Reinforcement of Thermoplastic Corn Starch with Crosslinked Starch/Chitosan Microparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Crosslinked Starch/Chitosan Microparticles
2.3. Preparation of Thermoplastic Starch
2.4. Characterization
3. Results and Discussion
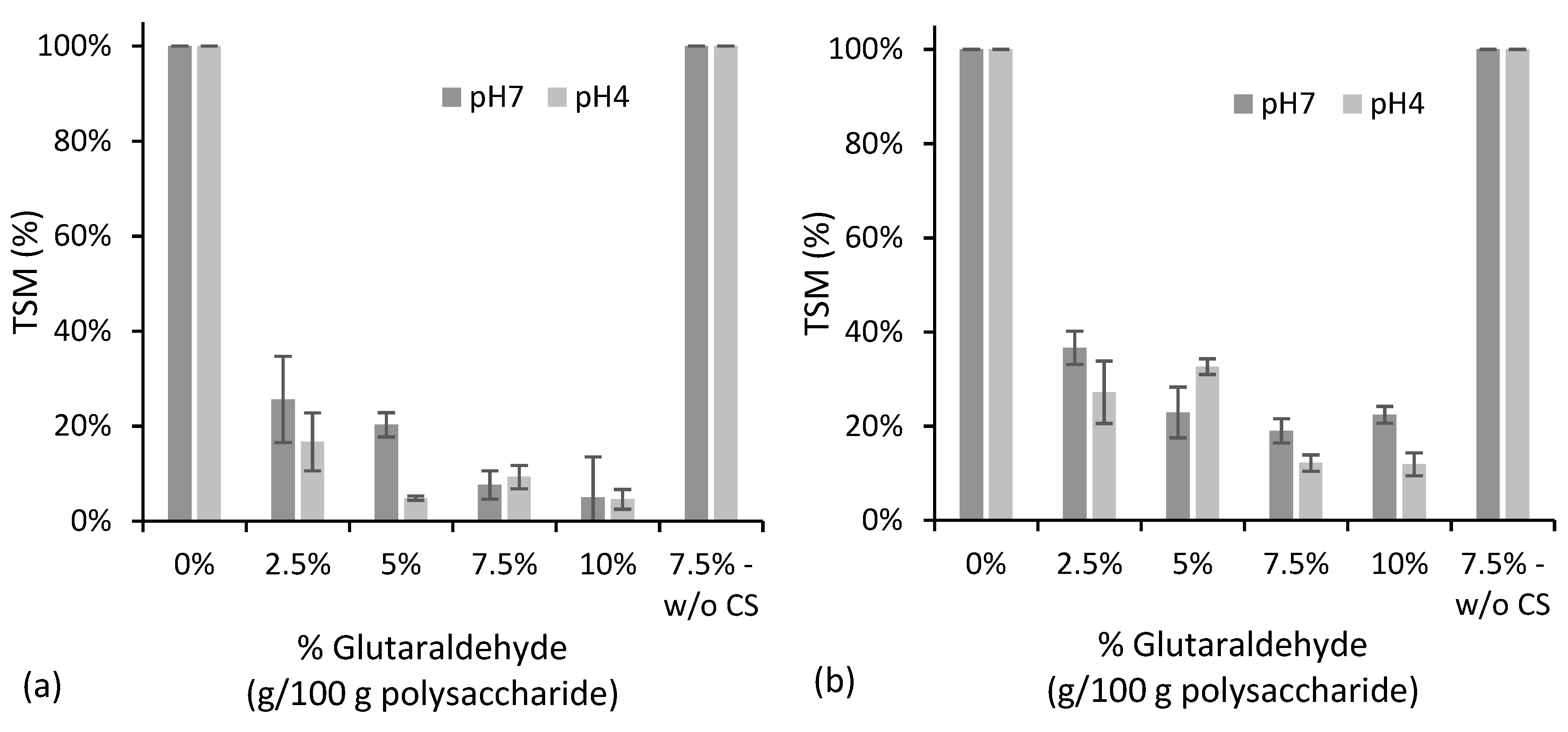
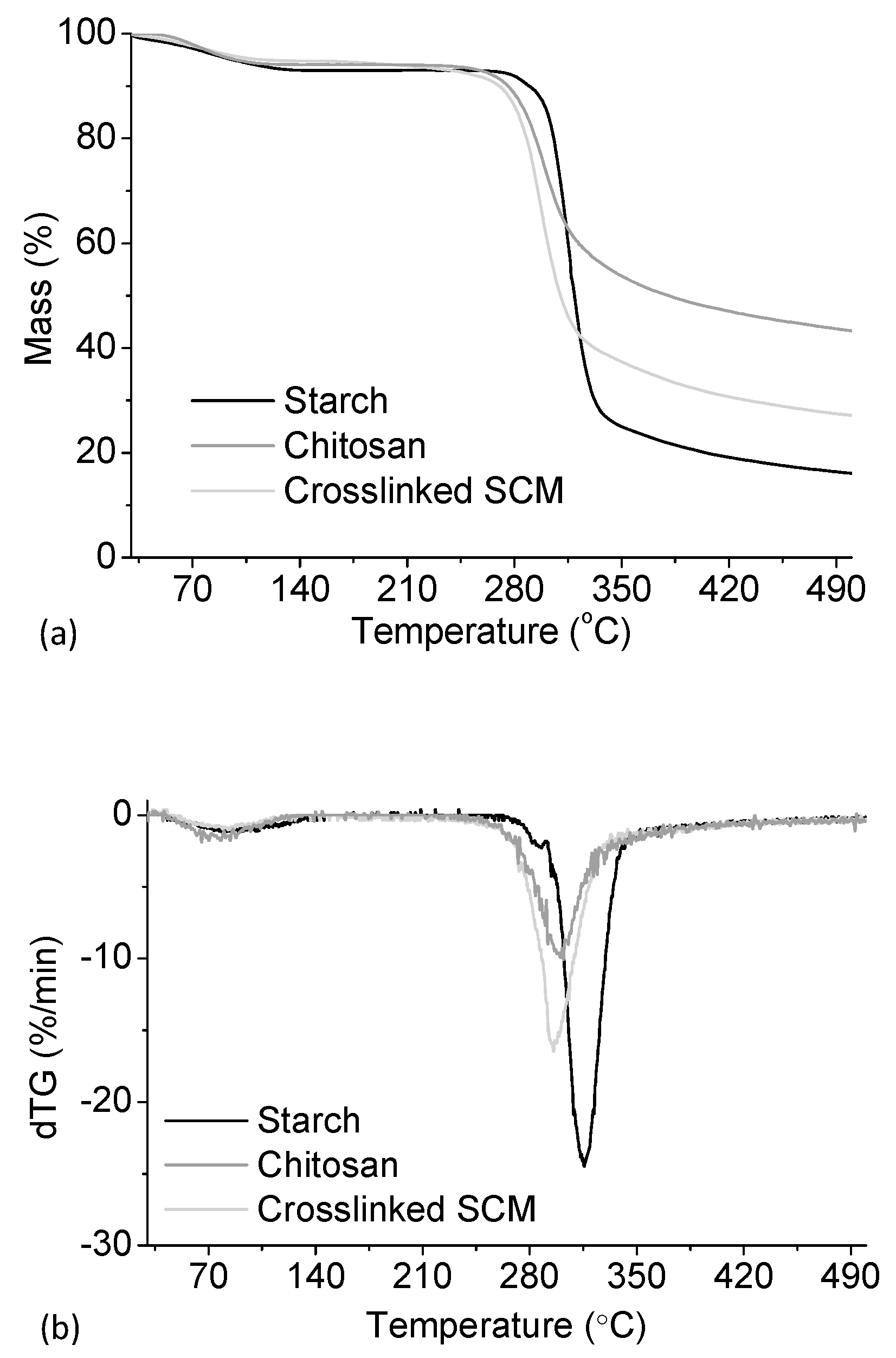
3.1. Starch/Chitosan Microparticles
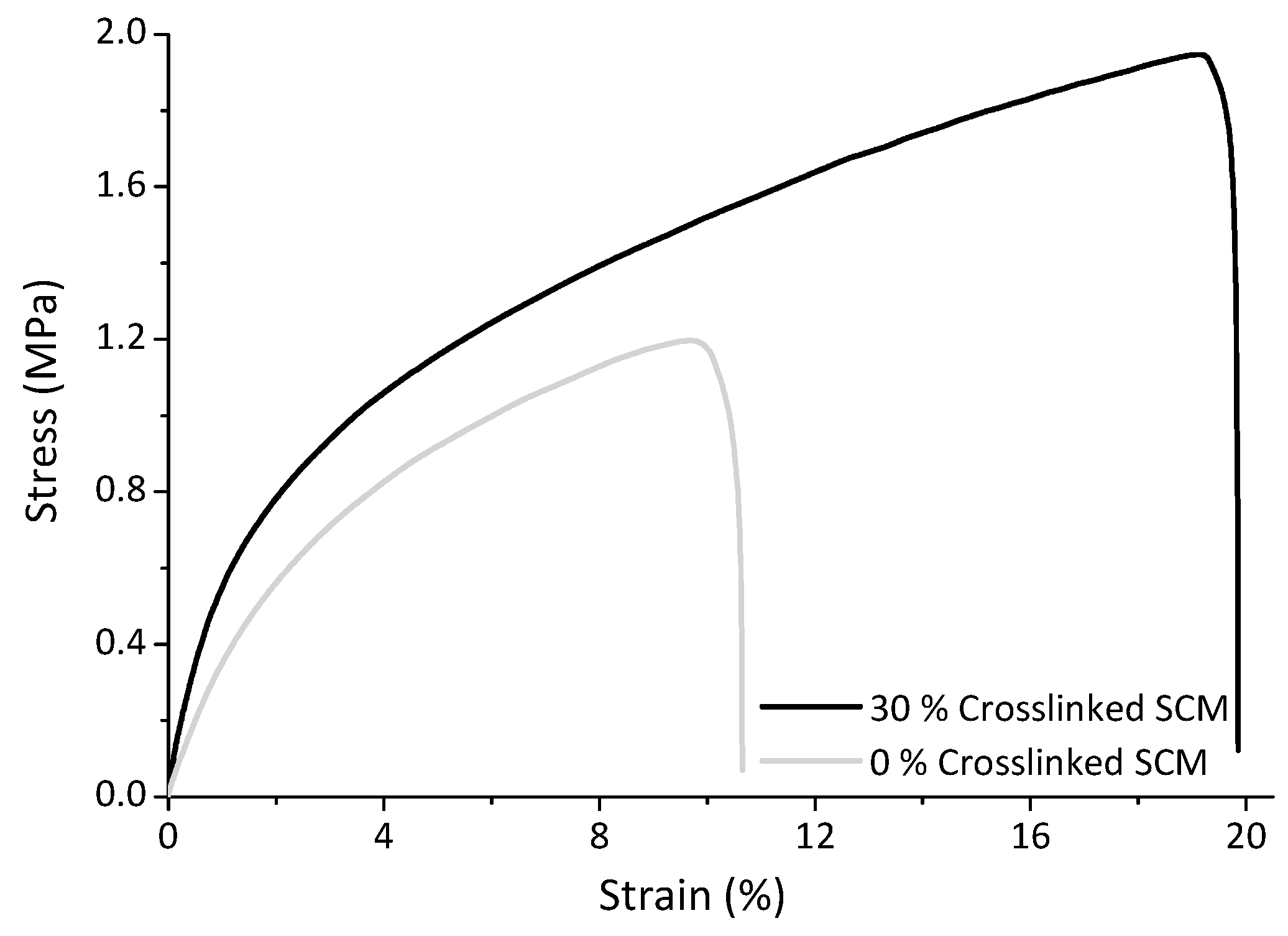
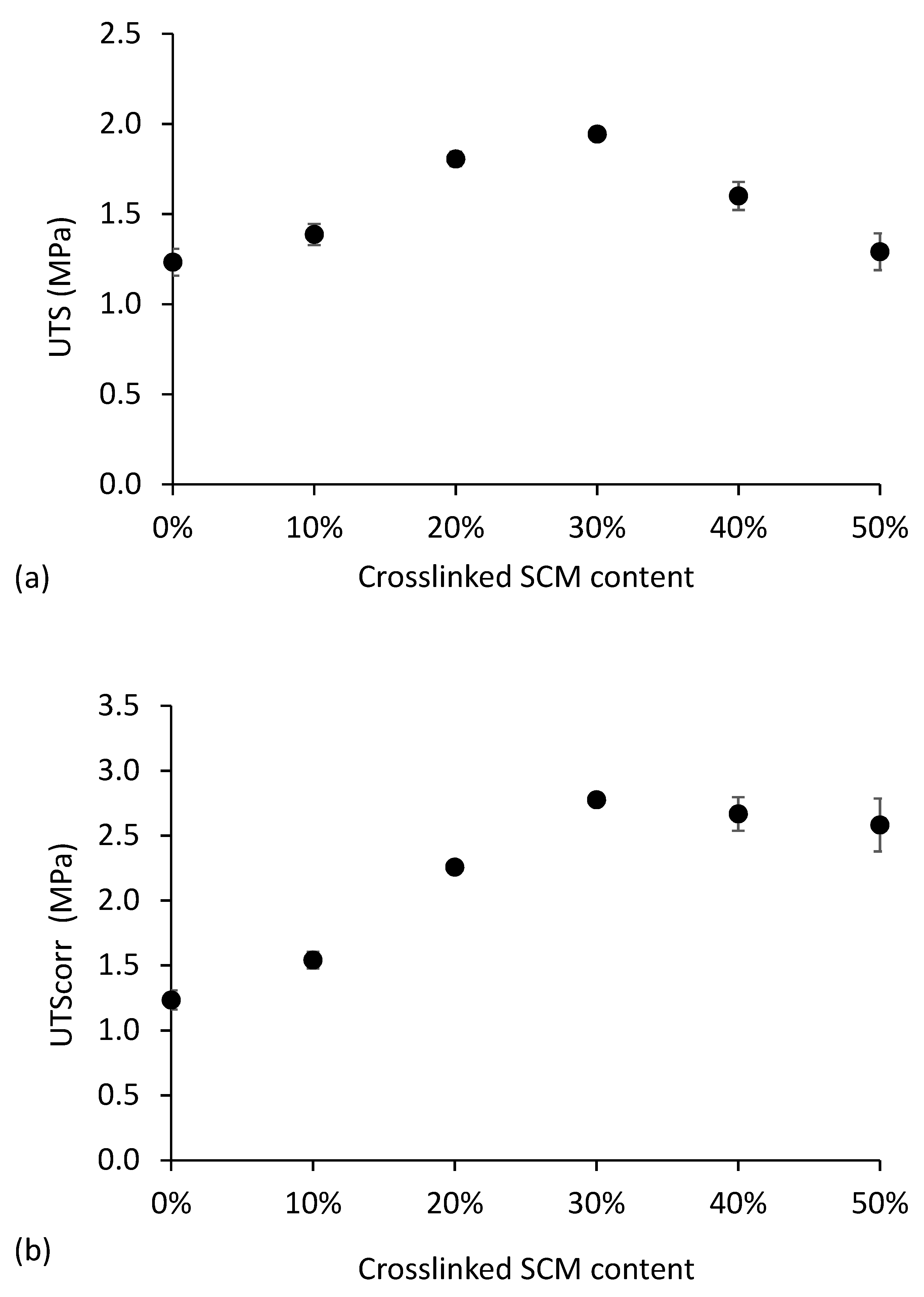
3.2. Reinforced Thermoplastic Starch
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shogren, R.L.; Swanson, C.L.; Thompson, A.R. Extrudates of cornstarch with urea and glycols: Structure/mechanical property relations. Starch-Stärke 1992, 44, 335–338. [Google Scholar] [CrossRef]
- Stevens, E.S.; Klamczynski, A.; Glenn, G.M. Starch-lignin foams. Express Polym. Lett. 2010, 4, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.V.; Venditti, R.A.; Pawlak, J.J. Dimensional changes of starch microcellular foam during the exchange of water with ethanol and subsequent drying. BioResources 2009, 5, 121–134. [Google Scholar]
- Jiankang, W.; Ruilin, M.; Liang, H. Recent research and patents on preparation and application of starch-based plastics. Recent Patents Mech. Eng. 2017, 10, 111–117. [Google Scholar]
- Carvalho, A.J.F. Chapter 6: Chemical Modification of Thermoplastic Starch. In RSC Green Chemistry; RSC: Cambridge, UK, 2016; pp. 217–235. [Google Scholar]
- Patel, S.; Venditti, R.A.; Pawlak, J.J.; Ayoub, A.; Rizvi, S.S.H. Development of cross-linked starch microcellular foam by solvent exchange and reactive supercritical fluid extrusion. J. Appl. Polym. Sci. 2009, 111, 2917–2929. [Google Scholar] [CrossRef]
- El-Tahlawy, K.; Venditti, R.; Pawlak, J. Effect of alkyl ketene dimer reacted starch on the properties of starch microcellular foam using a solvent exchange technique. Carbohydr. Polym. 2008, 73, 133–142. [Google Scholar] [CrossRef]
- Bastioli, C.; Magistrali, P.; Garcia, S.G. Starch. In Bio-Based Plastics: Materials and Applications; Kabasci, S., Ed.; Wiley: Chichester, UK, 2013; p. 392. [Google Scholar]
- Soykeabkaew, N.; Thanomsilp, C.; Suwantong, O. A review: Starch-based composite foams. Compos. Part A Appl. Sci. Manuf. 2015, 78, 246–263. [Google Scholar] [CrossRef]
- Blazek, J.; Gilbert, E.P. Application of small-angle X-ray and neutron scattering techniques to the characterisation of starch structure: A review. Carbohydr. Polym. 2011, 85, 281–293. [Google Scholar] [CrossRef]
- Carvalho, A.J.F. Starch: Major Sources, Properties and Applications as Thermoplastic Materials a2—Belgacem, Mohamed Naceur. In Monomers, Polymers and Composites from Renewable Resources; Gandini, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 321–342. [Google Scholar]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch retrogradation: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Mihai, M.; Huneault, M.A.; Favis, B.D.; Li, H. Extrusion foaming of semi-crystalline PLA and PLA/thermoplastic starch blends. Macromol. Biosci. 2007, 7, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.; Huneault, M.A.; Favis, B.D. Foaming of polystyrene/thermoplastic starch blends. J. Cell. Plast. 2007, 43, 215–236. [Google Scholar] [CrossRef]
- Chen, M.; Chen, B.; Evans, J.R.G. Novel thermoplastic starch-clay nanocomposite foams. Nanotechnology 2005, 16, 2334–2337. [Google Scholar] [CrossRef] [PubMed]
- Karger-Kocsis, J.; Kmetty, Á.; Lendvai, L.; Drakopoulos, S.; Bárány, T. Water-assisted production of thermoplastic nanocomposites: A review. Materials 2015, 8, 72–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahram, K.; Muhammad, B.K.N.; Ghufrana, S.; Zaib, J. Thermoplastic starch: A possible biodegradable food packaging material—A review. J. Food Process Eng. 2017, 40, e12447. [Google Scholar]
- Esmaeili, M.; Pircheraghi, G.; Bagheri, R. Optimizing mechanical and physical properties of thermoplastic starch via tuning the molecular microstructure through co-plasticization by sorbitol and glycerol. Polym. Int. 2017, 66, 809–819. [Google Scholar] [CrossRef]
- Graaf, R.A.; Karman, A.P.; Janssen, L.P.B.M. Material properties and glass transition temperatures of different thermoplastic starches after extrusion processing. Starch-Stärke 2003, 55, 80–86. [Google Scholar] [CrossRef]
- Mali, S.; Grossmann, M.V.E.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Effects of controlled storage on thermal, mechanical and barrier properties of plasticized films from different starch sources. J. Food Eng. 2006, 75, 453–460. [Google Scholar] [CrossRef]
- Xie, F.; Luckman, P.; Milne, J.; McDonald, L.; Young, C.; Tu, C.Y.; Pasquale, T.D.; Faveere, R.; Halley, P.J. Thermoplastic starch: Current development and future trends. J. Renew. Mater. 2014, 2, 95–106. [Google Scholar] [CrossRef]
- Salam, A.; Pawlak, J.J.; Venditti, R.A.; El-tahlawy, K. Synthesis and characterization of starch citrate−chitosan foam with superior water and saline absorbance properties. Biomacromolecules 2010, 11, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, T.J.; Alvarez, V.A. Cellulosic materials as natural fillers in starch-containing matrix-based films: A review. Polym. Bull. 2017, 74, 2401–2430. [Google Scholar] [CrossRef]
- Gironès, J.; López, J.P.; Mutjé, P.; Carvalho, A.J.F.; Curvelo, A.A.S.; Vilaseca, F. Natural fiber-reinforced thermoplastic starch composites obtained by melt processing. Compos. Sci. Technol. 2012, 72, 858–863. [Google Scholar] [CrossRef]
- Lomelí-Ramírez, M.G.; Kestur, S.G.; Manríquez-González, R.; Iwakiri, S.; de Muniz, G.B.; Flores-Sahagun, T.S. Bio-composites of cassava starch-green coconut fiber: Part ii—Structure and properties. Carbohydr. Polym. 2014, 102, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, E.; Lotti, C.; Corrêa, A.C.; Teodoro, K.B.R.; Marconcini, J.M.; Mattoso, L.H.C. Thermoplastic corn starch reinforced with cotton cellulose nanofibers. J. Appl. Polym. Sci. 2011, 120, 2428–2433. [Google Scholar] [CrossRef]
- Wattanakornsiri, A.; Pachana, K.; Kaewpirom, S.; Traina, M.; Migliaresi, C. Preparation and properties of green composites based on tapioca starch and differently recycled paper cellulose fibers. J. Polym. Environ. 2012, 20, 801–809. [Google Scholar] [CrossRef]
- Jose, J.; Al-Harthi, M.A.; AlMa’adeed, M.A.A.; Dakua, J.B.; De, S.K. Effect of graphene loading on thermomechanical properties of poly(vinyl alcohol)/starch blend. J. Appl. Polym. Sci. 2015, 132, 1–8. [Google Scholar] [CrossRef]
- Kellermayer, M.S.; Sun, Q.; Xi, T.; Li, Y.; Xiong, L. Characterization of corn starch films reinforced with caco3 nanoparticles. PLoS ONE 2014, 9, e106727. [Google Scholar]
- Chen, B.; Evans, J.R.G. Thermoplastic starch–clay nanocomposites and their characteristics. Carbohydr. Polym. 2005, 61, 455–463. [Google Scholar] [CrossRef]
- López, O.V.; Castillo, L.A.; García, M.A.; Villar, M.A.; Barbosa, S.E. Food packaging bags based on thermoplastic corn starch reinforced with talc nanoparticles. Food Hydrocoll. 2015, 43, 18–24. [Google Scholar] [CrossRef]
- Kumari, K.; Rani, U. Controlled release of metformin hydrochloride through crosslinked blends of chitosan-starch. Adv. Appl. Sci. Res. 2011, 2, 48–54. [Google Scholar]
- El-Tahlawy, K.; Venditti, R.A.; Pawlak, J.J. Aspects of the preparation of starch microcellular foam particles crosslinked with glutaraldehyde using a solvent exchange technique. Carbohydr. Polym. 2007, 67, 319–331. [Google Scholar] [CrossRef]
- Carvalho, A.J.F.; Curvelo, A.A.S.; Agnelli, J.A.M. A first insight on composites of thermoplastic starch and kaolin. Carbohydr. Polym. 2001, 45, 189–194. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The scherrer equation versus the ‘debye-scherrer equation’. Nat. Nanotechnol. 2011, 6, 534–534. [Google Scholar] [CrossRef] [PubMed]
- Jollet, V.; Chambon, F.; Rataboul, F.; Cabiac, A.; Pinel, C.; Guillon, E.; Essayem, N. Non-catalyzed and pt/γ-Al2O3-catalyzed hydrothermal cellulose dissolution–conversion: Influence of the reaction parameters and analysis of the unreacted cellulose. Green Chem. 2009, 11, 2052–2060. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Prachayawarakorn, J.; Hanchana, A. Effect of neem wood sawdust content on properties of biodegradable thermoplastic acetylated cassava starch/neem wood sawdust composites. Starch-Stärke 2017, 69, 1600113. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Heikkilä, M.I.; Willför, S.M.; Tenkanen, M. Films from glyoxal-crosslinked spruce galactoglucomannans plasticized with sorbitol. Int. J. Polym. Sci. 2012, 2012, 482810. [Google Scholar] [CrossRef]
- Patel, A.K.; Michaud, P.; de Baynast, H.; Grédiac, M.; Mathias, J.-D. Preparation of chitosan-based adhesives and assessment of their mechanical properties. J. Appl. Polym. Sci. 2013, 127, 3869–3876. [Google Scholar] [CrossRef]
- Yang, Q.; Dou, F.; Liang, B.; Shen, Q. Investigations of the effects of glyoxal cross-linking on the structure and properties of chitosan fiber. Carbohydr. Polym. 2005, 61, 393–398. [Google Scholar] [CrossRef]
- Monteiro, O.A.C.; Airoldi, C. Some studies of crosslinking chitosan–glutaraldehyde interaction in a homogeneous system. Int. J. Biol. Macromol. 1999, 26, 119–128. [Google Scholar] [CrossRef]
- Beppu, M.M.; Vieira, R.S.; Aimoli, C.G.; Santana, C.C. Crosslinking of chitosan membranes using glutaraldehyde: Effect on ion permeability and water absorption. J. Membr. Sci. 2007, 301, 126–130. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Yu, L.; Tong, Z.; Chen, L.; Liu, H.; Li, X. Thermal degradation and stability of starch under different processing conditions. Starch-Stärke 2013, 65, 48–60. [Google Scholar] [CrossRef]
- Liu, X.; Yu, L.; Liu, H.; Chen, L.; Li, L. Thermal decomposition of corn starch with different amylose/amylopectin ratios in open and sealed systems. Cereal Chem. 2009, 86, 383–385. [Google Scholar] [CrossRef]
- Ma, X.F.; Yu, J.G.; Wan, J.J. Urea and ethanolamine as a mixed plasticizer for thermoplastic starch. Carbohydr. Polym. 2006, 64, 267–273. [Google Scholar] [CrossRef]
- Zobel, H.F. Chapter IX—Gelatinization of Starch and Mechanical Properties of Starch Pastes. In Starch: Chemistry and Technology, 3rd ed.; BeMiller, J., Whistler, R., Eds.; Academic Press: London, UK, 2009. [Google Scholar]
- Arık Kibar, E.A.; Gönenç, İ.; Us, F. Modeling of retrogradation of waxy and normal corn starches. Int. J. Food Prop. 2011, 14, 954–967. [Google Scholar] [CrossRef]
- Cheetham, N.W.H.; Tao, L.P. Variation in crystalline type with amylose content in maize starch granules: An X-ray powder diffraction study. Carbohydr. Polym. 1998, 36, 277–284. [Google Scholar] [CrossRef]
- Zhang, C.-W.; Li, F.-Y.; Li, J.-F.; Wang, L.-M.; Xie, Q.; Xu, J.; Chen, S. A new biodegradable composite with open cell by combining modified starch and plant fibers. Mater. Des. 2017, 120, 222–229. [Google Scholar] [CrossRef]
- Van Soest, J.J.G.; Hulleman, S.H.D.; de Wit, D.; Vliegenthart, J.F.G. Crystallinity in starch bioplastics. Ind. Crop Prod. 1996, 5, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Shanks, R.; Kong, I. Thermoplastic starch. In Thermoplastic Elastomers; El-Sonbati, P.A., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Nafchi, A.M.; Moradpour, M.; Saeidi, M.; Alias, A.K. Thermoplastic starches: Properties, challenges, and prospects. Starch-Staerke 2013, 65, 61–72. [Google Scholar] [CrossRef]
- Roylance, D. Mechanics of Materials; Wiley: Hoboken, NJ, USA, 1996. [Google Scholar]
- Kvien, I.; Sugiyama, J.; Votrubec, M.; Oksman, K. Characterization of starch based nanocomposites. J. Mater. Sci. 2007, 42, 8163–8171. [Google Scholar] [CrossRef]
- Carvalho, A.J.F.; Curvelo, A.A.S.; Agnelli, J.A.M. Wood pulp reinforced thermoplastic starch composites. Int. J. Polym. Mater. Polym. Biomater. 2002, 51, 647–660. [Google Scholar] [CrossRef]
- Dufresne, A.; Vignon, M.R. Improvement of starch film performances using cellulose microfibrils. Macromolecules 1998, 31, 2693–2696. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paiva, D.; Pereira, A.M.; Pires, A.L.; Martins, J.; Carvalho, L.H.; Magalhães, F.D. Reinforcement of Thermoplastic Corn Starch with Crosslinked Starch/Chitosan Microparticles. Polymers 2018, 10, 985. https://doi.org/10.3390/polym10090985
Paiva D, Pereira AM, Pires AL, Martins J, Carvalho LH, Magalhães FD. Reinforcement of Thermoplastic Corn Starch with Crosslinked Starch/Chitosan Microparticles. Polymers. 2018; 10(9):985. https://doi.org/10.3390/polym10090985
Chicago/Turabian StylePaiva, Diana, André M. Pereira, Ana L. Pires, Jorge Martins, Luísa H. Carvalho, and Fernão D. Magalhães. 2018. "Reinforcement of Thermoplastic Corn Starch with Crosslinked Starch/Chitosan Microparticles" Polymers 10, no. 9: 985. https://doi.org/10.3390/polym10090985






