Crosslinked-Polymer Brushes with Switchable Capture and Release Capabilities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
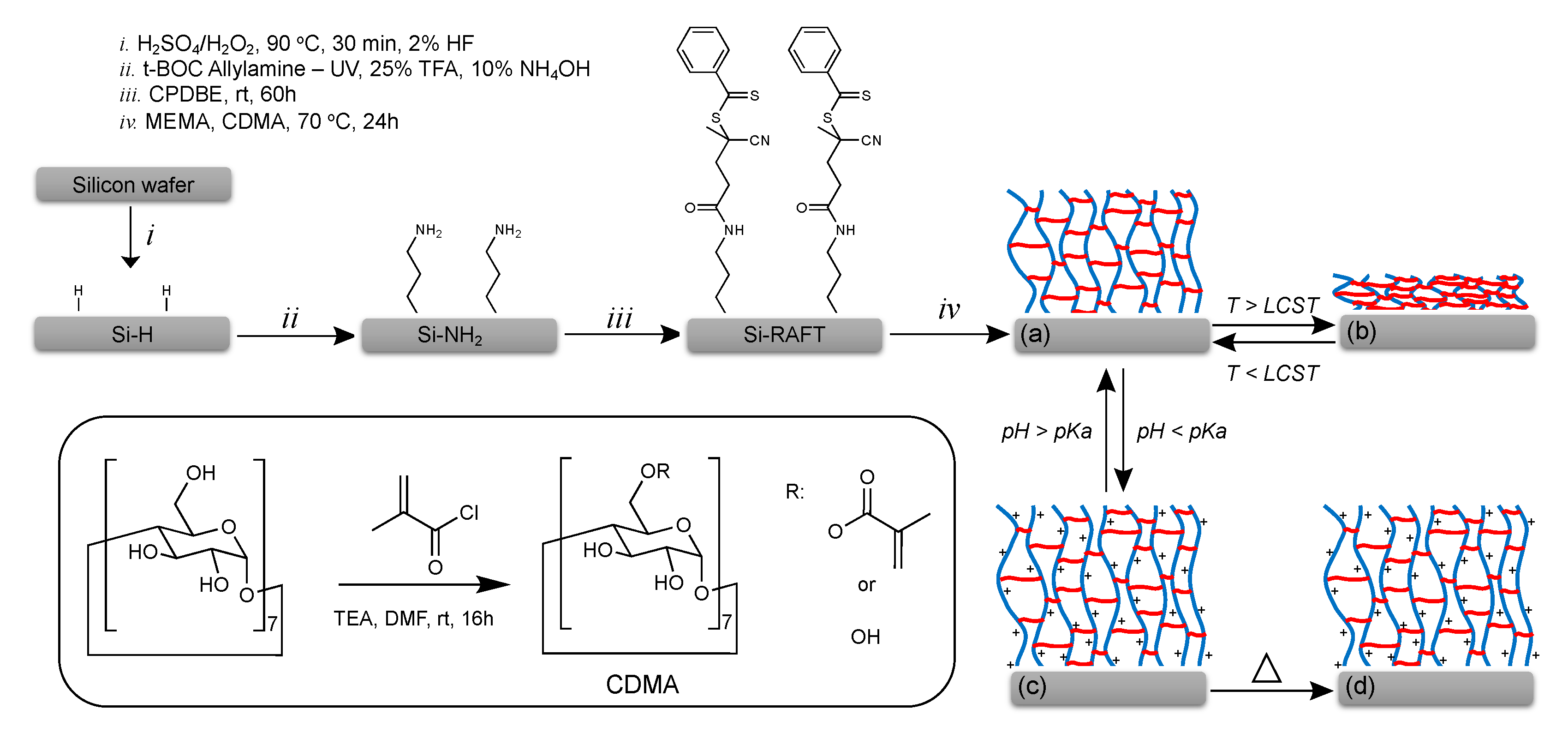
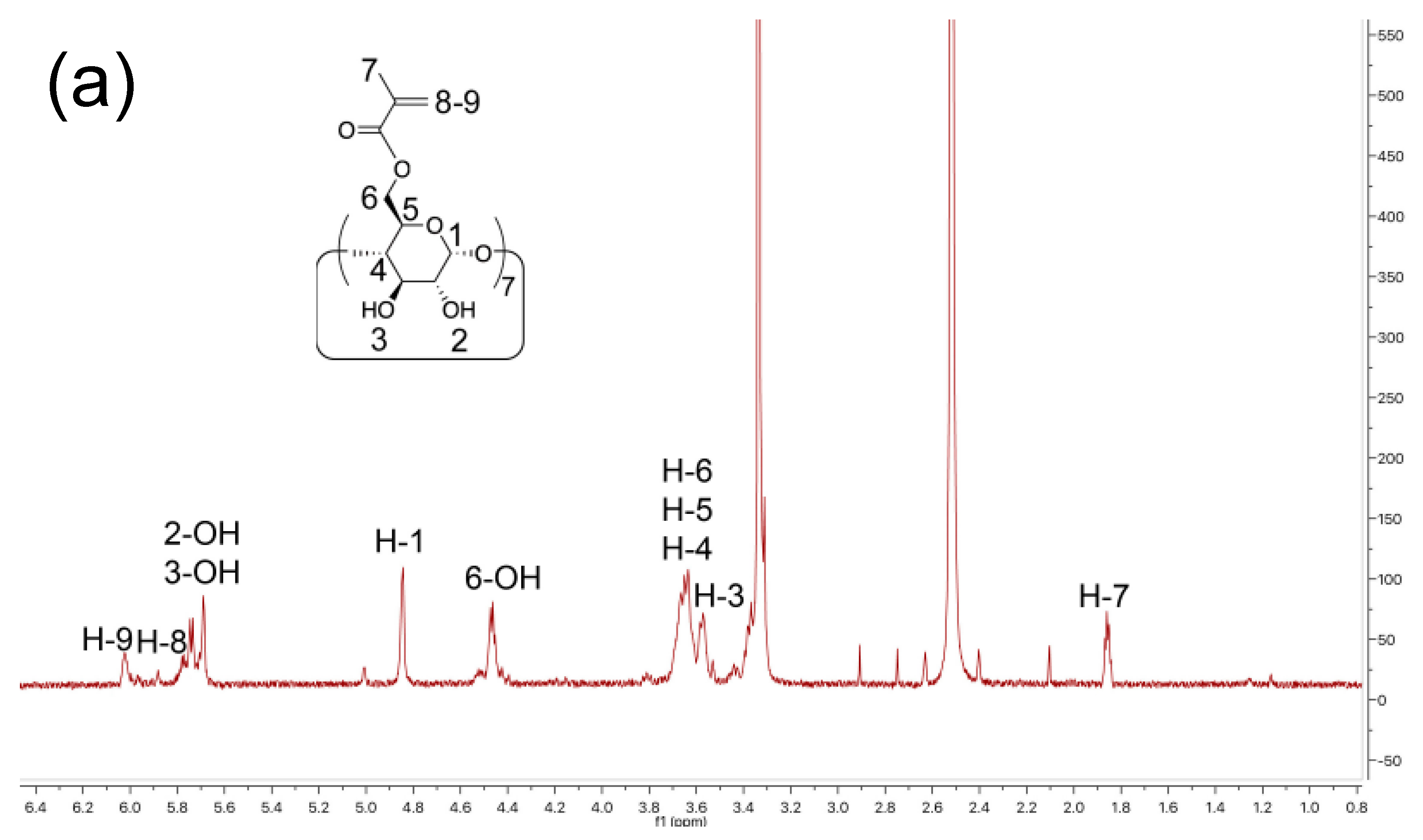
2.2. Synthesis of Cyclodextrin Methacrylate (CDMA)
2.3. Surface-Initiated Polymerization
2.4. Measurement of Methylene Blue Capture and Release
2.5. Characterization
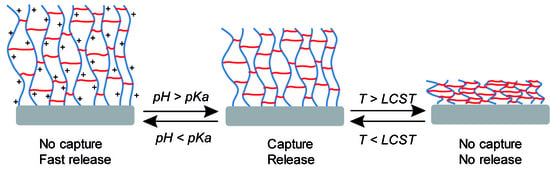
3. Results and Discussions
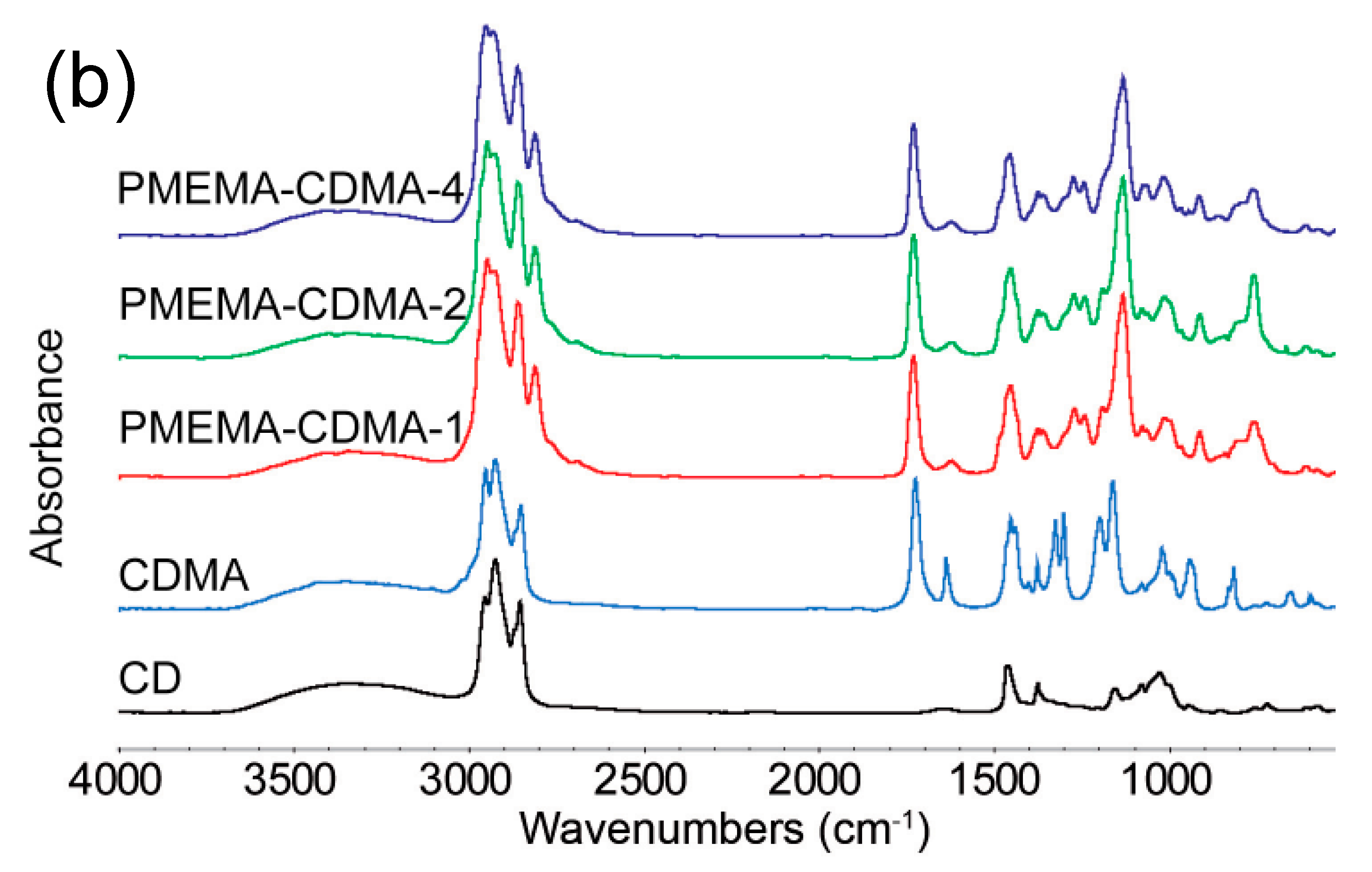
3.1. Surface Modification and Characterization
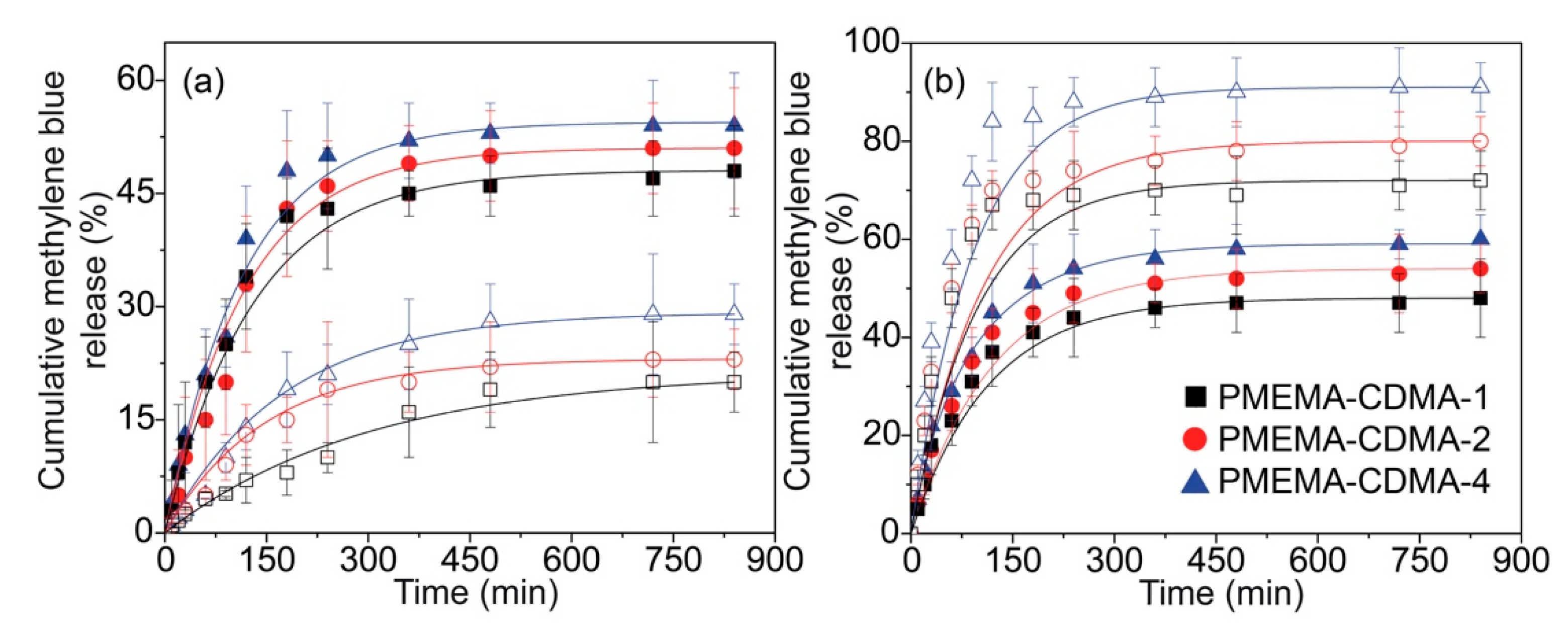
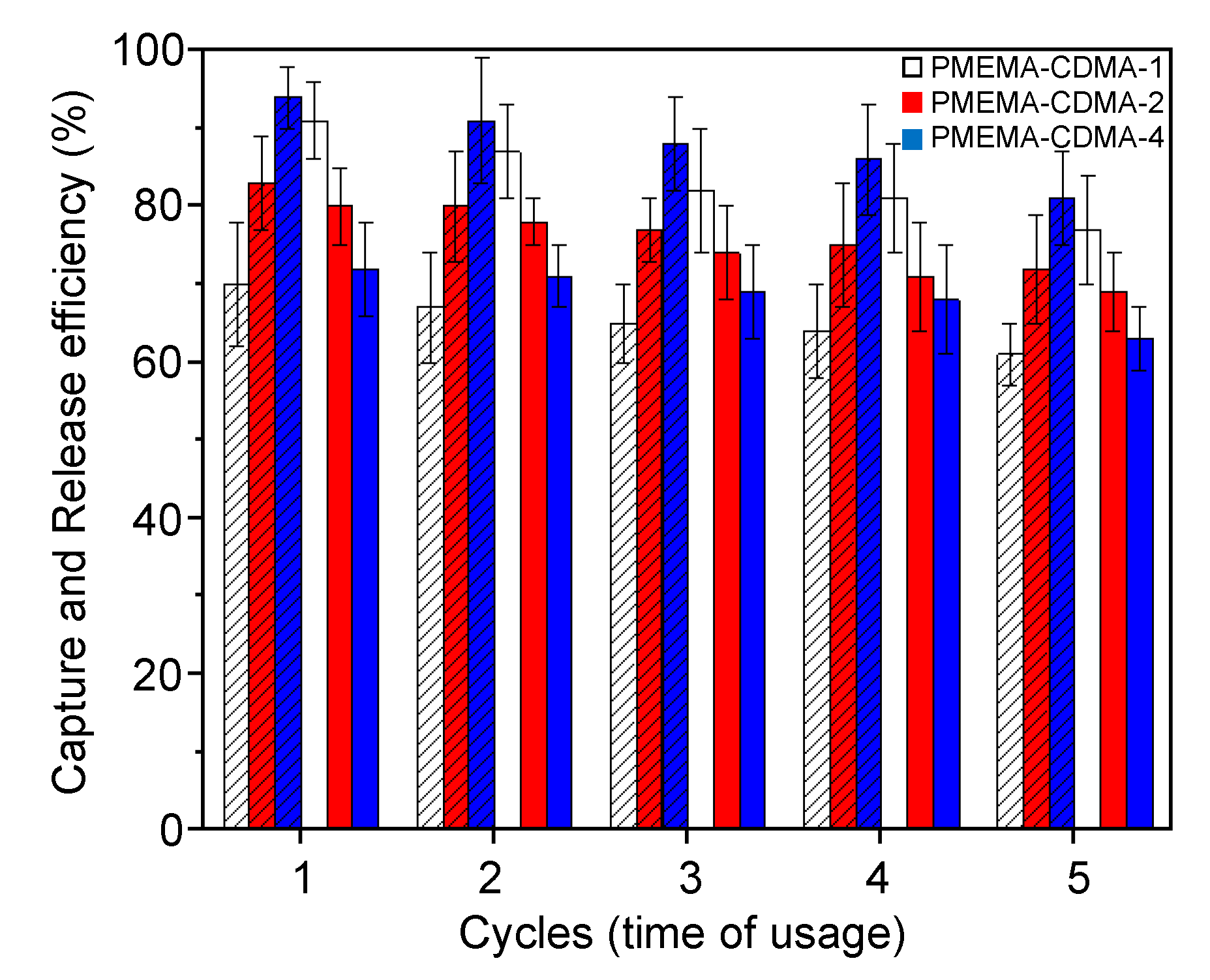
3.2. Reversible Capture and Release
4. Conclusions
Funding
Conflicts of Interest
References
- Lokuge, I.; Wang, X.; Bohn, P.W. Temperature-controlled flow switching in nanocapillary array membranes mediated by poly(N-isopropylacrylamide) polymer brushes grafted by atom transfer radical polymerization. Langmuir 2007, 23, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Bruening, M.L.; Dotzauer, D.M.; Jain, P.; Ouyang, L.; Baker, G.L. Creation of functional membranes using polyelectrolyte multilayers and polymer brushes. Langmuir 2008, 24, 7663–7673. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, F.; Chen, C.; Zhu, H.; Pan, C.; Wang, Y. Controlled protein adsorption on PMOXA/PAA based coatings by thermally induced immobilization. Appl. Surf. Sci. 2018, 439, 148–159. [Google Scholar] [CrossRef]
- Shen, Y.; Li, G.; Ma, Y.; Yu, D.; Sun, J.; Li, Z. Smart surfaces based on thermo-responsive polymer brushes prepared from L-alanine derivatives for cell capture and release. Soft Matter 2015, 11, 7502–7506. [Google Scholar] [CrossRef] [PubMed]
- Psarra, E.; König, U.; Ueda, Y.; Bellmann, C.; Janke, A.; Bittrich, E.; Eichhorn, K.-J.; Uhlmann, P. Nanostructured biointerfaces: nanoarchitectonics of thermoresponsive polymer brushes impact protein adsorption and cell adhesion. ACS Appl. Mater. Interfaces 2015, 7, 12516–12529. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Brash, J.L.; Zhu, S. Non-biofouling materials prepared by atom transfer radical polymerization grafting of 2-methacryloloxyethyl phosphorylcholine: Separate effects of graft density and chain length on protein repulsion. Biomaterials 2006, 27, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Ayres, N. Polymer brushes: Applications in biomaterials and nanotechnology. Polym. Chem. 2010, 1, 769–777. [Google Scholar] [CrossRef]
- Zhao, B.; Brittain, W. Polymer brushes: Surface-immobilized macromolecules. J. Prog. Polym. Sci. 2000, 25, 677–710. [Google Scholar] [CrossRef]
- Li, C.; Benicewicz, B.C. Synthesis of well-defined polymer brushes grafted onto silica nanoparticles via surface reversible addition-fragmentation chain transfer polymerization. Macromolecules 2005, 38, 5929–5936. [Google Scholar] [CrossRef]
- Demirci, S.; Caykara, T. Controlled grafting of cationic poly[(ar-vinylbenzyl)trimethylammonium chloride] on hydrogen-terminated silicon substrate by surface-initiated RAFT polymerization. React. Funct. Polym. 2012, 72, 588–595. [Google Scholar] [CrossRef]
- Huang, C.-J.; Chen, Y.-S.; Chang, Y. Counterion-activated nanoactuator: Reversibly switchable killing/releasing bacteria on polycation brushes. ACS Appl. Mater. Interfaces 2015, 7, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- Demirci, S.; Kinali-Demirci, S.; Caykara, T. Stimuli-responsive diblock copolymer brushes via combination of “click chemistry” and living radical polymerization. J. Polym. Sci. Part A 2013, 51, 2677–2685. [Google Scholar] [CrossRef]
- Suchao-in, N.; Chirachanchai, S.; Perrier, S. pH- and thermo-multi-responsive fluorescent micelles from block copolymers via reversible addition fragmentation chain transfer (RAFT) polymerization. Polymer 2009, 50, 4151–4158. [Google Scholar] [CrossRef]
- Grubbs, J.B.; Arnold, R.M.; Roy, A.; Brooks, K.; Bilbrey, J.A.; Gao, J.; Locklin, J. Degradable polycaprolactone and polylactide homopolymer and block copolymer brushes prepared by surface-initiated polymerization with triazabicyclodecene and zirconium catalysts. Langmuir 2015, 31, 10183–10189. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B. Synthesis of binary mixed homopolymer brushes by combining atom transfer radical polymerization and nitroxide-mediated radical polymerization. Polymer 2003, 44, 4079–4083. [Google Scholar] [CrossRef]
- Estillore, N.C.; Advincula, R.C. Stimuli-responsive binary mixed polymer brushes and free-standing films by LbL-SIP. Langmuir 2011, 27, 5997–6008. [Google Scholar] [CrossRef] [PubMed]
- Ionov, L.; Minko, S. Mixed polymer brushes with locking switching. ACS Appl. Mater. Interfaces 2012, 4, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Turan, E.; Demirci, S.; Caykara, T. Thermo-and pH-induced phase transitions and network parameters of poly(N-isopropylacrylamide-co-2-acrylamido-2-methyl-propanosulfonic acid) hydrogels. J. Polym. Sci. Part B 2008, 46, 1713–1724. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Dehghani, E.S.; Spencer, N.D.; Ramakrishna, S.N.; Benetti, M. Crosslinking polymer brushes with ethylene glycol-containing segments: Influence on physicochemical and antifouling properties. Langmuir 2016, 32, 10317–10327. [Google Scholar] [CrossRef] [PubMed]
- Denghani, E.S.; Aghion, S.; Anwand, W.; Consolat, G.; Ferragut, R.; Panzarasa, G. Investigating the structure of crosslinked polymer brushes (brush-gels) by means of positron annihilation spectroscopy. Eur. Polym. J. 2018, 99, 415–421. [Google Scholar] [CrossRef]
- Loveless, D.M.; Abu-Lail, N.I.; Kaholek, M.; Zauscher, S.; Craig, S.L. Reversibly cross-linked surface-grafted polymer brushes. Angew. Chem. Int. Ed. 2006, 45, 7812–7814. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Hedges, A. Industrial applications of cyclodextrins. Chem. Rev. 1998, 98, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Sharma, R.; Banerjee, U. Biotechnological applications of cyclodextrins. Biotechnol. Adv. 2002, 20, 341–359. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J. Cyclodextrins as food ingredients. Trends Food Sci. Technol. 2004, 15, 137–142. [Google Scholar] [CrossRef]
- Buschmann, H.-J.; Knittel, D.; Schollmeyer, E. New textile applications of cyclodextrins. J. Inclusion Phenom. Macrocyclic Chem. 2001, 40, 169–172. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Cyclodextrins as functional excipients: methods to enhance complexation efficiency. J. Pharm. Sci. 2012, 101, 3019–3032. [Google Scholar] [CrossRef] [PubMed]
- De Manakker, F.V.; Vermonden, T.; van Nostrum, C.F.; Hennink, W.E. Cyclodextrin-based polymeric materials: Synthesis, properties, and pharmaceutical/biomedical applications. Biomacromolecules 2009, 10, 3157–3175. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chen, G.-J.; Wang, Y.; Yuan, L.; Zhang, Q.; Haddleton, D.M.; Chen, H. Control the wettability of poly(N-isopropylacrylamide-co-1-adamantan-1-ylmethyl acrylate) modified surfaces: The more Ada, the bigger impact? Langmuir 2013, 29, 14188–14195. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Liu, X.; Shi, W.; Cheng, C.; He, C.; Zhao, C. Light-triggered switching of reversible and alterable biofunctionality via β-cyclodextrin/azobenzene-based host-guest interaction. ACS Macro Lett. 2014, 3, 1130–1133. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, L.; Tu, Q.; Wang, X.; Liu, R.; Li, L.; Wang, J. C.; Liu, W.; Xu, J.; Wang, J. Fabrication of reversible poly(dimethylsiloxane) surfaces via host-guest chemistry and their repeated utilization in cardiac biomarker analysis. Anal. Chem. 2011, 83, 9651–9659. [Google Scholar] [CrossRef] [PubMed]
- Kettel, M.J.; Schaefer, K.; Pich, A.; Moeller, M. Functional PMMA nanogels by cross-linking with cyclodextrin methacrylate. Polymer 2016, 86, 176–188. [Google Scholar] [CrossRef]
- Demirci, S.; Caykara, T. RAFT-mediated synthesis of cationic poly[(ar-vinylbenzyl)trimethyl ammonium chloride] brushes for quantitative DNA immobilization. Mater. Sci. Eng. C 2013, 33, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Demirci, S.; Kinali-Demirci, S.; Caykara, T. Novel pH-responsive mixed-charge copolymer brushes based on carboxylic acid and quaternary amine monomers. J. Polym. Sci. Part A 2013, 51, 1612–1619. [Google Scholar] [CrossRef]
- Tarbell, D.S.; Yamamoto, Y.; Pope, B.M. New method to prepare n-t-butoxycarbonyl derivatives and the corresponding sulfur analogs from di-t-butyl dicarbonate or di-t-butyl dithiol dicarbonates and amino acids. Proc. Natl. Acad. Sci. USA 1972, 69, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Plunkett, K.N.; Zhu, X.; Moore, J.S.; Leckband, D.E. PNIPAM chain collapse depends on the molecular weight and grafting density. Langmuir 2006, 22, 4259–4266. [Google Scholar] [CrossRef] [PubMed]
- Demirci, S.; Caykra, T. High density cationic polymer brushes from combined ‘‘click chemistry’’ and raft-mediated polymerization. J. Polym. Sci. Part A 2012, 50, 2999–3007. [Google Scholar] [CrossRef]
- Erel, I.; Karahan, H.E.; Tuncer, C.; Bütün, V.; Demirel, A.L. Hydrogen-bonded multilayers of micelles of a dually responsive dicationic block copolymer. Soft Matter 2012, 8, 827–836. [Google Scholar] [CrossRef]
- Demirci, S.; Kinali-Demirci, S.; Jiang, S. A switchable polymer brush system for antifouling and controlled detection. Chem. Comm. 2017, 53, 3713–3716. [Google Scholar] [CrossRef] [PubMed]


| Sample | O 1s | N 1s | C 1s | S 2p | Si 2p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| O–C=O | O–C–O | C–O | C–N | C–(C–H) | S–C | S=C | |||||
| Si–NH2 | Energy (eV) Conc. (%) | - - | 400.0 19.7 | - 64.1 | - - | - - | 285.7 | 285.0 | - - | - - | 99.1 16.2 |
| Si–RAFT | Energy (eV) Conc. (%) | 532.6 4.3 | 400.1 12.5 | 289.2 66.7 | - | - | 285.7 | 285.0 | 163.8 7.3 | 162.4 | 99.1 9.2 |
| PMEMA–CDMA–1 | Energy (eV) Conc. (%) | 532.1 21.9 | 400.1 7.1 | 289.2 69.7 | 287.6 | 286.5 | 285.7 | 285.0 | 163.9 1.3 | 162.3 | - - |
| PMEMA–CDMA–2 | Energy (eV) Conc. (%) | 532.2 27.7 | 400.1 5.8 | 289.3 65.6 | 287.8 | 286.7 | 285.3 | 285.0 | 163.7 0.9 | 162.4 | - - |
| PMEMA–CDMA–4 | Energy (eV) Conc. (%) | 532.1 30.7 | 400.2 4.4 | 289.1 64.1 | 287.9 | 286.6 | 285.5 | 285.0 | 163.9 0.8 | 162.2 | - - |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirci, S. Crosslinked-Polymer Brushes with Switchable Capture and Release Capabilities. Polymers 2018, 10, 956. https://doi.org/10.3390/polym10090956
Demirci S. Crosslinked-Polymer Brushes with Switchable Capture and Release Capabilities. Polymers. 2018; 10(9):956. https://doi.org/10.3390/polym10090956
Chicago/Turabian StyleDemirci, Serkan. 2018. "Crosslinked-Polymer Brushes with Switchable Capture and Release Capabilities" Polymers 10, no. 9: 956. https://doi.org/10.3390/polym10090956