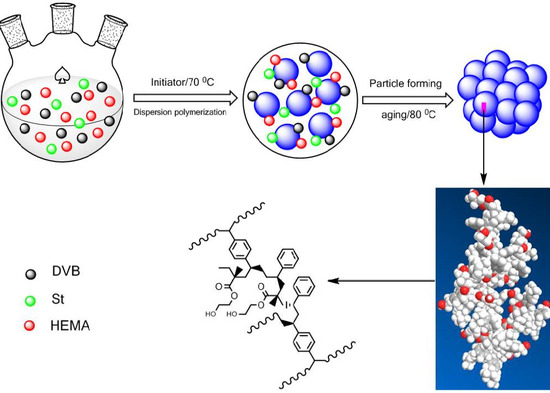
Feasibility Study on the Design and Synthesis of Functional Porous Organic Polymers with Tunable Pore Structure as Metallocene Catalyst Supports
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Functionalized POP Supports
2.3. Catalyst Immobilization
2.4. Characterization
3. Results and Discussion
3.1. Preparation of Poly(divinylbenzene) (PDVB)-Based Functionalized Porous Organic Polymers
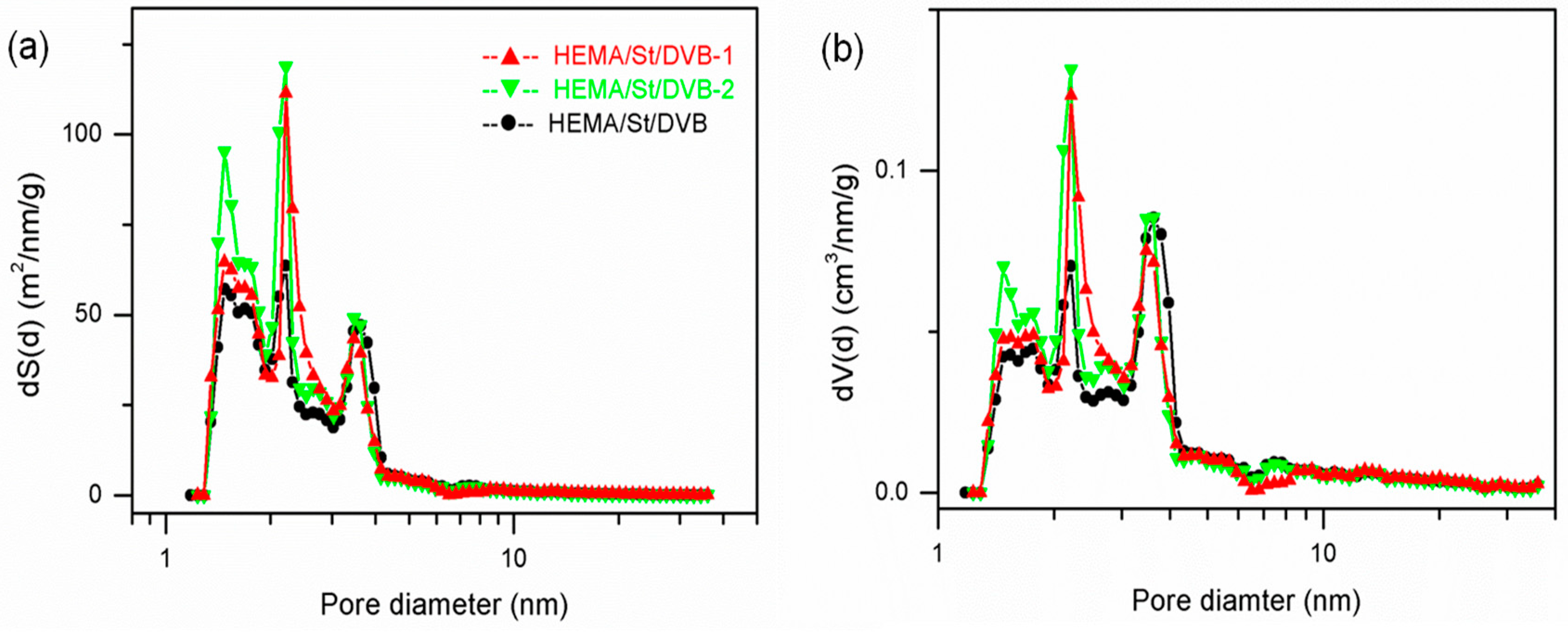
3.2. The Influence of Functional Comonomers on the Pore Structure of POPs
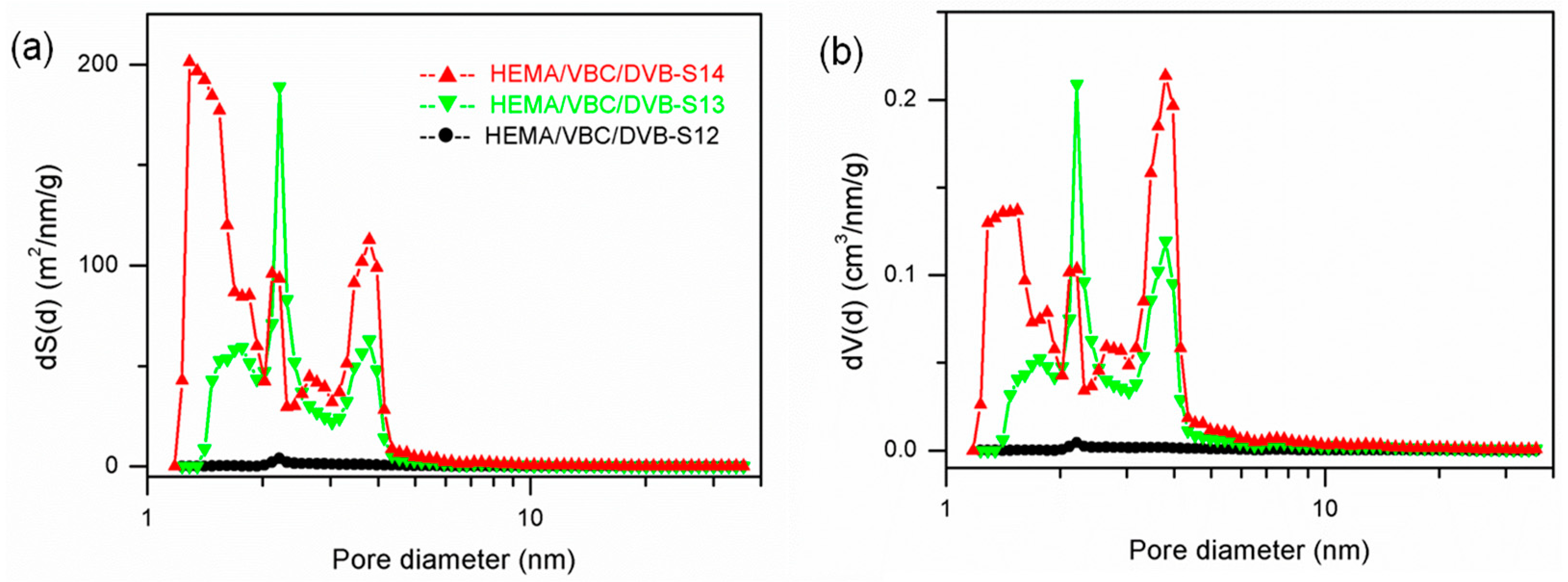
3.3. The Synthesis of PDVB-Based POPs with Dual Functional Comonomers
3.4. The Influence of a Template Agent on the Pore Structure of POPs
3.5. Cross-Linking Degree and Solvent on the Pore Structure of POPs
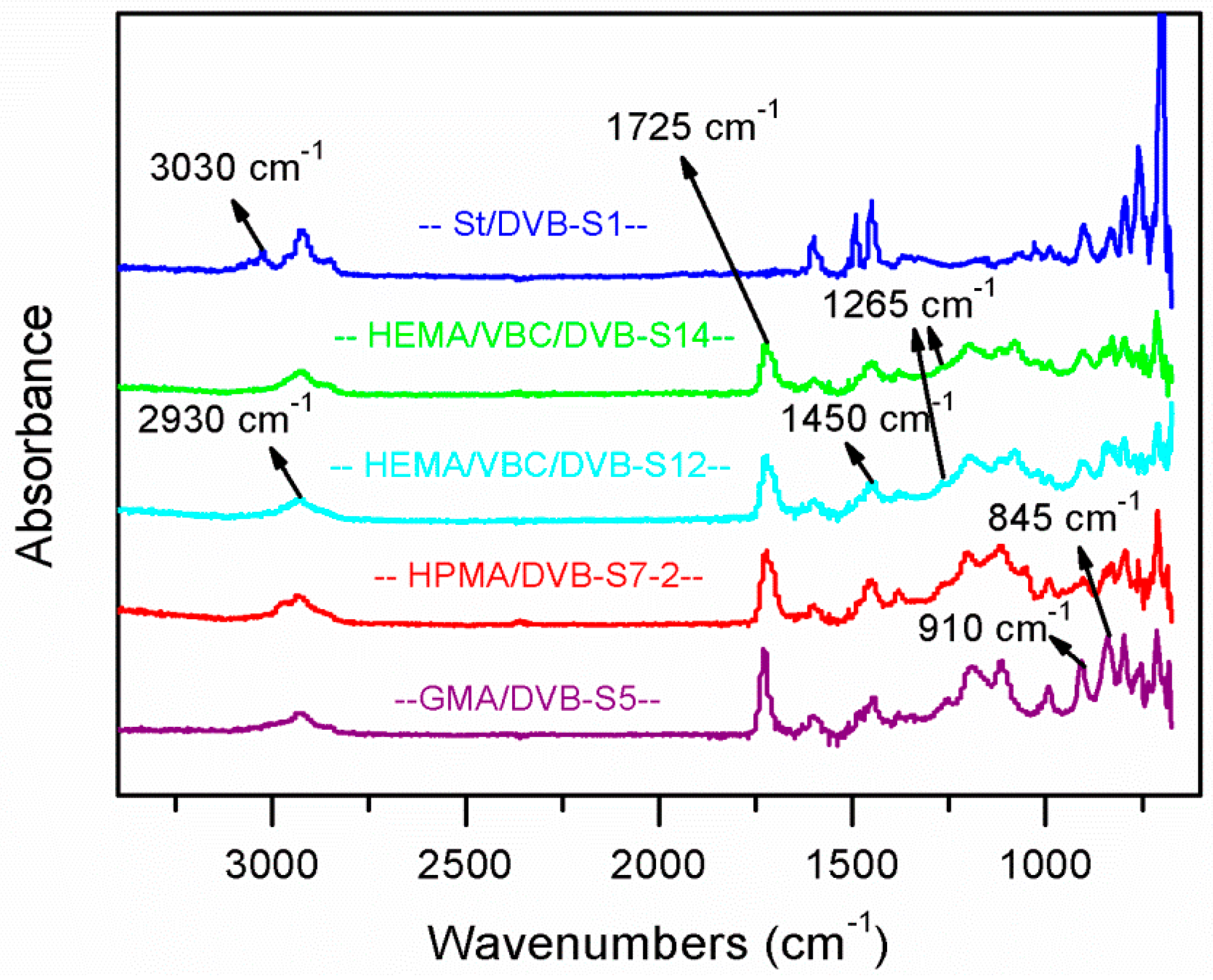
3.6. IR Analysis
3.7. Bulk Density and Surface Morphology of the POPs
3.8. Ethylene Polymerization of Supported Metallocene Catalysts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, X.-Y.; Chen, L.-H.; Li, Y.; Rooke, J.C.; Sanchez, C.; Su, B.-L. Hierarchically porous materials: Synthesis strategies and structure design. Chem. Soc. Rev. 2017, 46, 481–558. [Google Scholar] [CrossRef] [PubMed]
- Mondal, J.; Kundu, S.K.; Singuru, W.K.H.; Ng, R.; Borah, P.; Hirao, H.; Zhao, Y.; Bhaumik, A. Fabrication of ruthenium nanoparticles in porous organic polymers: Towards advanced heterogeneous catalytic nanoreactors. Chem. Eur. J. 2015, 21, 19016–19027. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Dai, Z.; Meng, X.; Xiao, F.-S. Porous polymer catalysts with hierarchical structures. Chem. Soc. Rev. 2015, 44, 6018–6034. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Shin, J.; Meng, Y.S.; Adelhardt, M.; Sutter, J.; Meyer, K.; Cohen, S.M. Reusable oxidation catalysis using metal-monocatecholato species in a robust metal–organic framework. J. Am. Chem. Soc. 2014, 136, 4965–4973. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lin, R.-B.; Krishna, R.; Wang, X.; Lin, B.; Wu, H.; Li, J.; Zhou, W.; Chen, B. Flexible–robust metal–organic framework for efficient removal of propyne from propylene. J. Am. Chem. Soc. 2017, 139, 7733–7736. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.; Cooper, A.I.; Adams, D.J. Nanoporous organic polymer networks. Prog. Polym. Sci. 2012, 37, 530–563. [Google Scholar] [CrossRef]
- Bildirir, H.; Osken, I.; Ozturk, T.; Thomas, A. Reversible doping of a dithienothiophene-based conjugated microporous polymer. Chem. Eur. J. 2015, 21, 9306–9311. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.; Bhaumik, A. Highly porous organic polymers bearing tertiary amine group and their exceptionally high CO2 uptake capacities. J. Solid State Chem. 2015, 222, 7–11. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.O.; Snurr, R.Q.; O’Keeffe, M.; Kim, J.; et al. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Ahn, S.; Yi, J.; Hupp, J.T.; Notestein, J.M.; Farha, O.K.; Lee, S.J. Ni(II) complex on a bispyridine-based porous organic polymer as a heterogeneous catalyst for ethylene oligomerization. Catal. Sci. Technol. 2017, 7, 4351–4354. [Google Scholar] [CrossRef]
- Ji, P.; Solomon, J.B.; Lin, Z.; Johnson, A.; Jordan, R.F.; Lin, W. Transformation of metal–organic framework secondary building units into hexanuclear Zr-alkyl catalysts for ethylene polymerization. J. Am. Chem. Soc. 2017, 139, 11325–11328. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, R.; Zhu, B.; Li, Y.; Han, X. Metal oxide as a template in the preparation of porous poly(2-hydroxyethylmethylacrylate-co-divinylbenzene) particles as a metallocene catalyst support. RSC Adv. 2016, 6, 52464–52474. [Google Scholar] [CrossRef]
- Damiani, D.E.; Ferreia, M.L.; Santos, J.H.Z.; Belelli, P.G.; Vayá, V.I. Influence of acidic support in metallocene catalysts for ethylene polymerization. J. Catal. 2001, 204, 1–10. [Google Scholar]
- Severn, J.R.; Chadwick, J.C.; Duchateau, R.; Friederichs, N. “Bound but not gagged”—Immobilizing single-site α-olefin polymerization catalysts. Chem. Rev. 2005, 105, 4073–4147. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Xu, K.; Feng, L.; Wang, L.; Yuan, Y. A novel zirconocene/ultradispersed diamond black powder supported catalytic system for ethylene polymerization. Eur. Polym. J. 2002, 38, 2125–2128. [Google Scholar]
- Sacchi, M.C.; Zucchi, D.; Tritto, I.; Locatelli, P. Silica-supported metallocenes: Stereochemical comparison between homogeneous and heterogeneous catalysis. Macromol. Rapid Commun. 1995, 16, 581–590. [Google Scholar] [CrossRef]
- Roscoe, S.B.; Fréchet, J.M.; Walzer, J.F.; Dias, A.J. Polyolefin spheres from metallocenes supported on noninteracting polystyrene. Science 1998, 280, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Nishida, H.; Uozumi, T.; Arai, T.; Soga, K. Polystyrene-supported metallocene catalysts for olefin polymerizations. Macromol. Rapid Commun. 1995, 16, 821–830. [Google Scholar] [CrossRef]
- Hong, S.C.; Teranishi, T.; Soga, K. Investigation on the polymer particle growth in ethylene polymerization with PS beads supported rac-Ph2Si(Ind)2ZrCl2 catalyst. Polymer 1998, 39, 7153–7157. [Google Scholar] [CrossRef]
- Kaur, P.; Hupp, J.T.; Nguyen, S.T. Porous organic polymers in catalysis: Opportunities and challenges. ACS Catal. 2011, 1, 819–835. [Google Scholar] [CrossRef]
- Lin, Z.; Lu, J.; Li, L.; Li, H.-F.; Cao, R. Defect porous organic frameworks (dPOFs) as a platform for chiral organocatalysis. J. Catal. 2017, 355, 131–138. [Google Scholar] [CrossRef]
- Zhang, Y.; Riduan, S.N. Functional porous organic polymers for heterogeneous catalysis. Chem. Soc. Rev. 2012, 41, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Diercks, C.S.; Zhang, Y.-B.; Kornienko, N.; Nichols, E.M.; Zhao, Y.; Paris, A.R.; Kim, D.; Yang, P.; Yaghi, O.M.; et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 2015, 349, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, Y.; Zhao, Y.; Sun, X.; Gandara, F.; Furukawa, H.; Liu, Z.; Zhu, H.; Zhu, C.; Suenaga, K.; et al. Weaving of organic threads into a crystalline covalent organic framework. Science 2016, 351, 365–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozhko, E.; Bavykina, A.; Osadchii, D.; Makkee, M.; Gascon, J. Covalent organic frameworks as supports for a molecular Ni based ethylene oligomerization catalyst for the synthesis of long chain olefins. J. Catal. 2017, 345, 270–280. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Ren, H.; Zhu, G. Topology-directed design of porous organic frameworks and their advanced applications. Chem. Commun. 2013, 49, 3925–3936. [Google Scholar] [CrossRef] [PubMed]
- Waller, P.J.; Gandara, F.; Yaghi, O.M. Chemistry of covalent organic frameworks. Acc. Chem. Res. 2015, 45, 3053–3063. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Krishna, R.; Jiang, D. Tailor-made pore surface engineering in covalent organic frameworks: Systematic functionalization for performance screening. J. Am. Chem. Soc. 2015, 137, 7079–7082. [Google Scholar] [CrossRef] [PubMed]
- Klet, R.C.; Tussupbayev, S.; Borycz, J.; Gallagher, J.R.; Stalzer, M.M.; Miller, J.T.; Gagliardi, L.; Hupp, J.T.; Marks, T.J.; Cramer, C.J.; et al. Single-site organozirconium catalyst embedded in a metal–organic framework. J. Am. Chem. Soc. 2015, 137, 15680–15683. [Google Scholar] [CrossRef] [PubMed]
- Comito, R.J.; Wu, Z.; Zhang, G.; Lawrence, J.A., III; Korzynski, M.D.; Kehl, J.A.; Miller, J.T.; Dinca, M. Stabilized vanadium catalyst for olefin polymerization by site isolation in a metal–organic framework. Angew. Chem. Int. Ed. 2018, 57, 8135–8139. [Google Scholar] [CrossRef] [PubMed]
- Taddei, M. When defects turn into virtues: The curious case of zirconium-based metal-organic frameworks. Coord. Chem. Rev. 2017, 343, 1–24. [Google Scholar] [CrossRef]
- Budd, P.M.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E. Polymers of intrinsic microporosity (PIMs): Robust, solution-processable, organic nanoporous materials. Chem. Commun. 2004, 4, 230–231. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-X.; Su, F.; Trewin, A.; Wood, C.D.; Campbell, N.L.; Niu, H.; Dickinson, C.; Ganin, A.Y.; Rosseinsky, M.J.; Khimyak, Y.Z.; et al. Conjugated microporous poly(aryleneethynylene) networks. Angew. Chem. Int. Ed. 2007, 46, 8574–8578. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A. Functional materials: From hard to soft porous frameworks. Angew. Chem. Int. Ed. 2010, 49, 8328–8344. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, R.; Zhu, B.; Li, Y.; Ma, Y. Synthesis and characterization of functional porous organic polymers as efficient metallocene catalyst supports. New J. Chem. 2016, 40, 8324–8333. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Han, X.; Han, Z.; Bai, Y. Highly tunable porous organic polymer (POP) supports for metallocene-based ethylene polymerization. Appl. Surf. Sci. 2017, 420, 496–503. [Google Scholar] [CrossRef]
- Wang, X.; Han, X.; Ren, F.; Xu, R.; Bai, Y. Porous organic polymers-supported metallocene catalysts for ethylene/1-hexene copolymerization. Catalysts 2018, 8, 146. [Google Scholar] [CrossRef]
- Paine, A.J. Dispersion polymerization of styrene in polar solvents. 7. A simple mechanistic model to predict particle size. Macromolecules 1990, 23, 3109–3117. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.; Xiao, A.; Yu, H.; Tan, Q.; Ding, J.; Ren, G. Unexpected behavior of 1-chlorodecane as a novel porogen in the preparation of high-porosity poly(divinylbenzene) microspheres. J. Phys. Chem. C 2008, 112, 13171–13174. [Google Scholar] [CrossRef]
- Bonini, F.; Fraajie, V.; Fink, G. Propylene polymerization through supported metallocene/MAO catalysts: Kinetic analysis and modeling. J. Polym. Sci. Part A 1995, 33, 2393–2402. [Google Scholar] [CrossRef]
- Fink, G.; Steinmetz, B.; Zechlin, J.; Przybyla, C.; Tesche, B. Propene polymerization with silica-supported metallocene/MAO catalysts. Chem. Rev. 2000, 100, 1377–1390. [Google Scholar] [CrossRef] [PubMed]

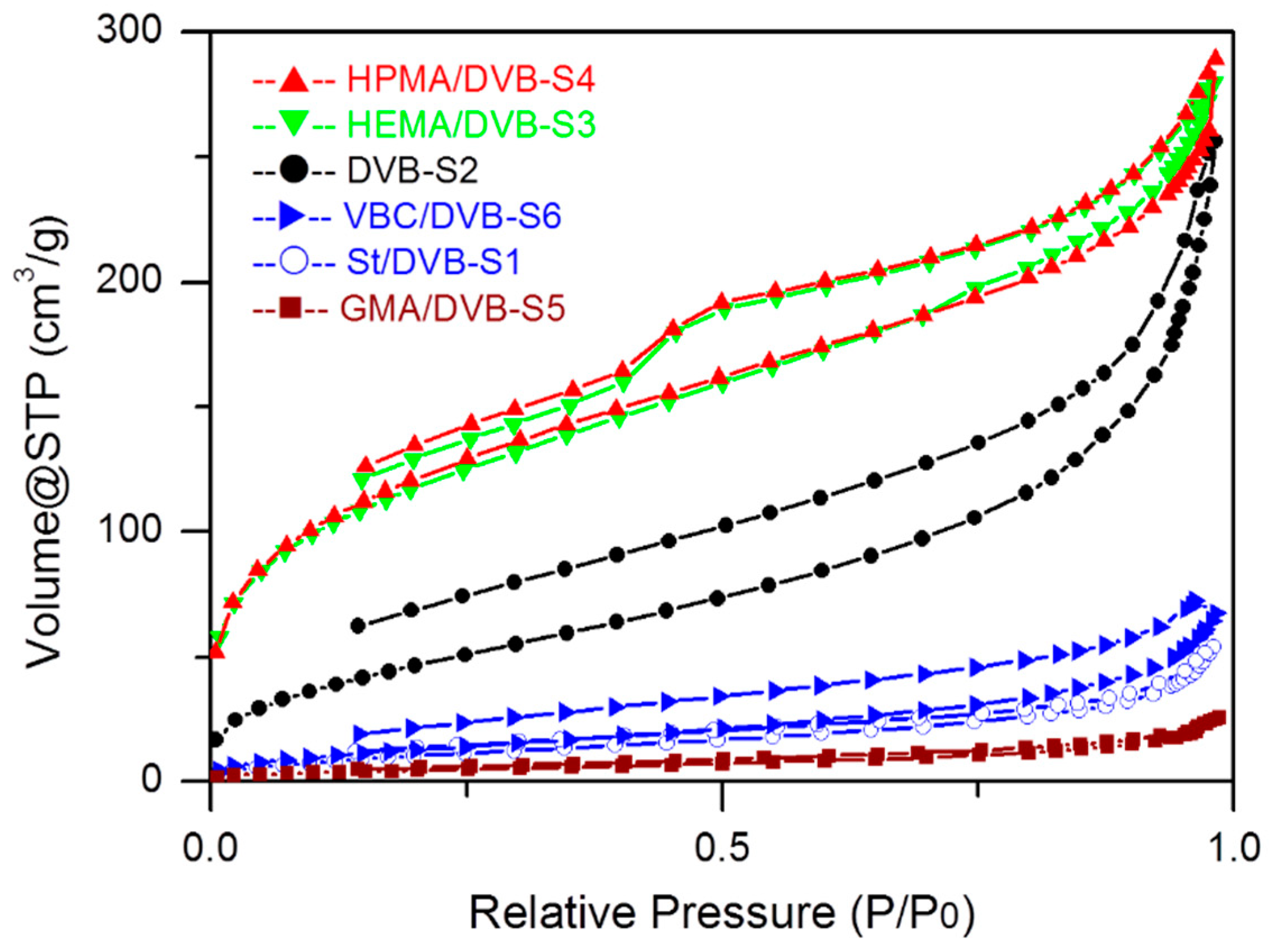
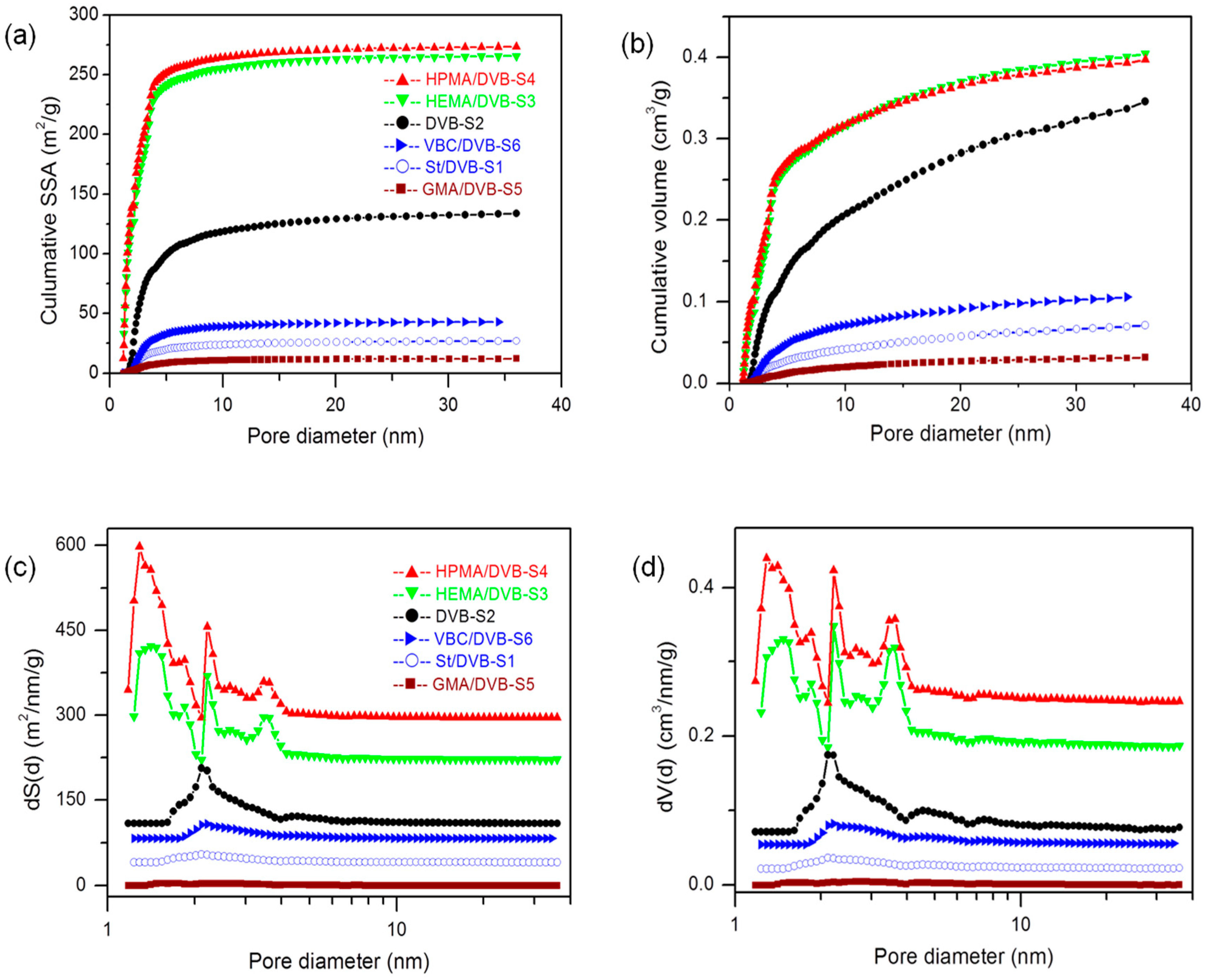


| No. | Functional Comonomer (FC) | Solvent | Divinylbenzene (DVB) | FC/DVB (Molar Ratio) | Template Agent | Specific Surface Area(Multi Point BET) [m2/g] | Total Pore Volume [cm3/g] | Average Pore Diameter [nm] | Bulk Density g/cm3 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | St | EtOH/H2O = 10:1 | 55% | 5:4 | - | 41.6 | 0.0836 | 8.03 | 0.08 |
| 2 | -- | EtOH/H2O = 9:1 | 80% | - | - | 178 | 0.396 | 8.89 | 0.02 |
| 3 | HEMA | EtOH/H2O = 9:1 | 80% | 2:3 | - | 417 | 0.434 | 4.16 | 0.20 |
| 4 | HPMA | EtOH/H2O = 9:1 | 80% | 2:3 | - | 430 | 0.447 | 4.16 | 0.24 |
| 5 | GMA | EtOH/H2O = 9:1 | 80% | 3:5 | - | 17.1 | 0.040 | 9.34 | 0.08 |
| 6 | VBC | EtOH/H2O =9:1 | 80% | 0.52:1 | - | 50.9 | 0.104 | 8.21 | 0.05 |
| 7-1 | HPMA | EtOH/H2O = 9:1 | 80% | 2:3 | Fe3O4-20 nm 3 wt % | 412 | 0.420 | 4.08 | 0.25 |
| 7-2 | - | 441 | 0.480 | 4.35 | 0.24 | ||||
| 8-1 | HEMA/St (FC1/FC2) | EtOH/H2O = 9:1 | 80% | FC1/FC2/DVB = 1.0/0.7/1.15 | Fe3O4-20 nm 3 wt % | 199 | 0.299 | 6.01 | 0.30 |
| 8-2 | 215 | 0.301 | 5.59 | 0.29 | |||||
| 9 | HEMA/St (FC1/FC2) | EtOH/H2O = 9:1 | 80% | 1.0/0.7/1.15 | - | 183 | 0.296 | 6.45 | 0.22 |
| 10 | HEMA/VBC | EtOH/H2O = 9:1 | 80% | 1:0.29:1.5 | - | 90.8 | 0.122 | 5.32 | 0.16 |
| 11 | HEMA/VBC | EtOH/H2O = 9:1 | 80% | 1:0.56:1.19 *a | - | 15.1 | 0.0383 | 10.1 | 0.39 |
| 12 | HEMA/VBC | ethanol | 80% | 1:0.32:1.19 *a | 4.88 | 0.0131 | 10.7 | 0.33 | |
| 13 | HEMA/VBC | ethanol | 80% | 1:0.147:1.19 | - | 210 | 0.244 | 4.63 | 0.28 |
| 14 | HEMA/VBC | ethanol | 80% | 1:0.17:1.5 | - | 380 | 0.400 | 4.21 | 0.22 |
| No. | Zr (μmol/g) | Cat (mg) | Al/Zr Molar Ratio | Yield (g) | Activity Kg PE/mol Zr h bar | Productivity g PE/g cat h | Bulk Density g/mL |
|---|---|---|---|---|---|---|---|
| S4 | 15.6 | 208 | 272 | 39.1 | 8033 | 376 | 0.30 |
| S5 | 2.3 | 192 | 179 | 1.45 | 2189 | 15.1 | 0.11 |
| S12 | 0.6 | 184 | 247 | - | - | - | - |
| S14 | 16.7 | 211 | 254 | 37.8 | 7152 | 358 | 0.29 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, C.; Liu, W.; Zhang, P. Feasibility Study on the Design and Synthesis of Functional Porous Organic Polymers with Tunable Pore Structure as Metallocene Catalyst Supports. Polymers 2018, 10, 944. https://doi.org/10.3390/polym10090944
Wang X, Zhang C, Liu W, Zhang P. Feasibility Study on the Design and Synthesis of Functional Porous Organic Polymers with Tunable Pore Structure as Metallocene Catalyst Supports. Polymers. 2018; 10(9):944. https://doi.org/10.3390/polym10090944
Chicago/Turabian StyleWang, Xiong, Cuiling Zhang, Wenxia Liu, and Pingsheng Zhang. 2018. "Feasibility Study on the Design and Synthesis of Functional Porous Organic Polymers with Tunable Pore Structure as Metallocene Catalyst Supports" Polymers 10, no. 9: 944. https://doi.org/10.3390/polym10090944