PA6 wt % | ||||
|---|---|---|---|---|---|
| 100/0/0 | 0 | 257.1 | 58 | 224.9 | −37 |
| 96.6/2.9/0.5 | 2.9 | 253.7 | 63(63) | 232.9 | −46 |
| 93/6/1 | 6 | 256.1 | 56(57) | 232.9 | −51 |
| 87/11/2 | 11 | 252.1 | 59(60) | 233.6 | −50 |
| 80/17/3 | 17 | 253.7 | 57(59) | 233.3 | −51 |
| 73/23/4 | 23 | 252.1 | 49(51) | 232.9 | −49 |
| Nanocomposite | Processing Method and Conditions | Pc (%) | Reference |
|---|---|---|---|
| PA410/CNT | Twin-screw extruder 270 °C, 200 rpm | 3.98 | Present study |
| PA410/PA6/CNT (PA6-based masterbatch) | Twin-screw extruder 270 °C, 200 rpm | 0.65 | Present study |
| PA6/CNT | Twin-screw mini-extruder 240 °C, 45 rpm | 0.4 | [33] |
| PA6/CNT | Twin-screw extruder 260 °C, 150 rpm | 2–3 | [34] |
| PA6/CNT | Twin-screw extruder Masterbatch 260 °C, 200 rpm | 4–6 | [21] |
| PA12/CNT | Twin-screw microcompounder 260 °C, 250 rpm | 1 (low matrix viscosity) 2–2.5 (medium matrix viscosity) 3.5 (high matrix viscosity) | [32] |
| PA12/CNT | Micro-extruder 190 °C, 180 rpm | 1.33 | [12] |
| aPA/CNT | Twin-screw extruder 265, 200 rpm | 2.97 | [35] |
| Composition | Young’s Modulus (MPa) | Tensile Strength (MPa) | Strain at Break (%) | |
|---|---|---|---|---|
| PA410/CNT NCs | 100/0 | 2880 ± 30 | 75.5 ± 0.4 | 100 ± 30 |
| 99/1 | 2850 ± 100 | 74.3 ± 1.2 | 7 ± 4 | |
| 98/2 | 2860 ± 60 | 75.6 ± 0.2 | 7 ± 1 | |
| 97/3 | 2870 ± 40 | 74.3 ± 1.2 | 5 ± 1 | |
| 96/4 | 2930 ± 40 | 75.1 ± 0.7 | 7 ± 1 | |
| 96/5 | 3010 ± 10 | 74.5 ± 0.8 | 5 ± 1 | |
| 94/6 | 3010 ± 50 | 76.3 ± 0.5 | 6 ± 0 | |
| PA410/PA6/CNT NCs | 96.6/2.9/0.5 | 2930 ± 90 | 74.3 ± 0.3 | 8 ± 4 |
| 93/6/1 | 2850 ± 40 | 73.5 ± 0.5 | 5 ± 1 | |
| 87/11/2 | 2930 ± 40 | 73.8 ± 1.0 | 6 ± 2 | |
| 80/17/3 | 3040 ± 80 | 71.8 ± 3.6 | 4 ± 1 | |
| 73/23/4 | 3180 ± 70 | 74.1 ± 2.0 | 3 ± 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otaegi, I.; Aramburu, N.; Müller, A.J.; Guerrica-Echevarría, G. Novel Biobased Polyamide 410/Polyamide 6/CNT Nanocomposites. Polymers 2018, 10, 986. https://doi.org/10.3390/polym10090986
Otaegi I, Aramburu N, Müller AJ, Guerrica-Echevarría G. Novel Biobased Polyamide 410/Polyamide 6/CNT Nanocomposites. Polymers. 2018; 10(9):986. https://doi.org/10.3390/polym10090986
Chicago/Turabian StyleOtaegi, Itziar, Nora Aramburu, Alejandro J. Müller, and Gonzalo Guerrica-Echevarría. 2018. "Novel Biobased Polyamide 410/Polyamide 6/CNT Nanocomposites" Polymers 10, no. 9: 986. https://doi.org/10.3390/polym10090986