A Facile Synthetic Route to Amphiphilic Poly(Meta-Phenylene Ethynylene) and Poly(Meta-Phenylene Ethynylene)-Block-Polyisocyanide Using a Single Catalyst
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Reagents
2.2. Instrumentation and Characterization
2.3. Representative Polymerization Procedure for PPE (Poly-125)
2.4. Representative Copolymerization Procedure for PPE-b-PPI (poly(125-b-260))
3. Results
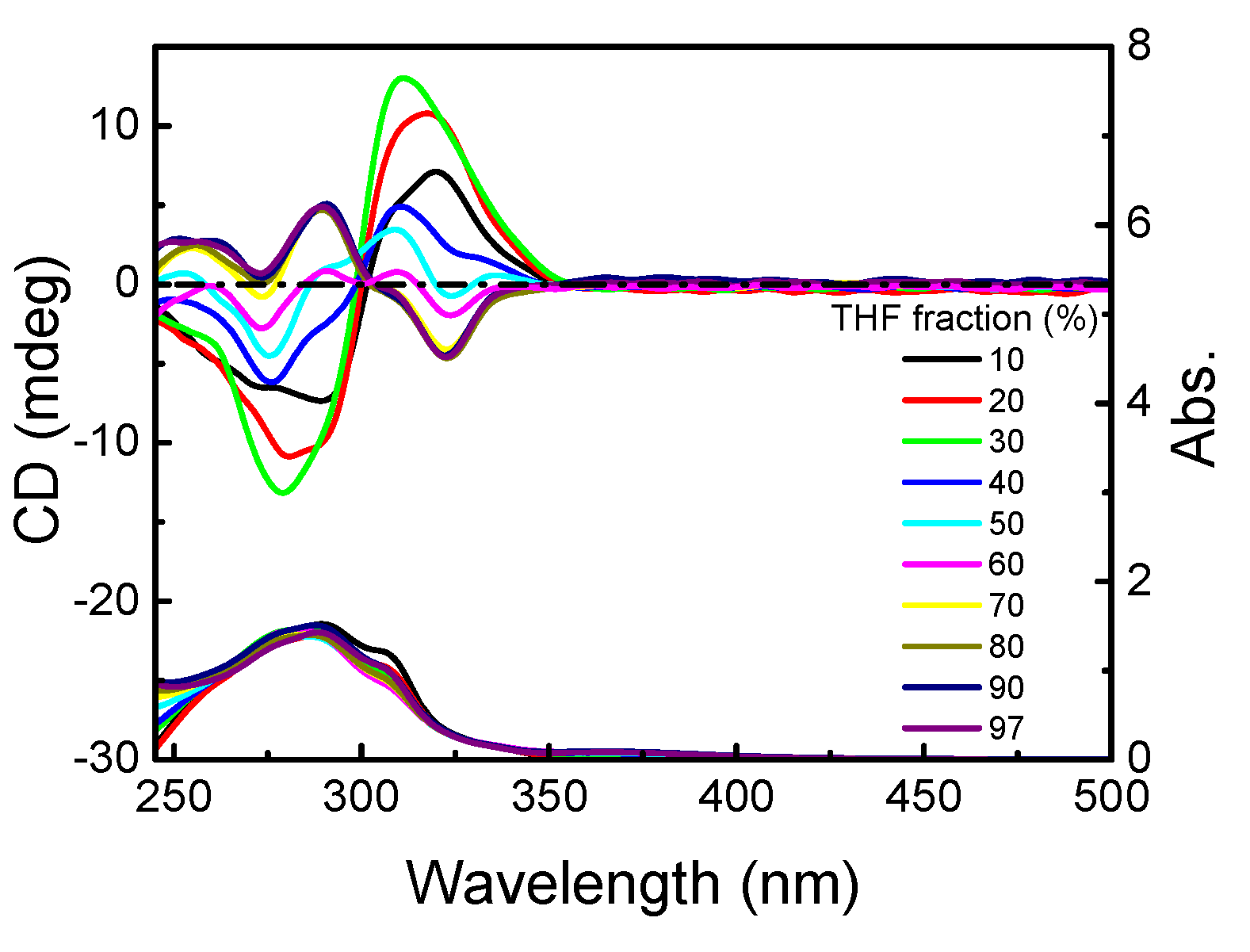
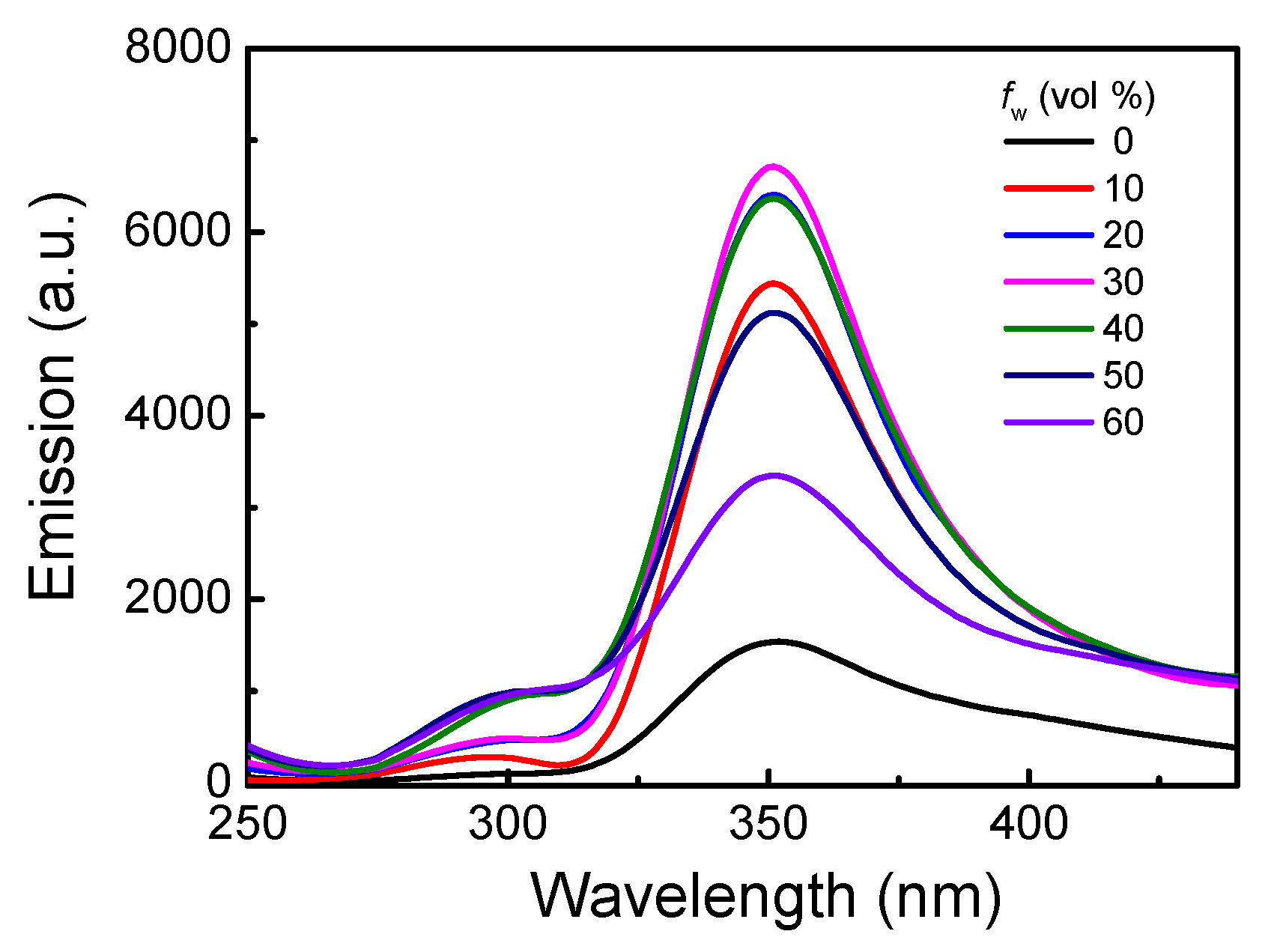
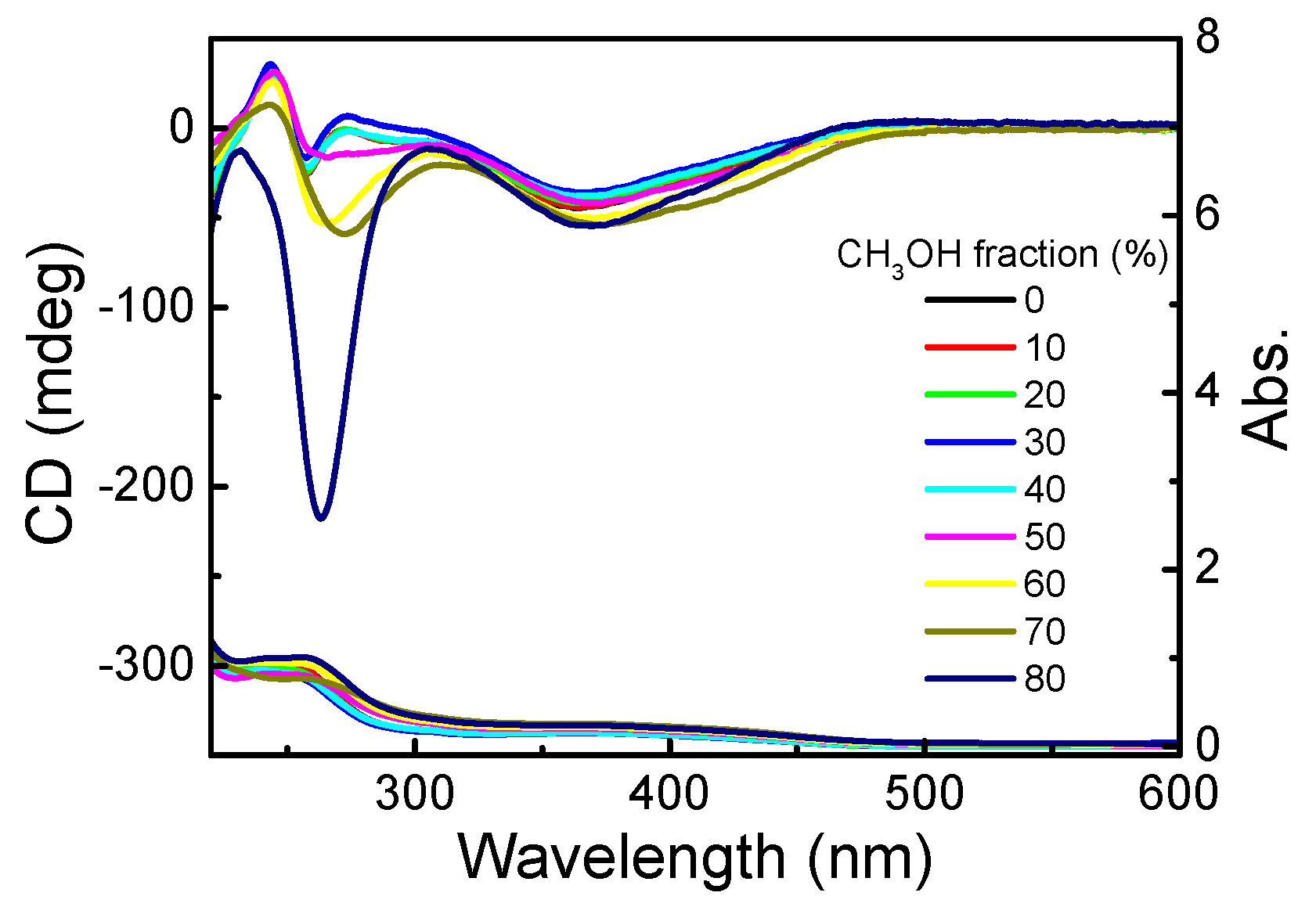
3.1. Homopolymerization of m-PPE and Its Chiroptical Properties
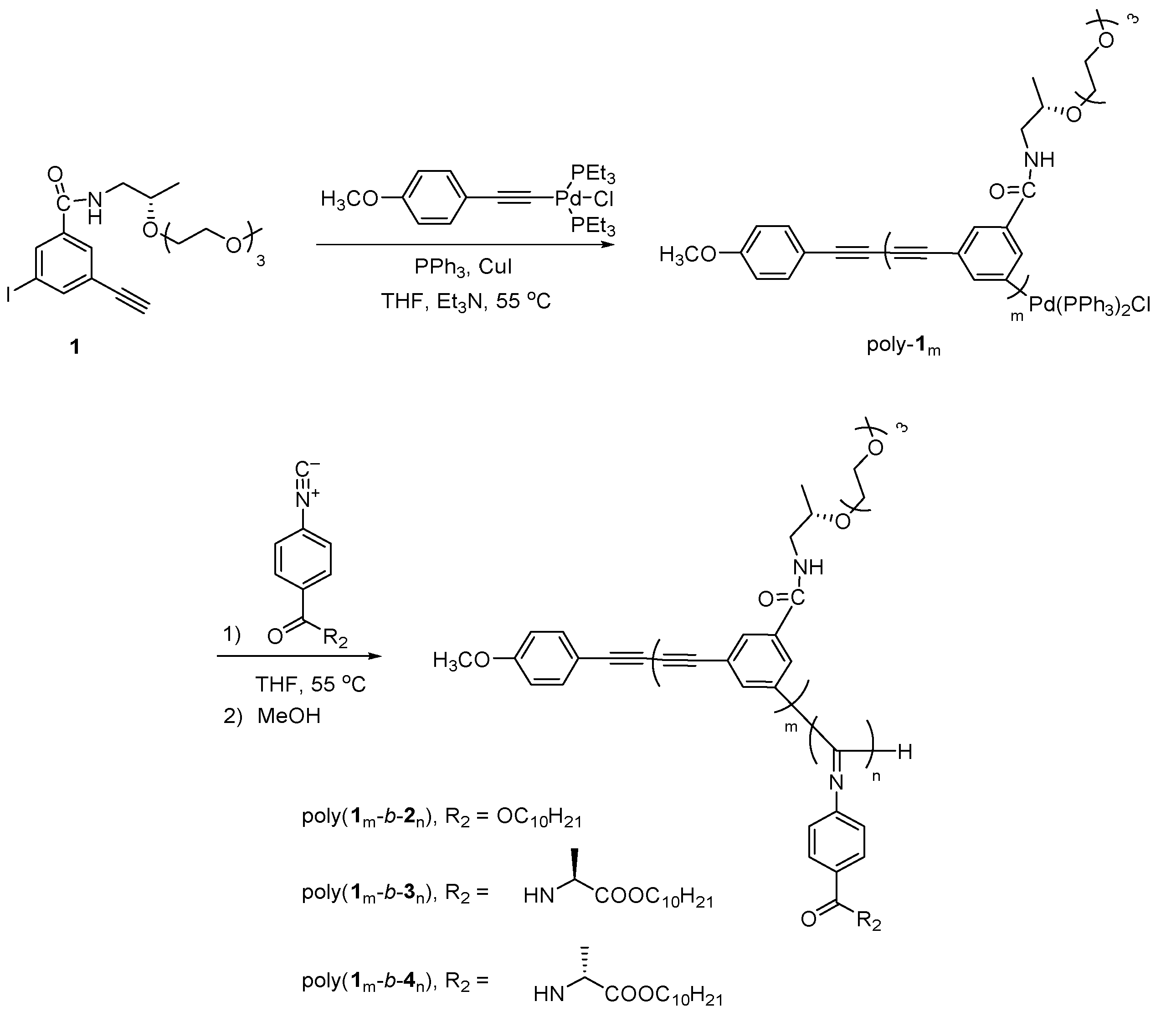
3.1.1. Synthesis and Polymerization of Optically Active m-Phenylene Ethynylenes
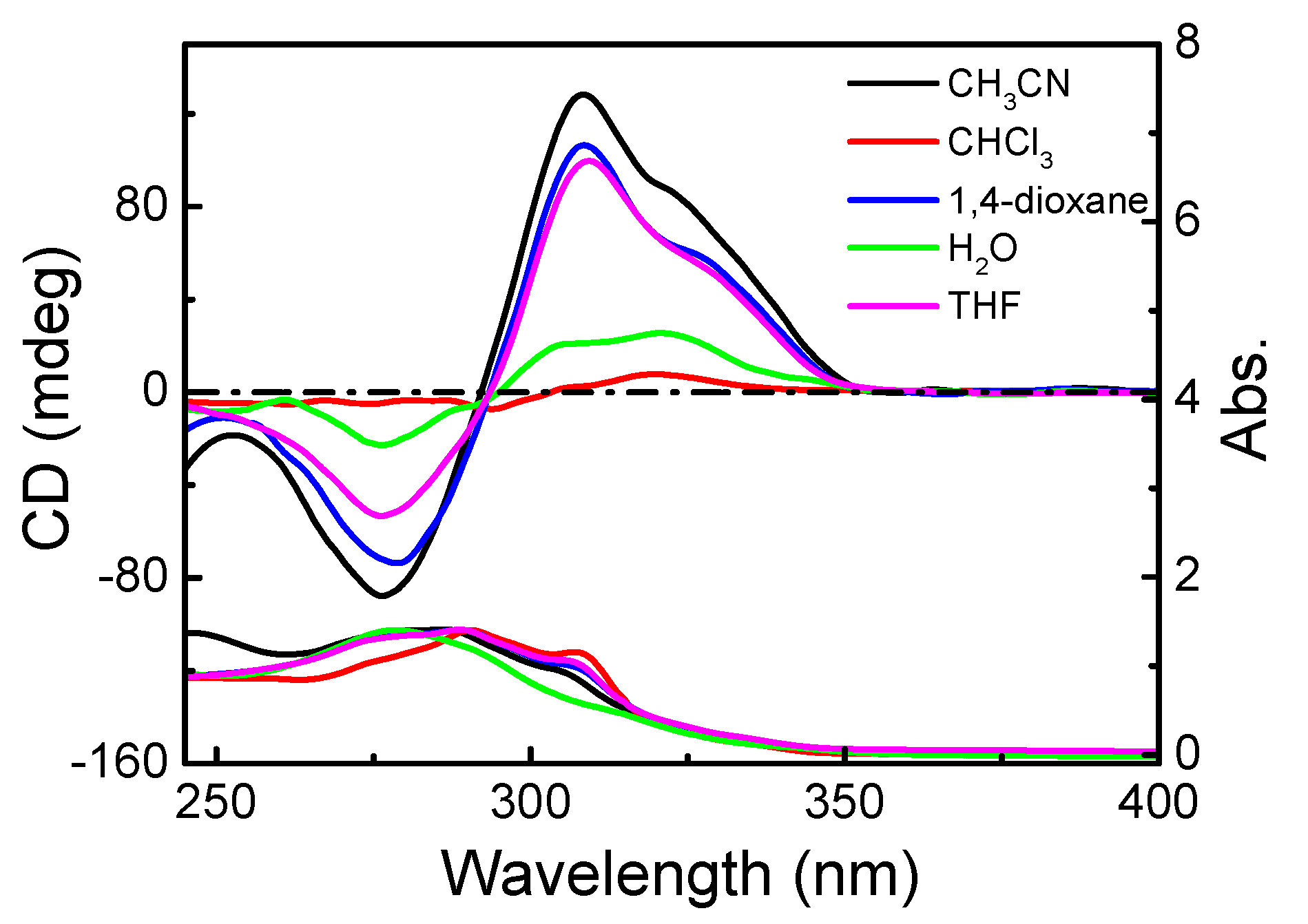
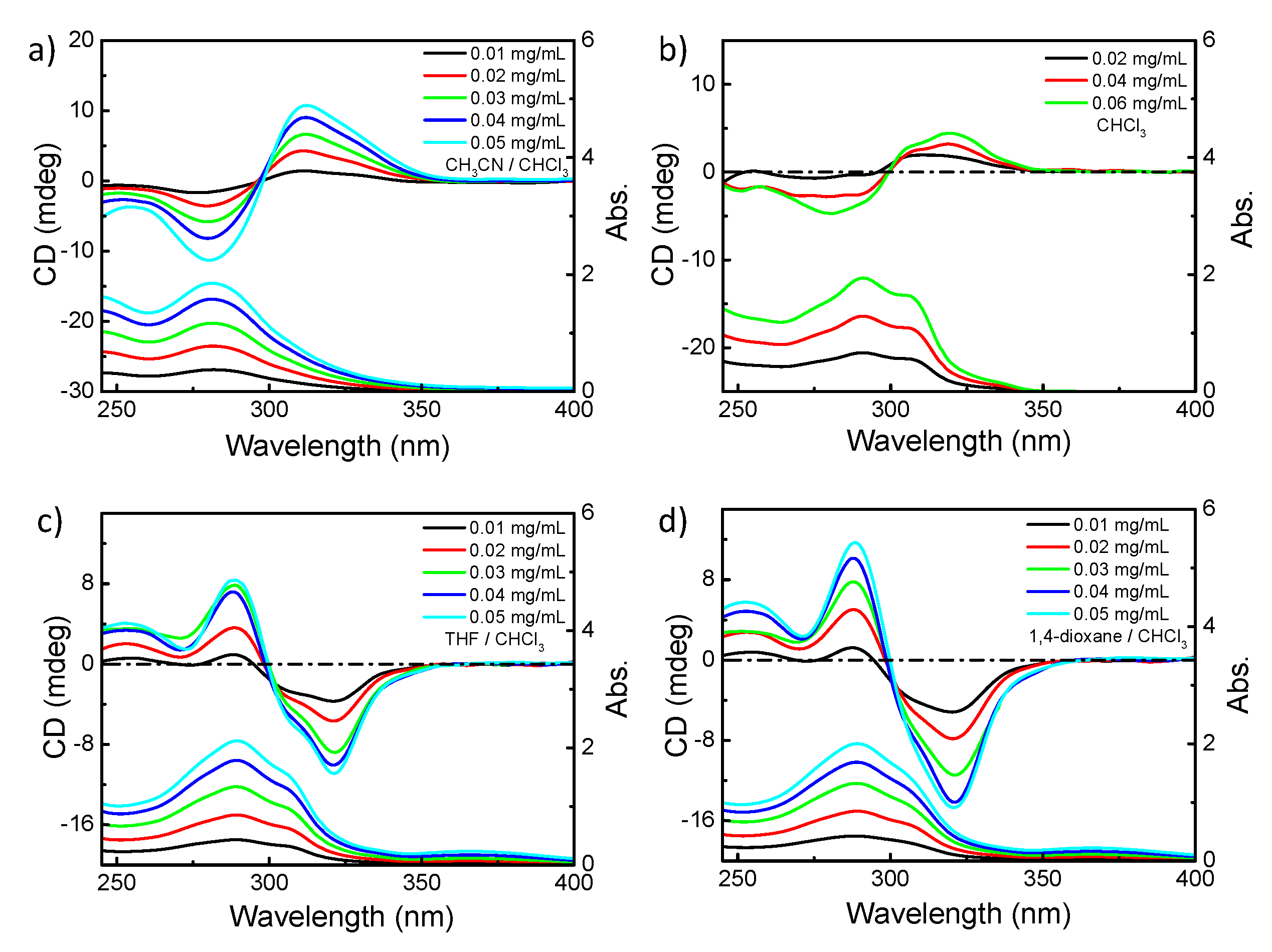
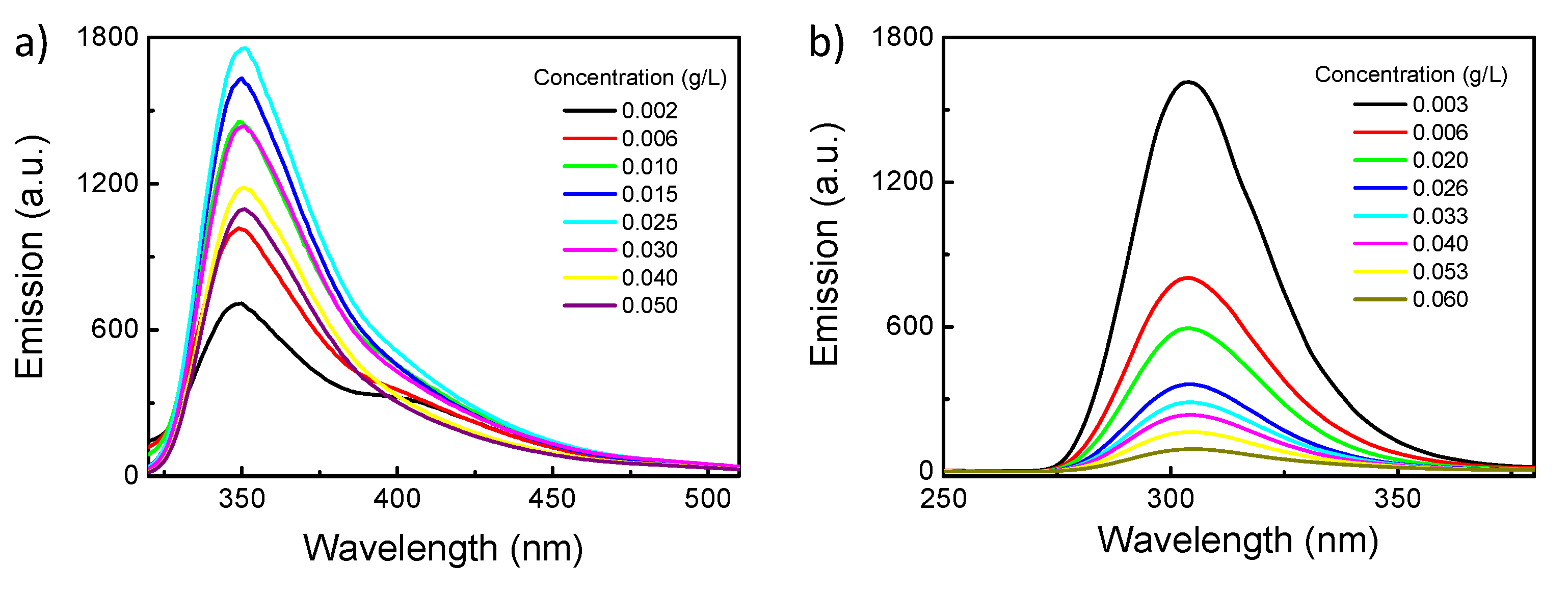
3.1.2. Chiroptical Properties in Dilute Solution
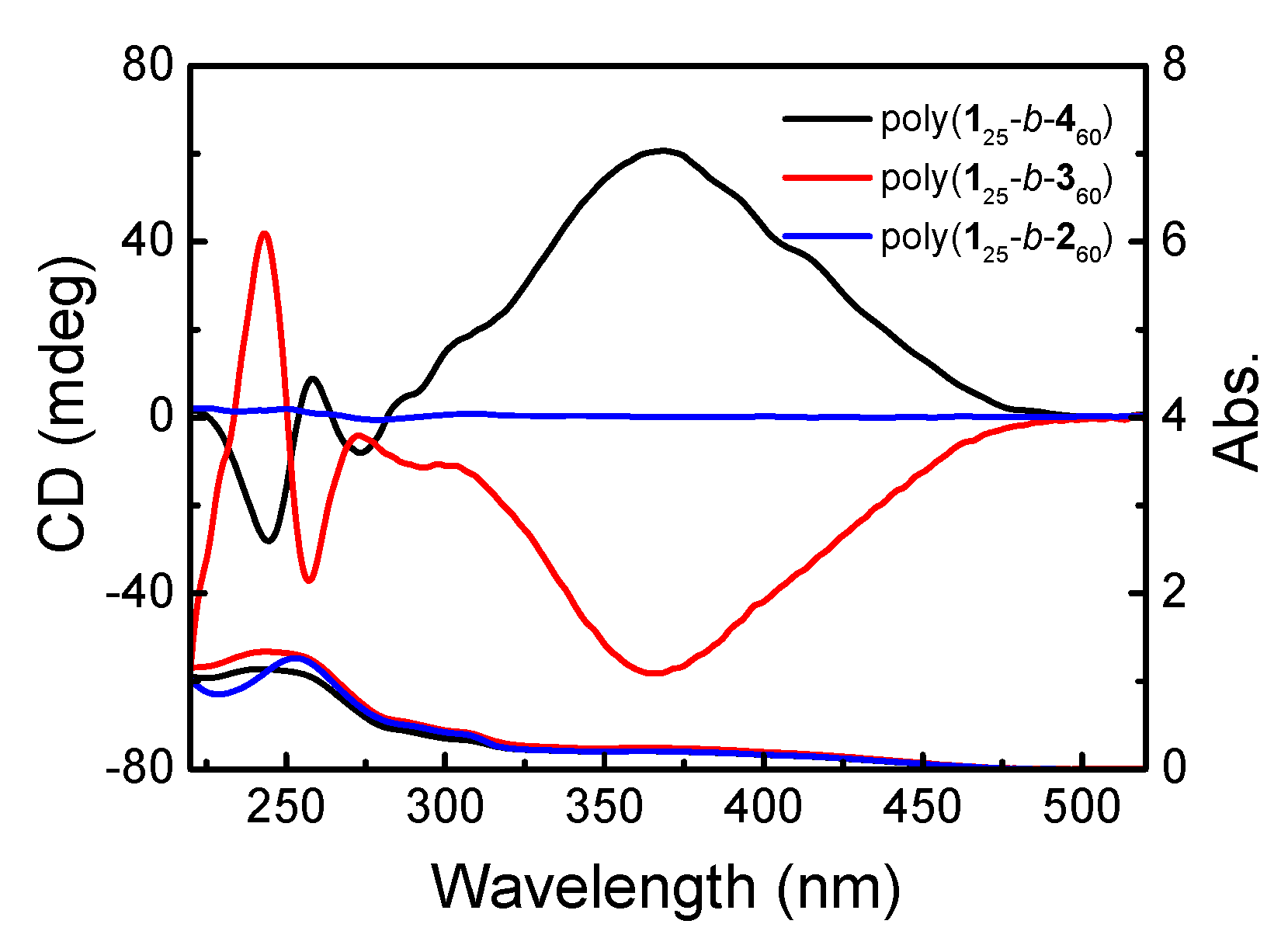
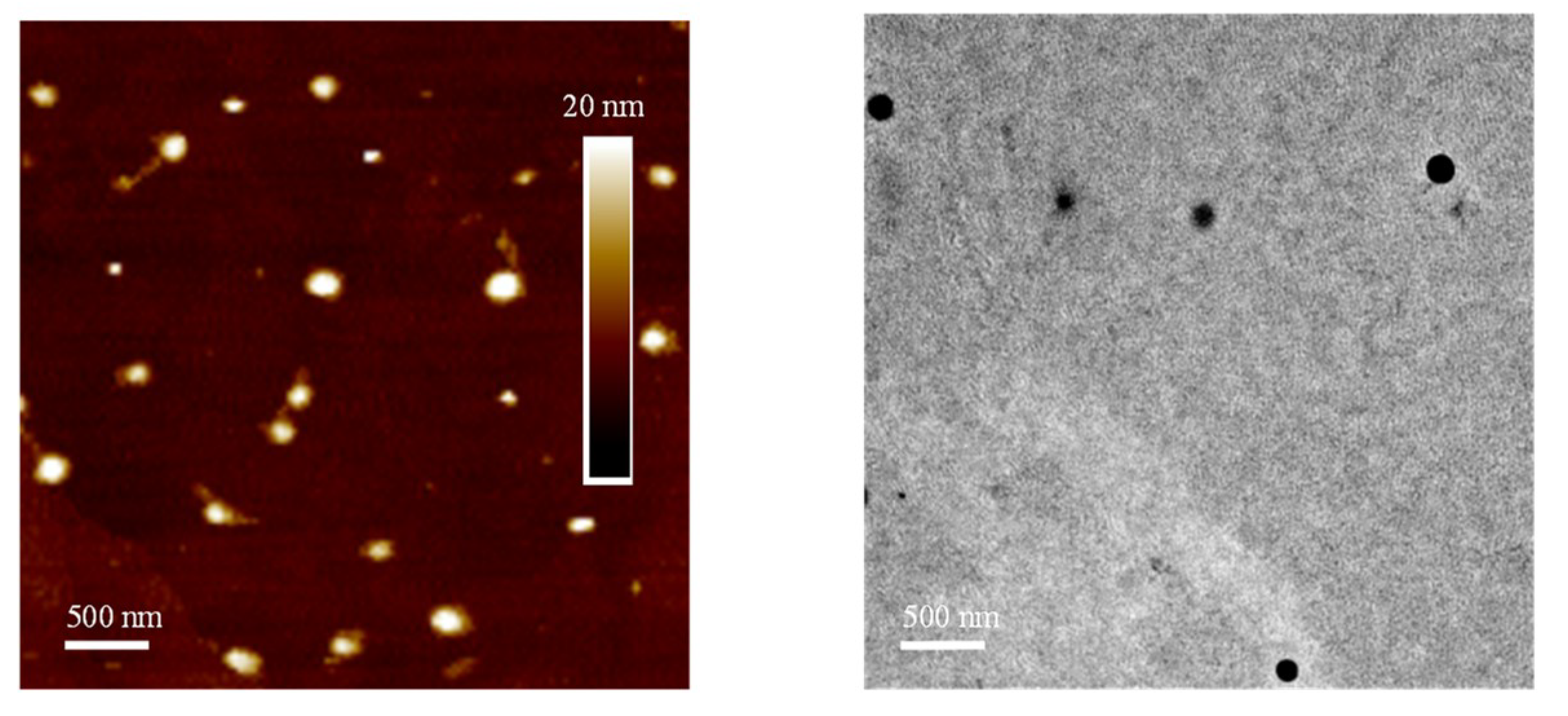
3.2. One-Pot Synthesis of a PPE-b-PPI Block Copolymer and Chiroptical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shen, J.; Okamoto, Y. Efficient Separation of Enantiomers Using Stereoregular Chiral Polymers. Chem. Rev. 2016, 116, 1094–1138. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Tang, Z.L.; Iida, H.; Miyabe, T.; Yashima, E. Enantioseparation on Helical Poly(phenylacetylene)s Bearing Cinchona Alkaloid Pendants as Chiral Stationary Phases for HPLC. Chem. Lett. 2012, 41, 809–811. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Chu, B.-F.; Xu, X.-Y.; Xu, L.; Liu, N.; Wu, Z.-Q. Significant Improvement on Enantioselectivity and Diastereoselectivity of Organocatalyzed Asymmetric Aldol Reaction Using Helical Polyisocyanides Bearing Proline Pendants. ACS Macro Lett. 2017, 6, 824–829. [Google Scholar] [CrossRef]
- Ke, Y.-Z.; Nagata, Y.; Yamada, T.; Suginome, M. Majority-Rules Type Helical Poly(quinoxaline-2,3-diyl)s as Highly Efficient ChiralityAmplification Systems for Asymmetric Catalysis. Angew. Chem. Int. Ed. 2015, 54, 9333–9337. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.L.; Iida, H.; Hu, H.Y.; Yashima, E. Remarkable Enhancement of the Enantioselectivity of an Organocatalyzed Asymmetric Henry Reaction Assisted by Helical Poly(phenylacetylene)s Bearing Cinchona Alkaloid Pendants via an Amide Linkage. ACS Macro Lett. 2012, 1, 261–265. [Google Scholar] [CrossRef]
- Zhou, L.; Shen, L.; Huang, J.; Liu, N.; Zhu, Y.-Y.; Wu, Z.-Q. Optically Active Helical Polyisocyanides Bearing Chiral Phosphine Pendants: Facile Synthesis and Application in Enantioselective Rauhut-Currier Reaction. Chin. J. Polym. Sci. 2018, 36, 163–170. [Google Scholar] [CrossRef]
- Maeda, K.; Yashima, E. Helical Polyacetylenes Induced via Noncovalent Chiral Interactions and Their Applications as Chiral Materials. Top. Curr. Chem. 2017, 375, 72. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, M. Supramolecular Chirality: Solvent Chirality Transfer in Molecular Chemistry and Polymer Chemistry. Symmetry 2014, 6, 677–703. [Google Scholar] [CrossRef] [Green Version]
- Nelson, J.C.; Saven, J.G.; Moore, J.S.; Wolynes, P.G. Solvophobically driven folding of nonbiological oligomers. Science 1997, 277, 1793–1796. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, S.; Thompson, J.L.; Moore, J.S. Solvophobically Driven π-Stacking of Phenylene Ethynylene Macrocycles and Oligomers. J. Am. Chem. Soc. 2000, 122, 11315–11319. [Google Scholar] [CrossRef]
- Stone, M.T.; Fox, J.M.; Moore, J.S. A helicene-containing foldamer displaying highly solvent-dependent CD spectra. Org. Lett. 2004, 6, 3317–3320. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.T.; Moore, J.S. A Water-Soluble m-Phenylene Ethynylene Foldamer. Org. Lett. 2004, 6, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Murayama, D.; Kayamori, F.; Inouye, M. Saccharide-Linked Ethynylpyridine Oligomers: Primary Structures Encode Chiral Helices. Macromolecules 2008, 41, 6903–6909. [Google Scholar] [CrossRef]
- Liu, R.; Shiotsuki, M.; Masuda, T.; Sanda, F. Synthesis and Chiroptical Properties of Hydroxy-phenylglycine-Based Poly(m-phenyleneethynylene-p-phenyleneethynylene)s. Macromolecules 2009, 42, 6115–6122. [Google Scholar] [CrossRef]
- Hill, D.J.; Mio, M.J.; Prince, R.B.; Hughes, T.S.; Moore, J.S. A Field Guide to Foldamers. Chem. Rev. 2001, 101, 3893–4012. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Okamoto, Y. Synthetic Helical Polymers: Conformation and Function. Chem. Rev. 2001, 101, 4013–4038. [Google Scholar] [CrossRef] [PubMed]
- Yashima, E.; Maeda, K.; Iida, H.; Furusho, Y.; Nagai, K. Helical Polymers: Synthesis, Structures, and Functions. Chem. Rev. 2009, 109, 6102–6211. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Okamoto, S.; Satoh, K.; Sakajiri, K.; Furuya, H.; Abe, A. Reversible Helix–helix Transition of Poly(β-phenylpropyll-aspartate) Involving a Screw-Sense Inversion in the Solid State. Macromolecules 1996, 29, 7084–7088. [Google Scholar] [CrossRef]
- Fujiki, M. Diversity and Selection in Self-Assembled Tetrameric Capsule. J. Am. Chem. Soc. 2000, 122, 3336–3343. [Google Scholar] [CrossRef]
- Tang, K.; Green, M.M.; Cheon, K.S.; Selinger, J.V.; Garetz, B.A. Chiral Conflict. The Effect of Temperature on the Helical Sense of a Polymer Controlled by the Competition between Structurally Different Enantiomers: From Dilute Solution to the Lyotropic Liquid Crystal State. J. Am. Chem. Soc. 2003, 125, 7313–7323. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Okamoto, Y. Synthesis and Conformational Characteristics of Poly(phenyl isocyanate)s Bearing an Optically Active Ester Group. Macromolecules 1999, 32, 974–980. [Google Scholar] [CrossRef]
- Pijper, D.; Feringa, B.L. Molecular Transmission: Controlling the Twist Sense of a Helical Polymer with a Single Light-Driven Molecular Motor. Angew. Chem. Int. Ed. 2007, 46, 3693–3696. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Schuster, G.B.; Cheon, K.S.; Green, M.M.; Selinger, J.V. Switching a Helical Polymer between Mirror Images Using Circularly Polarized Light. J. Am. Chem. Soc. 2000, 122, 2603–2612. [Google Scholar] [CrossRef]
- Otsuka, I.; Sakai, R.; Satoh, T.; Kakuchi, R.; Kaga, H.; Kakuchi, T. Metal-cation-induced chiroptical switching for poly(phenylacetylene) bearing a macromolecular ionophore as a graft chain. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 5855–5863. [Google Scholar] [CrossRef]
- Otsuka, I.; Sakai, R.; Kakuchi, R.; Satoh, T.; Kakuchi, T. Chiroptical switching system based on the host-guest interaction between metal cations and poly(phenylacetylene)s bearing polycarbohydrate ionophore. Eur. Polym. J. 2008, 44, 2971–2979. [Google Scholar] [CrossRef]
- Freire, F.; Seco, J.M.; Quinoa, E.; Riguera, R. Chiral Amplification and Helical-Sense Tuning by Mono- and Divalent Metals on Dynamic Helical Polymers. Angew. Chem. Int. Ed. 2011, 50, 11692–11696. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Nakano, T.; Ono, E.; Hatada, K. Synthesis and Reversible Stereomutation of Optically Active Poly[(S)-diphenyl(1-methylpyrrolidin-2-yl)methyl methacrylate]. Chem. Lett. 1991, 20, 525–528. [Google Scholar] [CrossRef]
- Sanda, F.; Terada, K.; Masuda, T. Synthesis, Chiroptical Properties, and pH Responsibility of Aspartic Acid- and Glutamic Acid-Based Helical Polyacetylenes. Macromolecules 2005, 38, 8149–8154. [Google Scholar] [CrossRef]
- Nakako, H.; Nomura, R.; Masuda, T. Helix Inversion of Poly(propiolic esters). Macromolecules 2001, 34, 1496–1502. [Google Scholar] [CrossRef]
- Yamazaki, K.; Yokoyama, A.; Yokozawa, T. Solvent and Temperature Effect on Chiral Conformation of Poly(m-benzamide)s. Macromolecules 2006, 39, 2432–2434. [Google Scholar] [CrossRef]
- Morino, K.; Maeda, K.; Yashima, E. Helix-Sense Inversion of Poly(phenylacetylene) Derivatives Bearing an Optically Active Substituent Induced by External Chiral and Achiral Stimuli. Macromolecules 2003, 36, 1480–1486. [Google Scholar] [CrossRef]
- Freire, F.; Quiñoá, E.; Riguera, R. Supramolecular Assemblies from Poly(phenylacetylene)s. Chem. Rev. 2015, 116, 1242–1271. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Ono, R.J.; Bielawski, C.W. Controlled Catalyst Transfer Polycondensation and Surface-Initiated Polymerization of a p-Phenyleneethynylene-Based Monomer. J. Am. Chem. Soc. 2013, 135, 4984–4987. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.R.; Müller, P.; Swager, T.M. Interrupted Energy Transfer: Highly Selective Detection of Cyclic Ketones in the Vapor Phase. J. Am. Chem. Soc. 2011, 133, 12910–12913. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wilson, J.N.; Smith, M.D.; Bunz, U.H.F. TEMPO-Substituted PPEs: Polystyrene-PPE Graft Copolymers and Double Graft Copolymers. Macromolecules 2004, 37, 9701–9708. [Google Scholar] [CrossRef]
- Lee, J.K.; Jung, Y.H.; Tok, J.B.-H.; Bao, Z.N. Syntheses of Organic Molecule-DNA Hybrid Structures. ACS Nano 2011, 5, 2067–2074. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Ramakrishnan, S. Folding of a Donor-Containing Ionene by Intercalation with an Acceptor. Chem. Asian J. 2011, 6, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Banno, M.; Yamaguchi, T.; Nagai, K.; Kaiser, C.; Hecht, S.; Yashima, E. Optically Active, Amphiphilic Poly(meta-phenylene ethynylene)s: Synthesis, Hydrogen-Bonding Enforced Helix Stability, and Direct AFM Observation of Their Helical Structures. J. Am. Chem. Soc. 2012, 134, 8718–8728. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.-X.; Zhu, Y.-Y.; Gao, L.-M.; He, X.-Y.; Liu, N.; Zhang, W.-Y.; Yin, J.; Ding, Y.S.; Zhou, H.; Wu, Z.-Q. Air-Stable (Phenylbuta-1,3-diynyl)palladium(II) Complexes: Highly Active Initiators for Living Polymerization of Isocyanides. J. Am. Chem. Soc. 2014, 136, 4706–4713. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Liu, N.; Wang, Q.; Wang, H.-Q.; Yin, J.; Wu, Z.-Q. Facile Synthesis of Poly(phenyleneethynylene)-block-Polyisocyanide Copolymers via Two Mechanistically Distinct, Sequential Living Polymerizations Using a Single Catalyst. Macromolecules 2016, 49, 110–119. [Google Scholar] [CrossRef]
- Xue, Y.-X.; Chen, J.-L.; Jiang, Z.-Q.; Yu, Z.; Liu, N.; Yin, J.; Zhu, Y.-Y.; Wu, Z.-Q. Living polymerization of arylisocyanide initiated by the phenylethynyl palladium (II) complex. Polym. Chem. 2014, 5, 6435–6438. [Google Scholar] [CrossRef]
- Matsuda, K.; Stone, M.T.; Moore, J.S. Helical Pitch of m-Phenylene Ethynylene Foldamers by Double Spin Labeling. J. Am. Chem. Soc. 2002, 124, 11836–11837. [Google Scholar] [CrossRef] [PubMed]
- Brunsveld, L.; Meijer, E.W.; Prince, R.B.; Moore, J.S. Self-Assembly of Folded m-Phenylene Ethynylene Oligomers into Helical Columns. J. Am. Chem. Soc. 2001, 123, 7978–7984. [Google Scholar] [CrossRef] [PubMed]
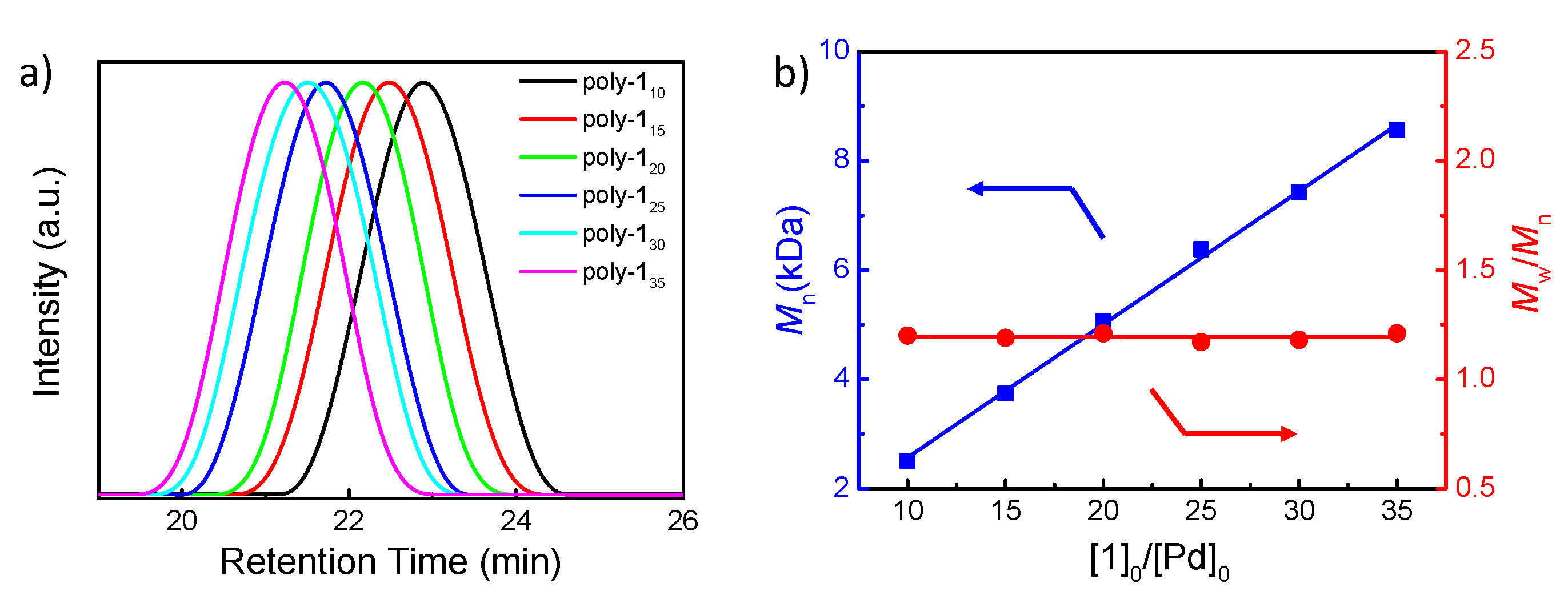

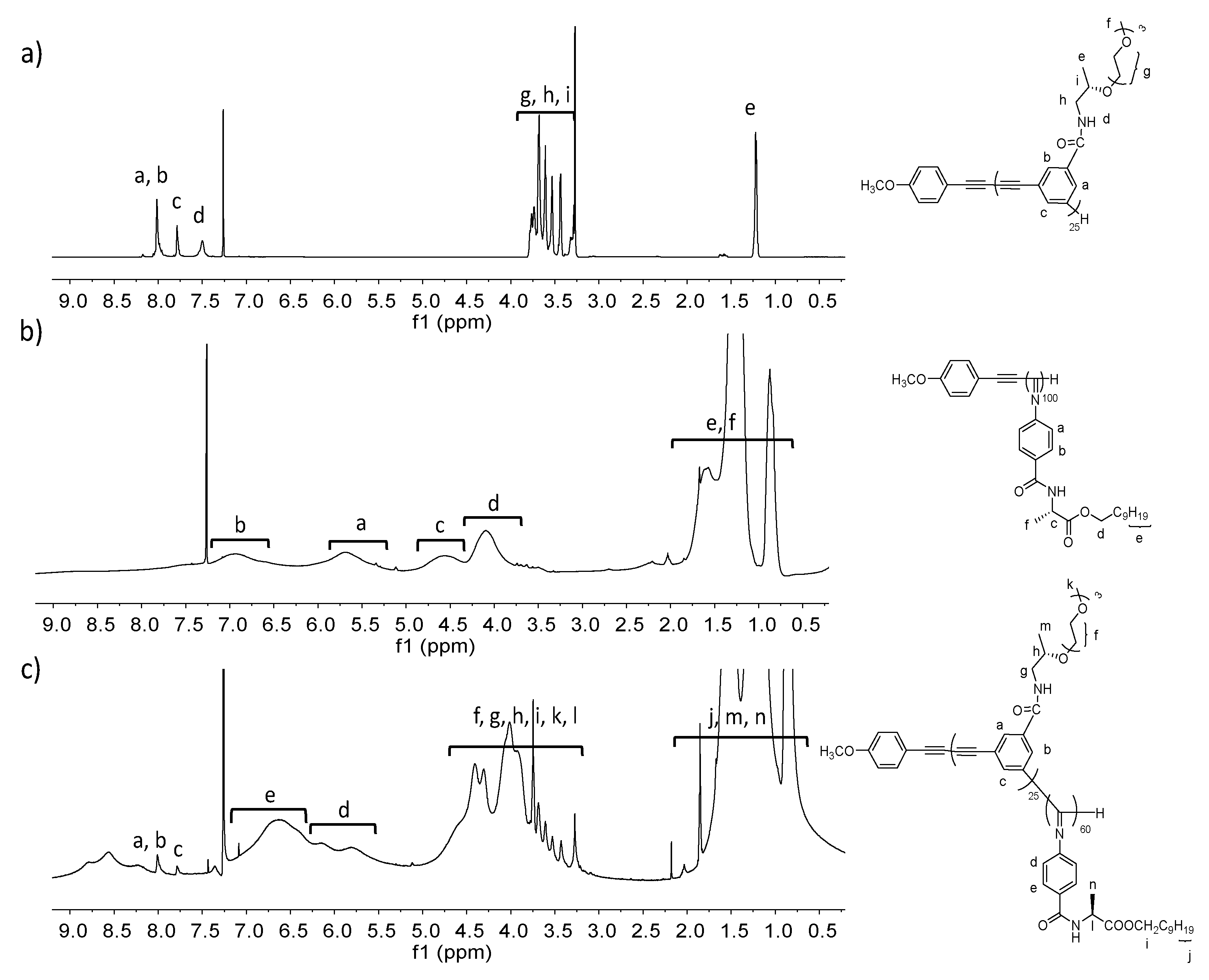
| Run | Polymer b | [1]0/[Pd]0 c | Mn(kDa) d | Mw/Mnd | Yield e (%) |
|---|---|---|---|---|---|
| 1 | poly-110 | 10 | 2.51 | 1.20 | 67 |
| 2 | poly-115 | 15 | 3.74 | 1.19 | 67 |
| 3 | poly-120 | 20 | 5.07 | 1.21 | 70 |
| 4 | poly-125 | 25 | 6.38 | 1.17 | 70 |
| 5 | poly-130 | 30 | 7.42 | 1.18 | 73 |
| 6 | poly-135 | 35 | 8.57 | 1.21 | 78 |
| Run | Block Polymer b | Mn (kDa) c | Mw/Mnc | Yield d (%) |
|---|---|---|---|---|
| 1 | poly(125-b-215) | 9.08 | 1.15 | 73 |
| 2 | poly(125-b-250) | 17.80 | 1.21 | 78 |
| 3 | poly(125-b-2100) | 31.75 | 1.17 | 81 |
| 4 | poly(125-b-320) | 11.64 | 1.14 | 78 |
| 5 | poly(125-b-360) | 25.33 | 1.09 | 81 |
| 6 | poly(125-b-420) | 11.16 | 1.16 | 77 |
| 7 | poly(125-b-430) | 14.15 | 1.15 | 81 |
| 8 | poly(125-b-460) | 22.86 | 1.21 | 80 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Xu, X.; Xu, L.; Liu, N. A Facile Synthetic Route to Amphiphilic Poly(Meta-Phenylene Ethynylene) and Poly(Meta-Phenylene Ethynylene)-Block-Polyisocyanide Using a Single Catalyst. Polymers 2018, 10, 936. https://doi.org/10.3390/polym10090936
Li C, Xu X, Xu L, Liu N. A Facile Synthetic Route to Amphiphilic Poly(Meta-Phenylene Ethynylene) and Poly(Meta-Phenylene Ethynylene)-Block-Polyisocyanide Using a Single Catalyst. Polymers. 2018; 10(9):936. https://doi.org/10.3390/polym10090936
Chicago/Turabian StyleLi, Chonglong, Xunhui Xu, Lei Xu, and Na Liu. 2018. "A Facile Synthetic Route to Amphiphilic Poly(Meta-Phenylene Ethynylene) and Poly(Meta-Phenylene Ethynylene)-Block-Polyisocyanide Using a Single Catalyst" Polymers 10, no. 9: 936. https://doi.org/10.3390/polym10090936





