Phase Separation Driven On-Demand Debondable Waterborne Pressure-Sensitive Adhesives
Abstract
:1. Introduction
2. Experimental Section
2.1. Latex Synthesis
2.2. Adhesive Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Onusseit, H. The influence of adhesives on recycling. Resour. Conserv. Recycl. 2006, 46, 168–181. [Google Scholar] [CrossRef]
- Kirsten, C.; Ferencz, A.; Hirthammer, M. Detachable Adhesives. DE Patent Application No. 19904835A1, 10 August 2000. [Google Scholar]
- Kawate, K.; Kanki, T. Heat Debondable Adhesive Composition and Adhesion Structure. WO Patent Application No. 2000040648A1, 13 July 2000. [Google Scholar]
- Paul, C.W.; Carter, J.T.; Morrison, B.D. Hot Melt Adhesive for Packaging Applications. EP Patent Application No. 1847579A1, 24 Octobers 2007. [Google Scholar]
- Rule, J.D.; Lewandowski, K.M.; Determan, M.D. Debondable Adhesive Article. U.S. Patent Application No. 20100316845A1, 16 December 2010. [Google Scholar]
- Gurney, R.S.; Dupin, D.; Nunes, J.S.; Ouzineb, K.; Siband, E.; Asua, J.M.; Armes, S.P.; Keddie, J.L. Switching off the tackiness of a nanocomposite adhesive in 30 s via infrared sintering. ACS Appl. Mater. Interfaces 2012, 4, 5442–5452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, B.S.; Gurney, R.S.; Siband, E.; Dupin, D.; Keddie, J.L. Power Density Threshold for Switching Off the Tack Adhesion of Colloidal Nanocomposites. Macromol. Chem. Phys. 2014, 215, 998–1003. [Google Scholar] [CrossRef]
- Chivers, R.A. Easy removal of pressure sensitive adhesives for skin applications. Int. J. Adhes. Adhes. 2001, 21, 381–388. [Google Scholar] [CrossRef]
- Webster, I. The development of a pressure-sensitive adhesive for trauma-free removal. Int. J. Adhes. Adhes. 1999, 19, 29–34. [Google Scholar] [CrossRef]
- Boyne, J.M.; Millan, E.J.; Webster, I. Peeling performance of a novel light switchable pressure-sensitive adhesive. Int. J. Adhes. Adhes. 2001, 21, 49–53. [Google Scholar] [CrossRef]
- Trenor, S.R.; Long, T.E.; Love, B.J. Development of a Light-Deactivatable PSA Via Photodimerization. J. Adhes. 2005, 81, 213–229. [Google Scholar] [CrossRef]
- Lee, S.H.; You, R.; Yoon, Y.I.; Park, W.H. Preparation and characterization of acrylic pressure-sensitive adhesives based on UV and heat curing systems. Int. J. Adhes. Adhes. 2017, 75, 190–195. [Google Scholar] [CrossRef]
- Therriault, D.J.; Workinger, J.E. Water-inactivatable Pressure Sensitive Adhesive. U.S. Patent 5032637A, 16 July 1991. [Google Scholar]
- Inui, T.; Sato, E.; Matsumoto, A. Pressure-Sensitive Adhesion System Using Acrylate Block Copolymers in Response to Photoirradiation and Postbaking as the Dual External Stimuli for On-Demand Dismantling. ACS Appl. Mater. Interfaces 2012, 4, 2124–2132. [Google Scholar] [CrossRef] [PubMed]
- Yamanishi, K.; Sato, E.; Matsumoto, A. Precise Synthesis of Acrylic Block Copolymers and Application to On-Demand Dismantlable Adhesion Systems in Response to Photoirradiation and Postbaking. J. Photopolym. Sci. Technol. 2013, 26, 239–244. [Google Scholar] [CrossRef]
- Inui, T.; Yamanishi, K.; Sato, E.; Matsumoto, A. Organotellurium-Mediated Living Radical Polymerization (TERP) of Acrylates Using Ditelluride Compounds and Binary Azo Initiators for the Synthesis of High-Performance Adhesive Block Copolymers for On-Demand Dismantlable Adhesion. Macromolecules 2013, 46, 8111–8120. [Google Scholar] [CrossRef]
- Sato, E.; Tamura, H.; Matsumoto, A. Cohesive Force Change Induced by Polyperoxide Degradation for Application to Dismantable Adhesion. ACS Appl. Mater. Interfaces 2010, 2, 2594–2601. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Uto, N.; Sato, C.; Sakurai, H. Dismantlement Behavior and Strength of Dismantlable Adhesive Including Thermally Expansive Particles. Int. J. Adhes. Adhes. 2003, 23, 377–382. [Google Scholar] [CrossRef]
- Ishikawa, H.; Seto, K.; Shimotuma, S.; Kishi, N.; Sato, C. Bond Strength and Disbonding Behavior of Elastomer and Emulsion-Type Dismantlable Adhesives Used for Building Materials. Int. J. Adhes. Adhes. 2005, 25, 193–199. [Google Scholar] [CrossRef]
- Stewart, R.F. Skin-activated Temperature-sensitive Adhesive Assemblies. U.S. Patent 5156911A, 20 October 1992. [Google Scholar]
- Agirre, A.; Heras-Alarcón, C.D.L.; Wang, T.; Keddie, J.L.; Asua, J.M. Waterborne, semicrystalline, pressure-sensitive adhesives with temperature-responsiveness and optimum properties. ACS Appl. Mater. Interfaces 2010, 2, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Mehravar, E.; Gross, M.A.; Reck, B.; Leiza, J.R.; Asua, J.M. Importance of film morphology on the performance of thermo-responsive waterborne pressure sensitive adhesives. Eur. Polym. J. 2018, 98, 63–71. [Google Scholar] [CrossRef]
- Goikoetxea, M.; Reyes, Y.; De Las Heras Alarcon, C.; Minari, R.J.; Beristain, I.; Paulis, M.; Barandiaran, M.J.; Keddie, J.L.; Asua, J.M. Transformation of Waterborne Hybrid Polymer Particles into Films: Morphology Development and Modeling. Polymer 2012, 53, 1098–1108. [Google Scholar] [CrossRef] [Green Version]
- Mehravar, E.; Leiza, J.R.; Asua, J.M. Performance of latexes containing nano-sized crystalline domains formed by comb-like polymers. Polymer 2016, 96, 121–129. [Google Scholar] [CrossRef]
- Mehravar, E.; Leswin, J.; Reck, B.; Leiza, J.R.; Asua, J.M. Waterborne paints containing nano-sized crystalline domains formed by comb-like polymers. Prog. Org. Coat. 2017, 106, 11–19. [Google Scholar] [CrossRef]
- Lestriez, B.; Lakrout, H.; Chiche, A.; Roos, A.; Creton, C. Probe tack tests as a characterization tool in pressure-sensitive-adhesives. In Proceedings of the PSTC Technical Seminar TECH XXIV, Orlando, FL, USA, 2–4 May 2001. [Google Scholar]
- FTM 1 Peel adhesion (180°) at 300 mm per minute. In FINAT Technical Handbook, 6th ed.; FINAT: The Hague, The Netherlands, 2001.
- Plate, N.A.; Shibaev, V.P. Comb-like polymers. Structure and properties. J. Polym. Sci. Macromol. Rev. 1974, 8, 117–253. [Google Scholar] [CrossRef]
- Cook, A.G.; Inkster, R.T.; Martinez-Felipe, A.; Ribes-Greus, A.; Hamley, I.W.; Imrie, C.T. Synthesis and phase behaviour of a homologous series of polymethacrylate-based side-chain liquid crystal polymers. Eur. Polym. J. 2012, 48, 821–829. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Ling, A.; Zhang, H.L. Synthesis and phase behaviors of side-chain liquid-crystalline polymers containing azobenzene mesogen with the different length alkyl tail. J. Polym. Sci. Part. A Polym. Chem. 2013, 51, 2759–2768. [Google Scholar] [CrossRef]
- Mehravar, E.; Iturrospe, A.; Arbe, A.; Asua, J.M.; Leiza, J.R. Phase behavior of side-chain liquid-crystalline polymers containing biphenyl mesogens with different spacer lengths synthesized via miniemulsion polymerization. Polym. Chem. 2016, 7, 4736–4750. [Google Scholar] [CrossRef]
- Mehravar, E.; Iturrospe, A.; Arbe, A.; Leiza, J.R.; Asua, J.M. Acrylic-based composite latexes containing nano-sized liquid crystalline domains. Polymer 2017, 108, 288–300. [Google Scholar] [CrossRef]

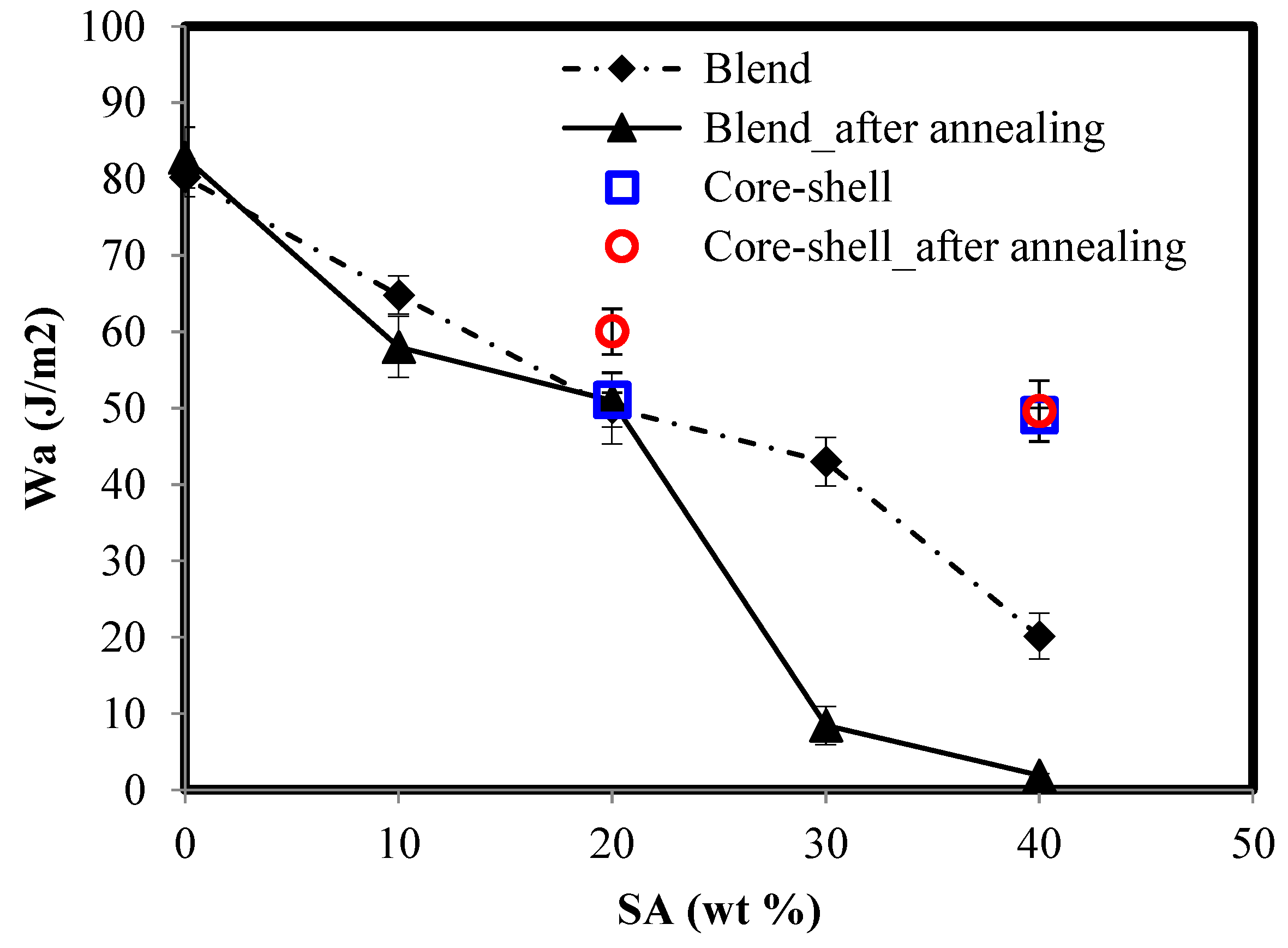


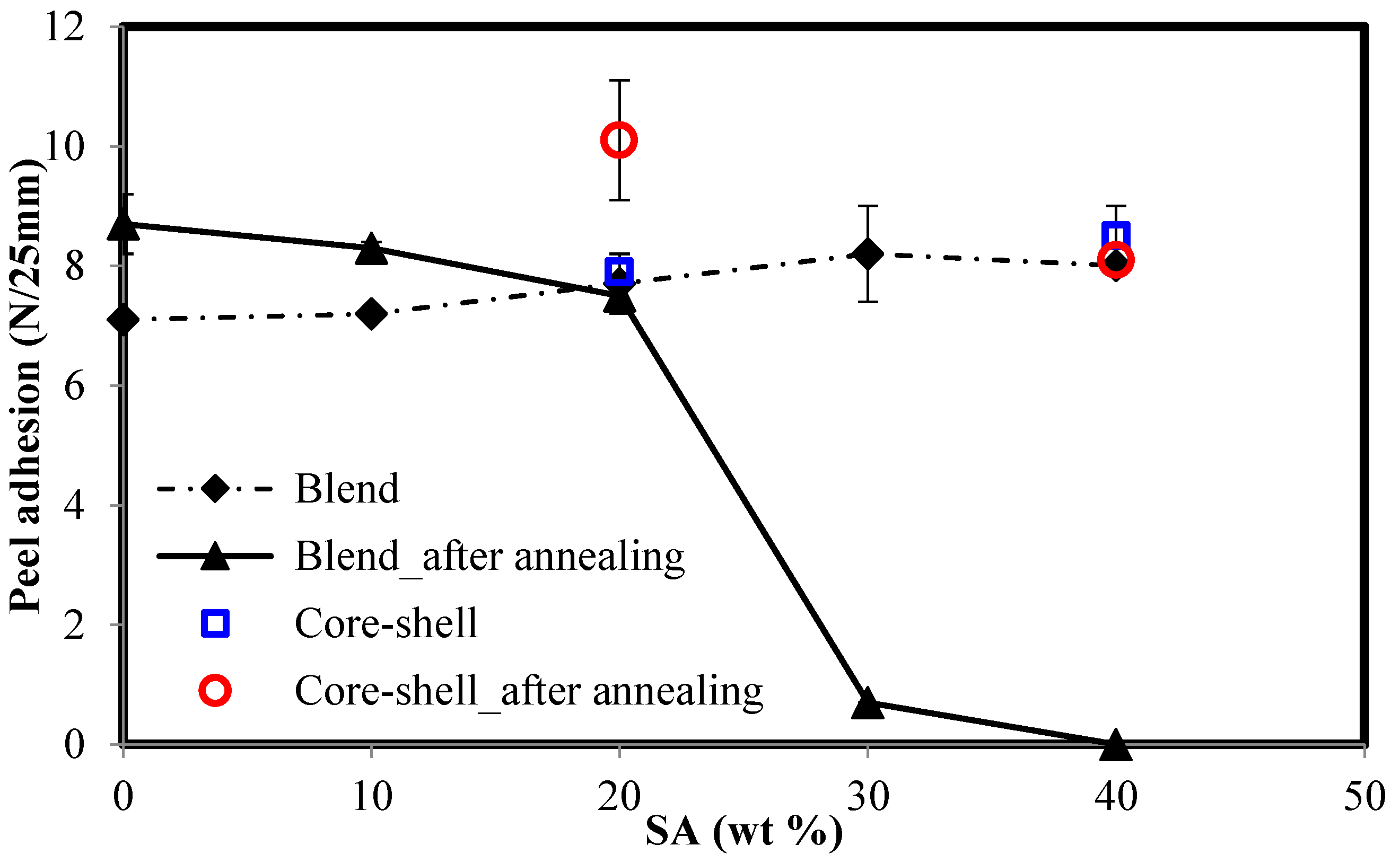


| Latex | SA/SC(M)A a (wt/wt) | Comment |
|---|---|---|
| 1 | 100/0 | batch miniemulsion |
| 2 | 0/100 | seeded semicountinuous emulsion copolymerization |
| 3 | 10/90 | Blends of latexes 1 and 2 |
| 4 | 20/80 | |
| 5 | 30/70 | |
| 6 | 40/60 | |
| 7 | 20/80 | Core-shell latexes |
| 8 | 40/60 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehravar, E.; Gross, M.A.; Leal, G.P.; Reck, B.; Leiza, J.R.; Asua, J.M. Phase Separation Driven On-Demand Debondable Waterborne Pressure-Sensitive Adhesives. Polymers 2018, 10, 975. https://doi.org/10.3390/polym10090975
Mehravar E, Gross MA, Leal GP, Reck B, Leiza JR, Asua JM. Phase Separation Driven On-Demand Debondable Waterborne Pressure-Sensitive Adhesives. Polymers. 2018; 10(9):975. https://doi.org/10.3390/polym10090975
Chicago/Turabian StyleMehravar, Ehsan, Michael A. Gross, Gracia Patricia Leal, Bernd Reck, Jose R. Leiza, and José M. Asua. 2018. "Phase Separation Driven On-Demand Debondable Waterborne Pressure-Sensitive Adhesives" Polymers 10, no. 9: 975. https://doi.org/10.3390/polym10090975





