Efficient Adsorption on Benzoyl and Stearoyl Cellulose to Remove Phenanthrene and Pyrene from Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
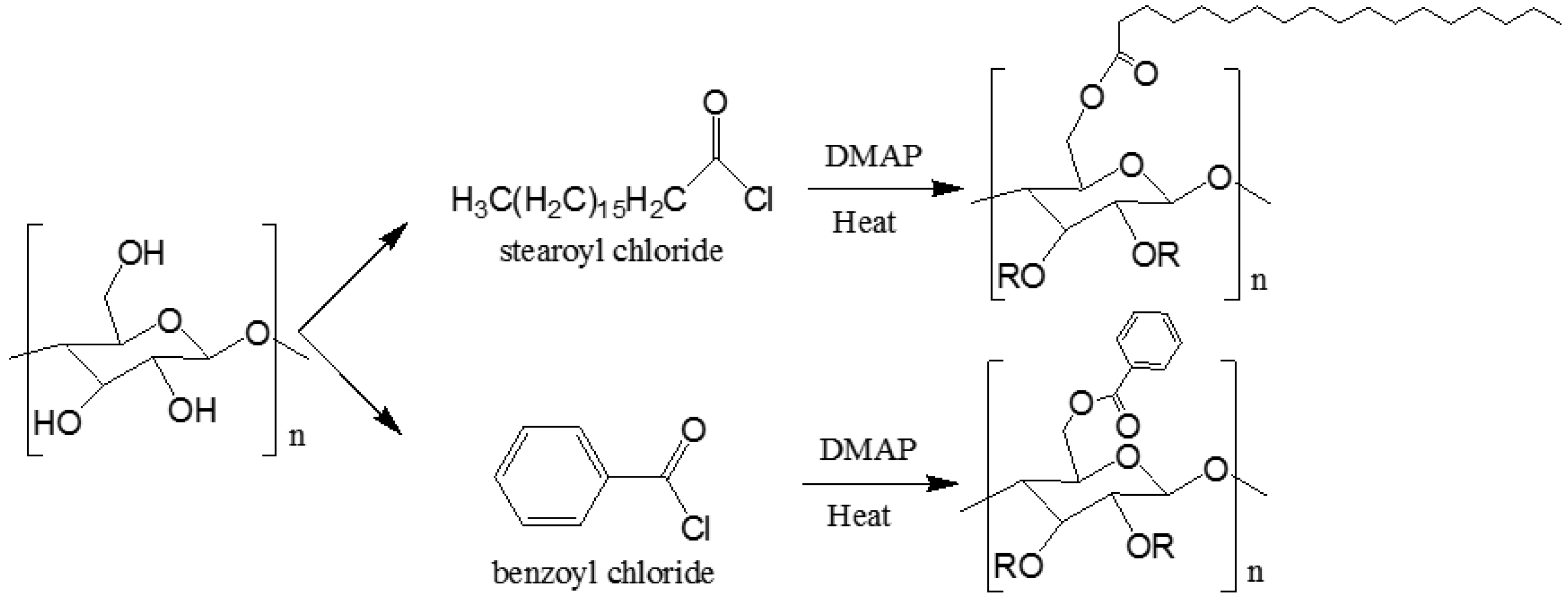
2.2. Synthesis of Benzoyl Cellulose (Bz–Cell) and Stearoyl Cellulose (St–Cell)
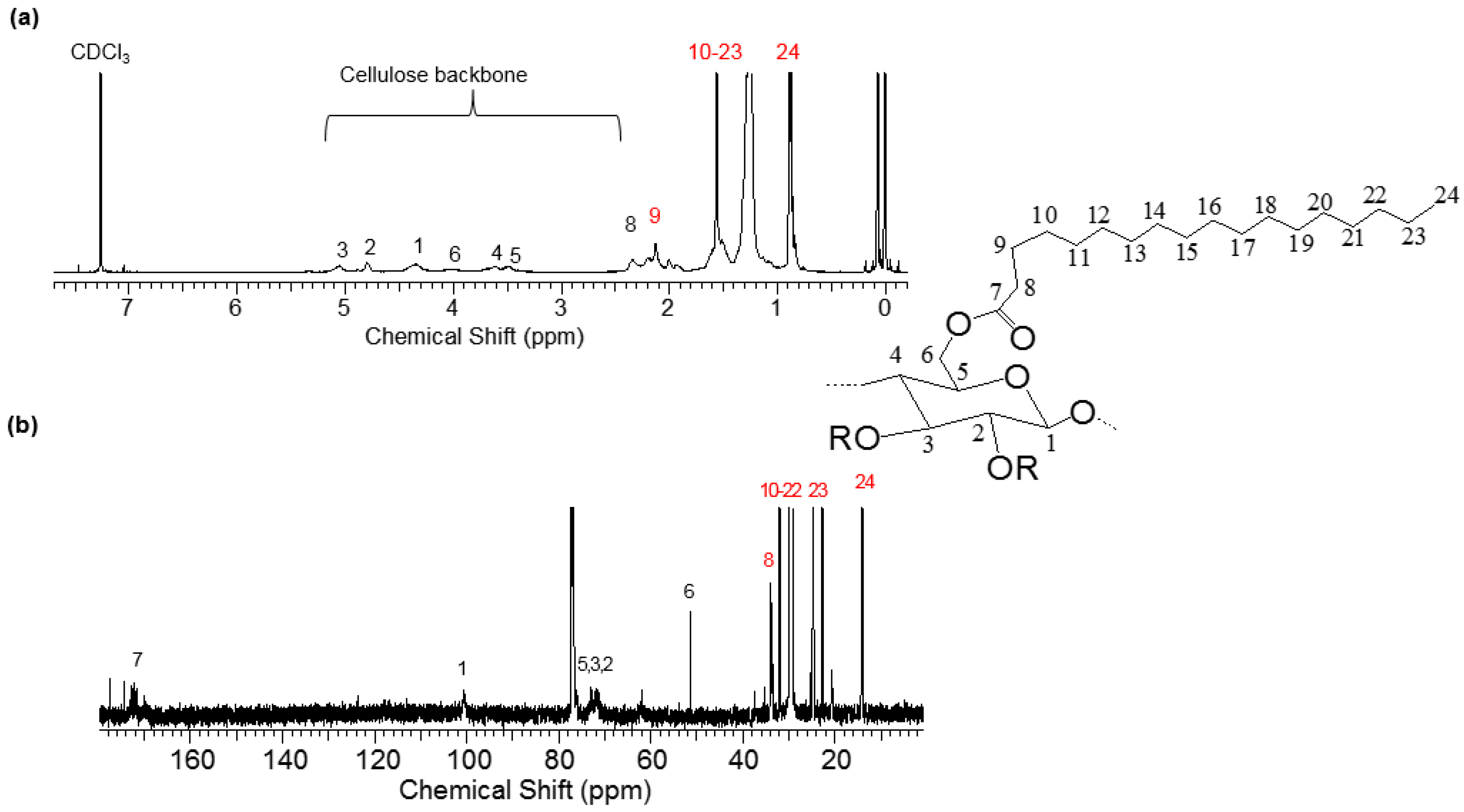
2.3. NMR Spectroscopy
2.4. Fourier Transform Infrared (FT-IR) Spectroscopy
2.5. Field Emission Scanning Electron Microscopy (FE-SEM)
2.6. X-ray Diffraction Analysis (XRD)
2.7. Thermal Gravimetric Analysis (TGA)
2.8. Adsorption of Phenanthrene and Pyrene
2.9. Recyclability of Bz–Cell and St–Cell
3. Results
3.1. Characterization of Bz–Cell and St–Cell
3.2. FT-IR Spectroscopic Analysis
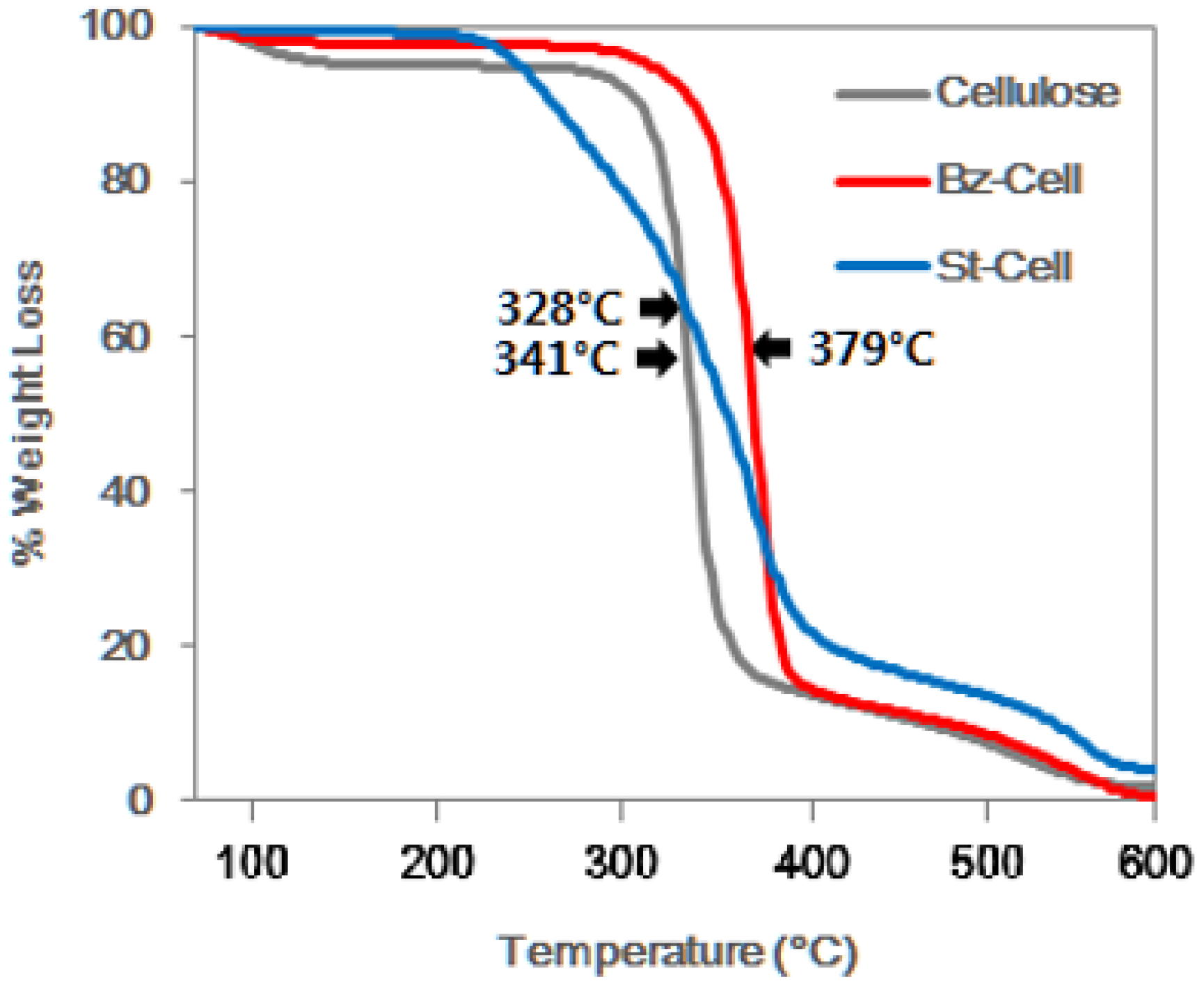
3.3. Thermal Gravimetric Analysis (TGA)
3.4. XRD Analysis
3.5. FE-SEM Analysis
3.6. Emission Spectra and Removal Test
3.7. Reusability of Bz–Cell and St–Cell
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, X.; Shi, S.; Wei, J.; Lv, F.; Zhao, H.; Kong, J.; He, Q.; Ni, J. Electrochemical oxidation characteristics of p-substituted phenols using a boron-doped diamond electrode. Environ. Sci. Technol. 2007, 41, 6541–6546. [Google Scholar] [CrossRef] [PubMed]
- San Miguel, G.; Lambert, S.; Graham, N. The regeneration of field-spent granular-activated carbons. Water Res. 2001, 35, 2740–2748. [Google Scholar] [CrossRef]
- Thakur, S.; Sharma, B.; Verma, A.; Chaudhary, J.; Tamulevicius, S.; Thakur, V.K. Recent progress in sodium alginate based sustainable hydrogels for environmental applications. J. Clean. Prod. 2018, 198, 143–159. [Google Scholar] [CrossRef]
- Thakur, V.K.; Voicu, S.I. Recent advances in cellulose and chitosan based membranes for water purification: A concise review. Carbohydr. Polym. 2016, 146, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Govender, P.P.; Mamo, M.A.; Tamulevicius, S.; Mishra, Y.K.; Thakur, V.K. Progress in lignin hydrogels and nanocomposites for water purification: Future perspectives. Vacuum 2017, 146, 342–355. [Google Scholar] [CrossRef]
- Thakur, S.; Govender, P.P.; Mamo, M.A.; Tamulevicius, S.; Thakur, V.K. Recent progress in gelatin hydrogel nanocomposites for water purification and beyond. Vacuum 2017, 146, 396–408. [Google Scholar] [CrossRef]
- Thakur, M.K.; Thakur, V.K.; Gupta, R.K.; Pappu, A. Synthesis and applications of biodegradable soy based graft copolymers: A review. Sustain. Chem. Eng. 2015, 4, 1–17. [Google Scholar] [CrossRef]
- White, P.A.; Claxton, L.D. Mutagens in contaminated soil: A review. Rev. Mutat. Res. 2004, 567, 227–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-T.; Miao, Y.; Zhang, Y.; Li, Y.-C.; Wu, M.-H.; Yu, G. Polycyclic aromatic hydrocarbons (pahs) in urban soils of the megacity shanghai: Occurrence, source apportionment and potential human health risk. Sci. Total Environ. 2013, 447, 80–89. [Google Scholar] [CrossRef] [PubMed]
- May, W.E.; Wasik, S.P.; Freeman, D.H. Determination of the solubility behavior of some polycyclic aromatic hydrocarbons in water. Anal. Chem. 1978, 50, 997–1000. [Google Scholar] [CrossRef]
- Holman, H.-Y.N.; Nieman, K.; Sorensen, D.L.; Miller, C.D.; Martin, M.C.; Borch, T.; McKinney, W.R.; Sims, R.C. Catalysis of pah biodegradation by humic acid shown in synchrotron infrared studies. Environ. Sci. Technol. 2002, 36, 1276–1280. [Google Scholar] [CrossRef] [PubMed]
- Mastral, A.; García, T.; Callén, M.; Navarro, M.; Galbán, J. Removal of naphthalene, phenanthrene, and pyrene by sorbents from hot gas. Environ. Sci. Technol. 2001, 35, 2395–2400. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, K.; Tao, S.; Xing, B. Sorption of aromatic organic contaminants by biopolymers: Effects of ph, copper (ii) complexation, and cellulose coating. Environ. Sci. Technol. 2007, 41, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008, 99, 6709–6724. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Boving, T.B.; Xing, B. Sorption of pahs by aspen wood fibers as affected by chemical alterations. Environ. Sci. Technol. 2006, 40, 3279–3284. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, T.; Shimura, Y. Removal of mercuric ions by systems based on cellulose derivatives. J. Appl. Polym. Sci. 1982, 27, 747–756. [Google Scholar] [CrossRef]
- Maekawa, E.; Koshijima, T. Properties of 2,3-dicarboxy cellulose combined with various metallic ions. J. Appl. Polym. Sci. 1984, 29, 2289–2297. [Google Scholar] [CrossRef]
- Jonker, M.T. Absorption of polycyclic aromatic hydrocarbons to cellulose. Chemosphere 2008, 70, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Saliba, R.; Gauthier, H.; Gauthier, R. Adsorption of heavy metal ions on virgin and chemically-modified lignocellulosic materials. Adsorpt. Sci. Technol. 2005, 23, 313–322. [Google Scholar] [CrossRef]
- Gurdag, G.; Yasar, M.; Gurkaynak, M. Graft copolymerization of acrylic acid on cellulose: Reaction kinetics of copolymerization. J. Appl. Polym. Sci. 1997, 66, 929–934. [Google Scholar] [CrossRef]
- Rao, S.R.; Kapur, S. Grafting of acrylonitrile onto cellulose initiated by ceric ion. J. Appl. Polym. Sci. 1969, 13, 2649–2656. [Google Scholar] [CrossRef]
- Gourson, C.; Benhaddou, R.; Granet, R.; Krausz, P.; Saulnier, L.; Thibault, J.-F. Preparation of biodegradable plastic in microwave oven and solvent-free conditions. C. R. Acad. Sci. Ser. Chem. 1999, 2, 75–78. [Google Scholar] [CrossRef]
- Jadhav, A.H.; Mai, X.T.; Appiah-Ntiamoah, R.; Lee, H.; Momade, F.W.; Seo, J.G.; Kim, H. Preparation and characterization of palmitoyl grafted cellulose nano absorbent for the efficient adsorption of pyrene from aqueous solution. J. Nanosci. Nanotechnol. 2015, 15, 7980–7987. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.; Hornsby, A.; Kilcrease, D.; Nkedi-Kizza, P. Sorption and transport of hydrophobic organic chemicals in aqueous and mixed solvent systems: Model development and preliminary evaluation 1. J. Environ. Qual. 1985, 14, 376–383. [Google Scholar] [CrossRef]
- Dawsey, T.; McCormick, C.L. The lithium chloride/dimethylacetamide solvent for cellulose: A literature review. J. Macromol. Sci. Rev. Macromol. Chem. Phys. 1990, 30, 405–440. [Google Scholar] [CrossRef]
- Williamson, S.L.; McCormick, C.L. Cellulose derivatives synthesized via isocyanate and activated ester pathways in homogeneous solutions of lithium chloride/N,N-dimethylacetamide. J. Macromol. Sci. 1998, 35, 1915–1927. [Google Scholar] [CrossRef]
- Hameed, B. Equilibrium and kinetic studies of methyl violet sorption by agricultural waste. J. Hazard. Mater. 2008, 154, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Tahir, M.N.; Choi, J.M.; Kim, H.; Yu, J.-H.; Jung, S. Novel magnetic nanoparticles coated by benzene-and β-cyclodextrin-bearing dextran, and the sorption of polycyclic aromatic hydrocarbon. Carbohydr. Polym. 2015, 133, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.K.; Kim, Y.M.; Woo, S.H.; Park, J.M. Selective adsorption of phenanthrene dissolved in surfactant solution using activated carbon. Chemosphere 2007, 69, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-C.; Cao, Y.-H. Adsorption behavior of phenanthrene on ctab-modified polystyrene microspheres. Colloids Surf. A 2018, 553, 689–694. [Google Scholar] [CrossRef]
- Ren, X.; Shao, D.; Yang, S.; Hu, J.; Sheng, G.; Tan, X.; Wang, X. Comparative study of pb (ii) sorption on xc-72 carbon and multi-walled carbon nanotubes from aqueous solutions. Chem. Eng. J. 2011, 170, 170–177. [Google Scholar] [CrossRef]
- Hameed, B.; Krishni, R.; Sata, S. A novel agricultural waste adsorbent for the removal of cationic dye from aqueous solutions. J. Hazard. Mater. 2009, 162, 305–311. [Google Scholar] [CrossRef] [PubMed]

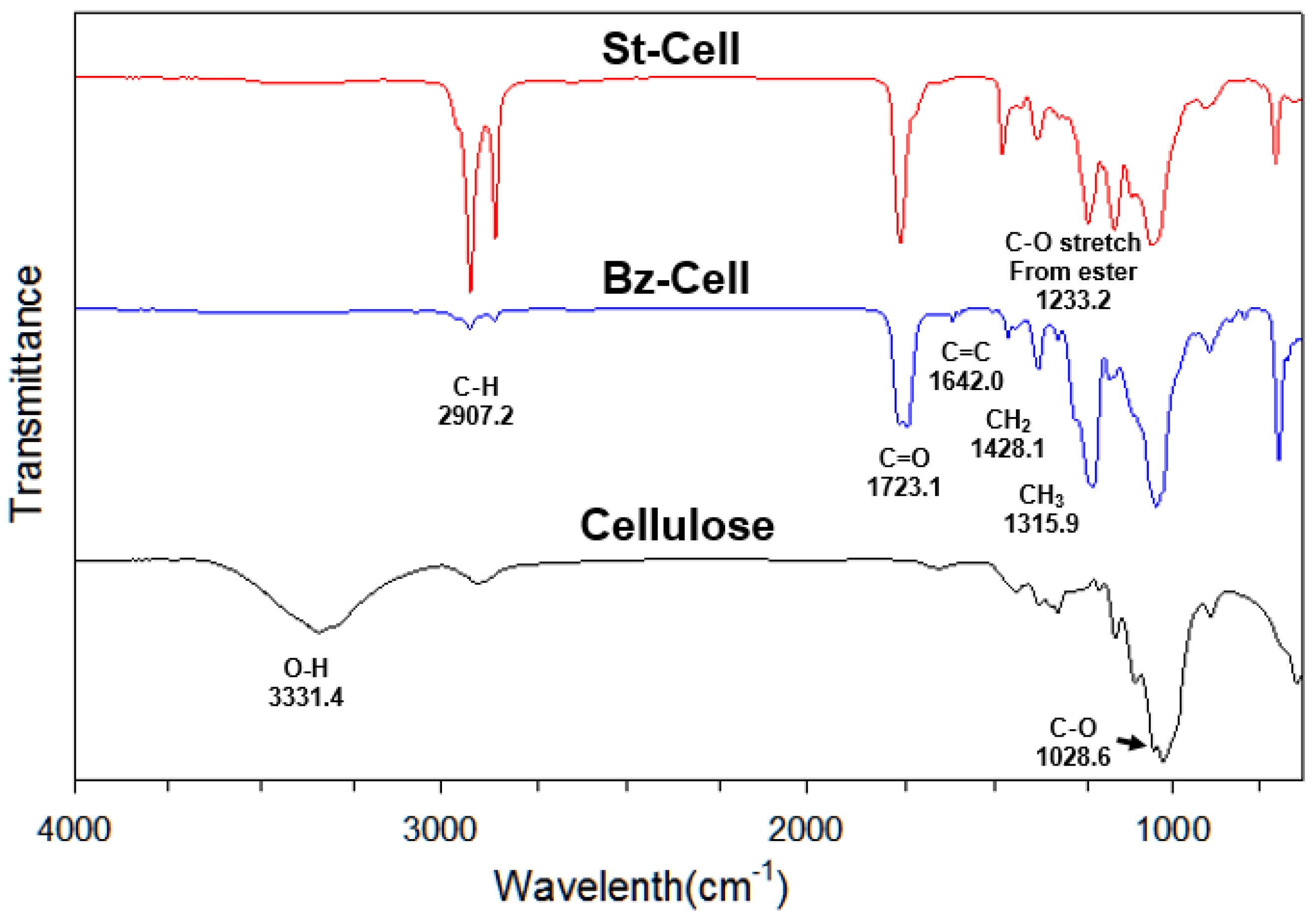





| Assignment | Cellulose (cm−1) | Bz–Cell (cm−1) | St–Cell (cm−1) |
|---|---|---|---|
| ν (O–H) | 3331.4 | - | - |
| ν (C–H) | 2897.6 | 2907.6 | 2917.8 |
| ν (C=O) | - | 1723.1 | 1743.3 |
| ν (C–O–C) | 1028.6 | 1062.6 | 1052.9 |
| ν (C–C(=O)–O–C) | - | 1220.0 | 1233.2 |
| C=C aromatic ring | - | 1642.0 | - |
| Sample | Initial Decomposition Temperature, Ti (°C) | Glass Transition Temperature, Tg (°C) | 50% Weight Loss Temperature (°C) |
|---|---|---|---|
| Cellulose | 200 | 328 | 364 |
| Bz–Cell | 278 | 379 | 385 |
| St–Cell | 268 | 341 | 350 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Jeong, D.; Park, K.H.; Yu, J.-H.; Jung, S. Efficient Adsorption on Benzoyl and Stearoyl Cellulose to Remove Phenanthrene and Pyrene from Aqueous Solution. Polymers 2018, 10, 1042. https://doi.org/10.3390/polym10091042
Kim Y, Jeong D, Park KH, Yu J-H, Jung S. Efficient Adsorption on Benzoyl and Stearoyl Cellulose to Remove Phenanthrene and Pyrene from Aqueous Solution. Polymers. 2018; 10(9):1042. https://doi.org/10.3390/polym10091042
Chicago/Turabian StyleKim, Yohan, Daham Jeong, Kyeong Hui Park, Jae-Hyuk Yu, and Seunho Jung. 2018. "Efficient Adsorption on Benzoyl and Stearoyl Cellulose to Remove Phenanthrene and Pyrene from Aqueous Solution" Polymers 10, no. 9: 1042. https://doi.org/10.3390/polym10091042





