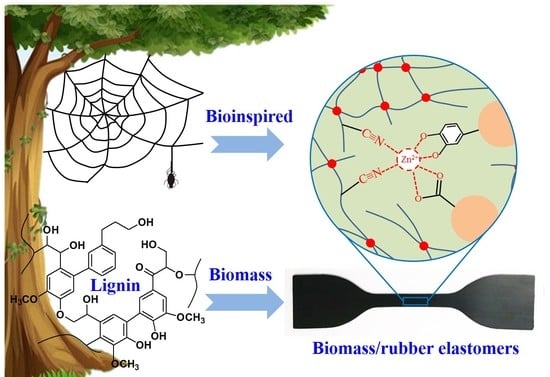
Bioinspired Engineering towards Tailoring Advanced Lignin/Rubber Elastomers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
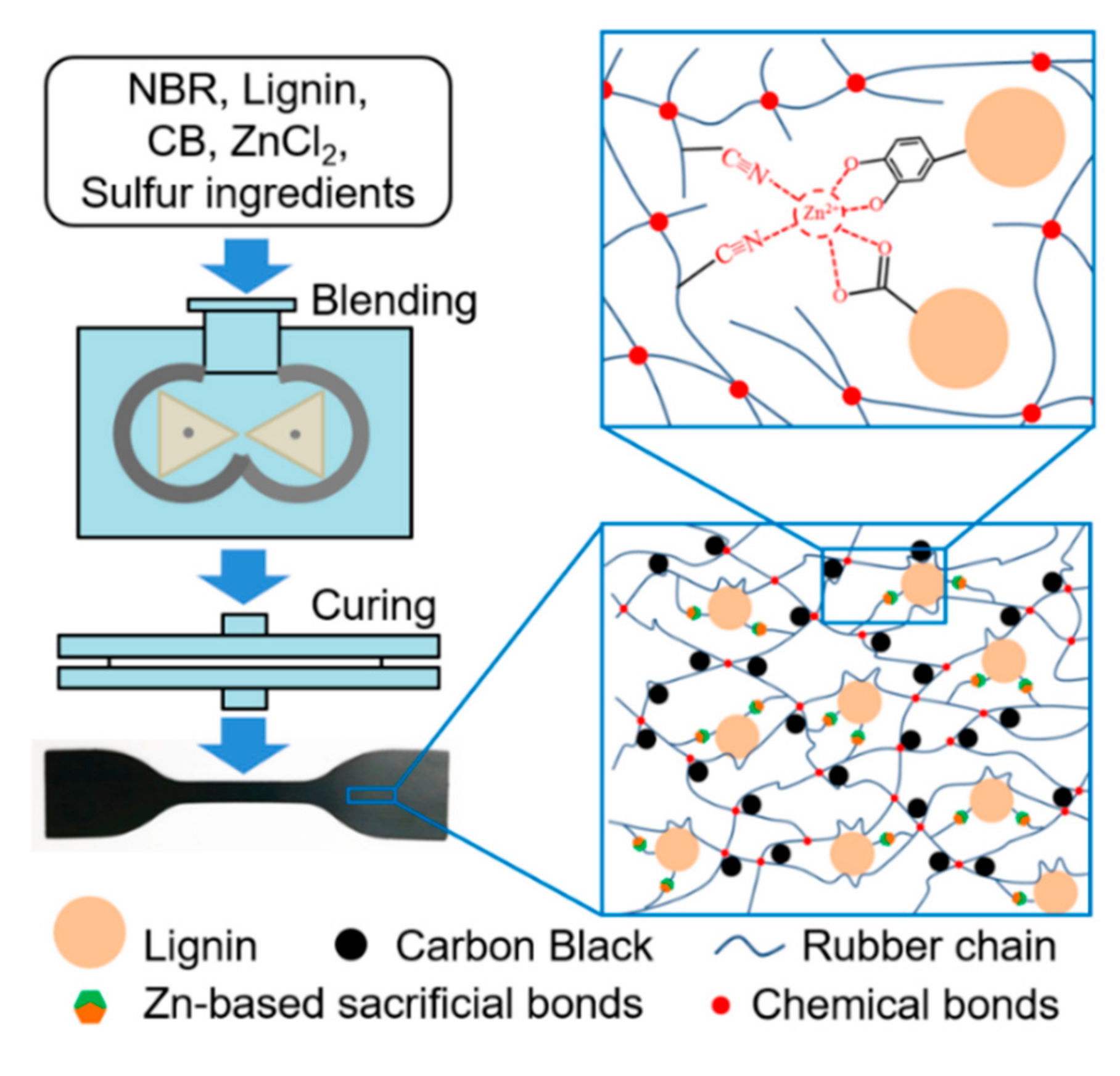
2.2. Sample Preparation
2.3.Characterization
3. Results
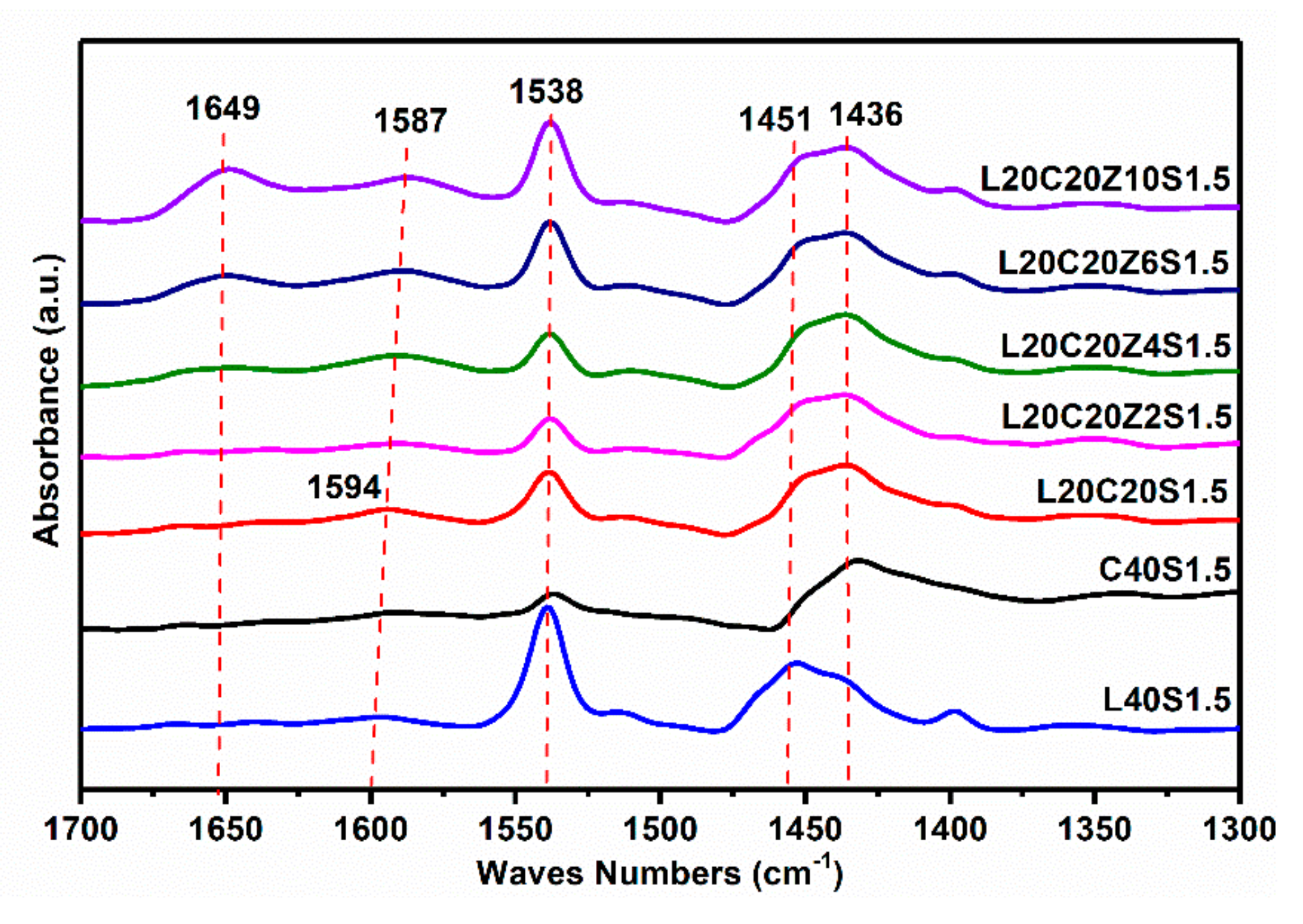
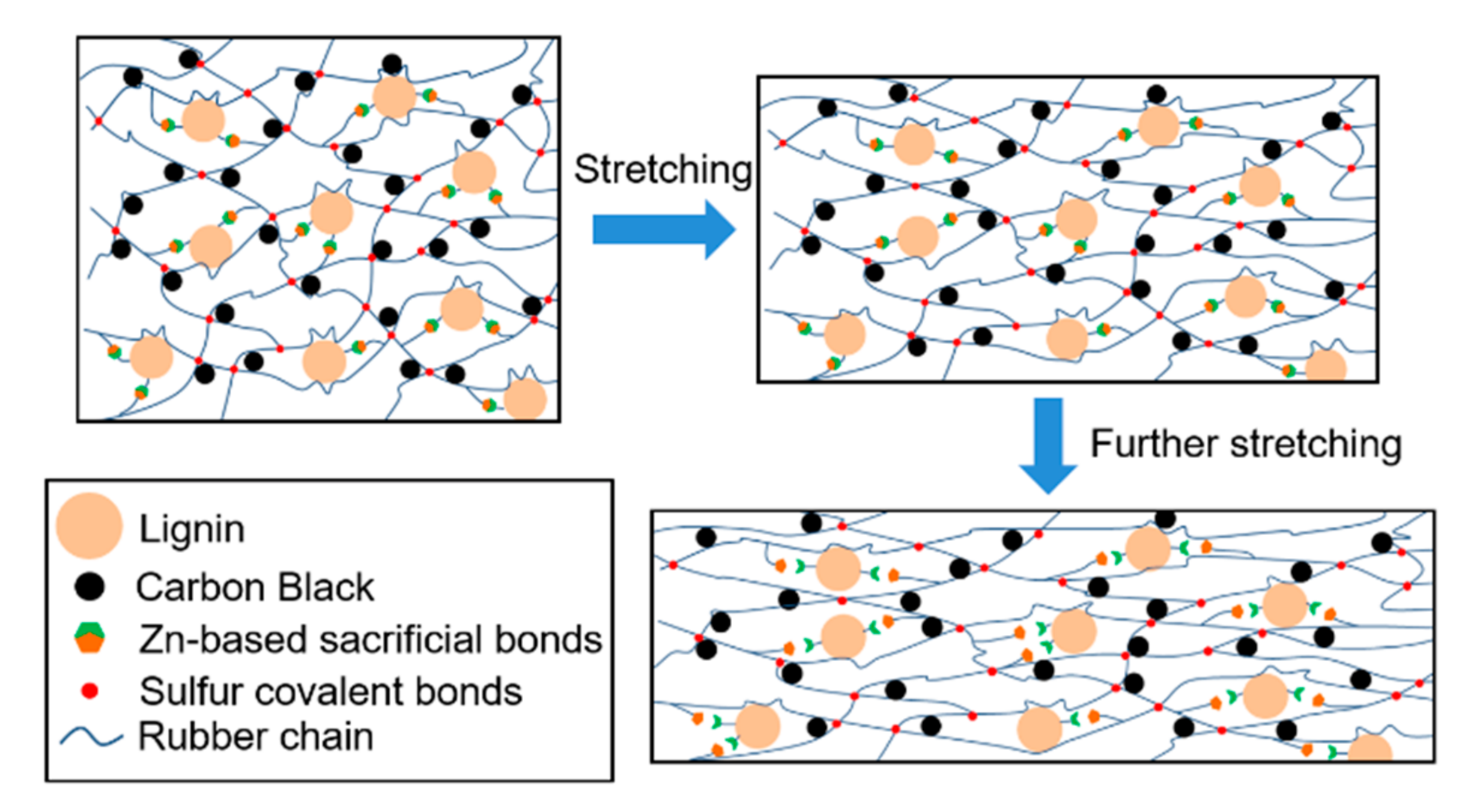
3.1. Introduction of Zn-Based Sacrificial Bonds into Lignin/CB/NBR Elastomers
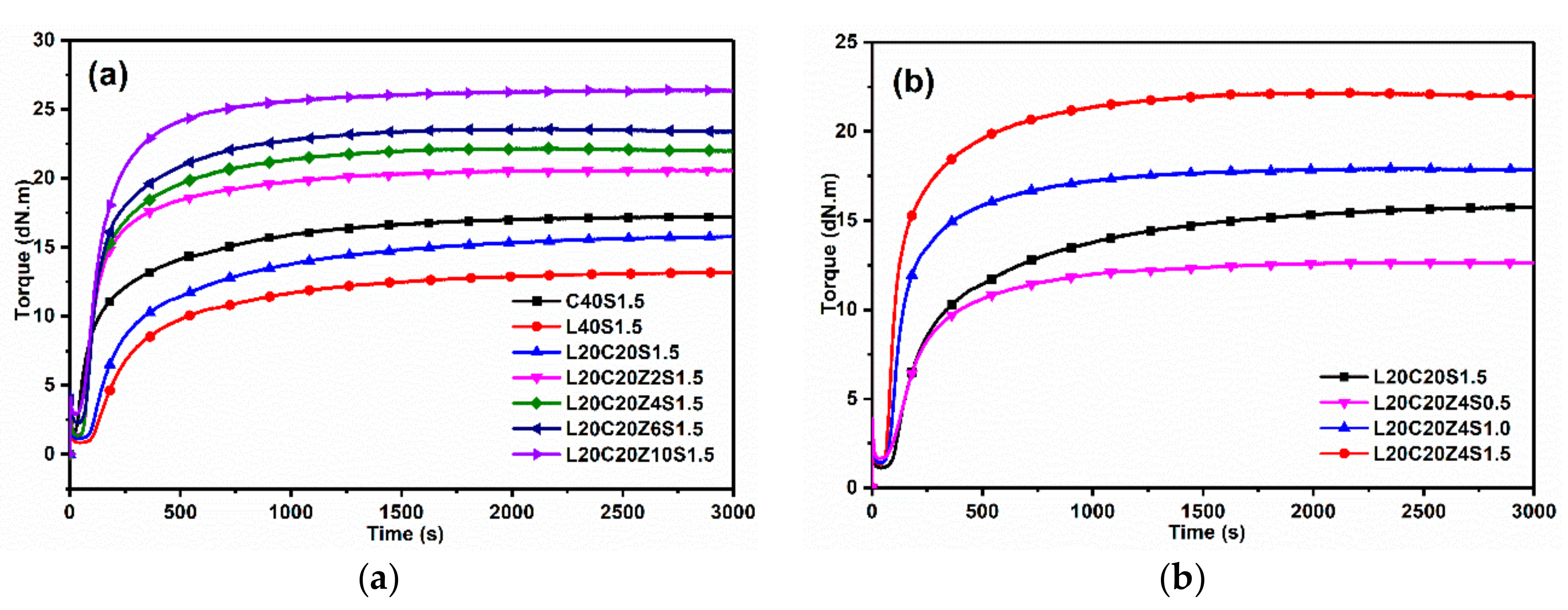
3.2. Curing Characteristics of Lignin/CB/NBR Elastomers
3.3. Mechanical Performance of Lignin/CB/NBR Elastomers
3.4. Dynamic Features of Lignin/CB/NBR Elastomers
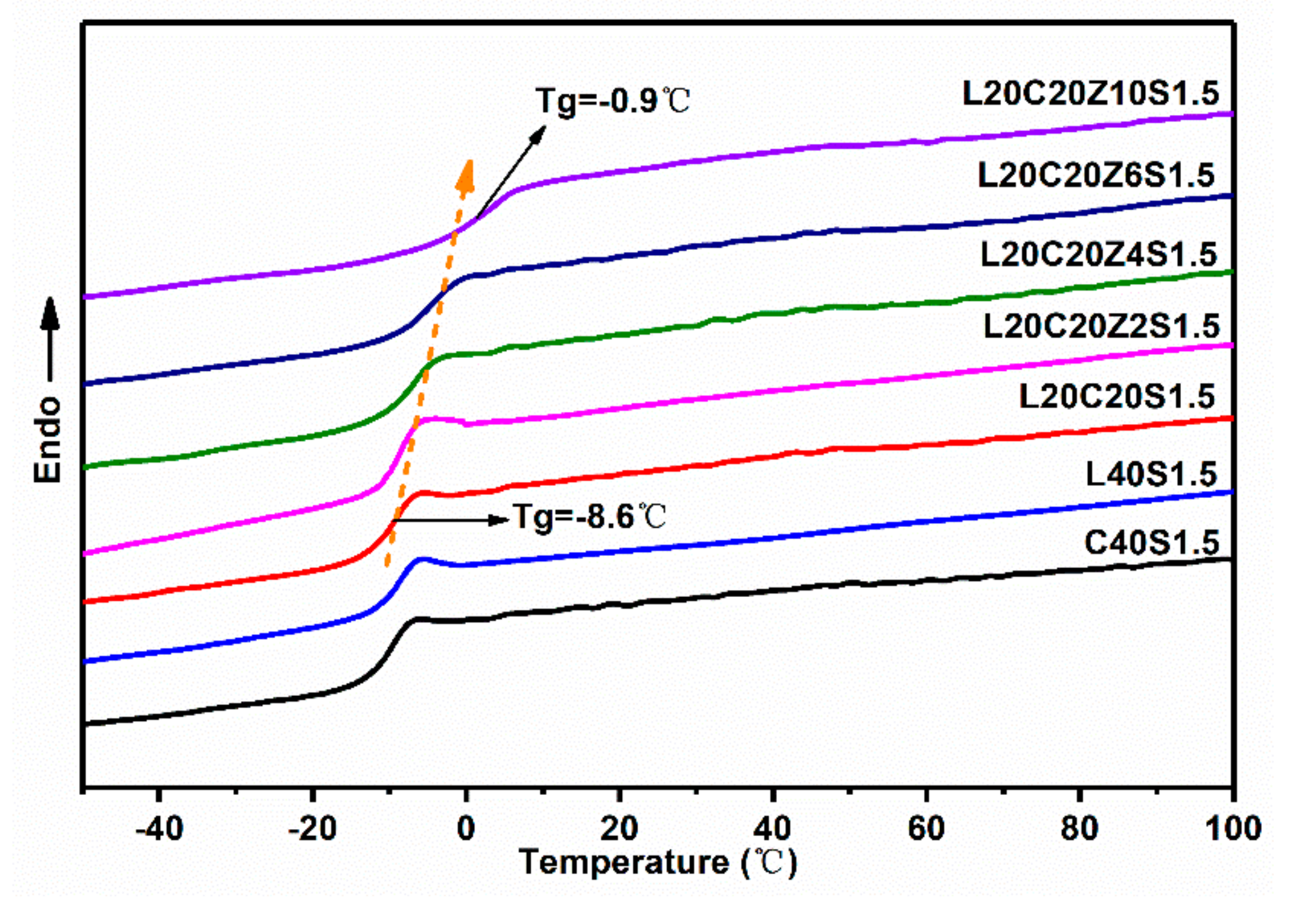
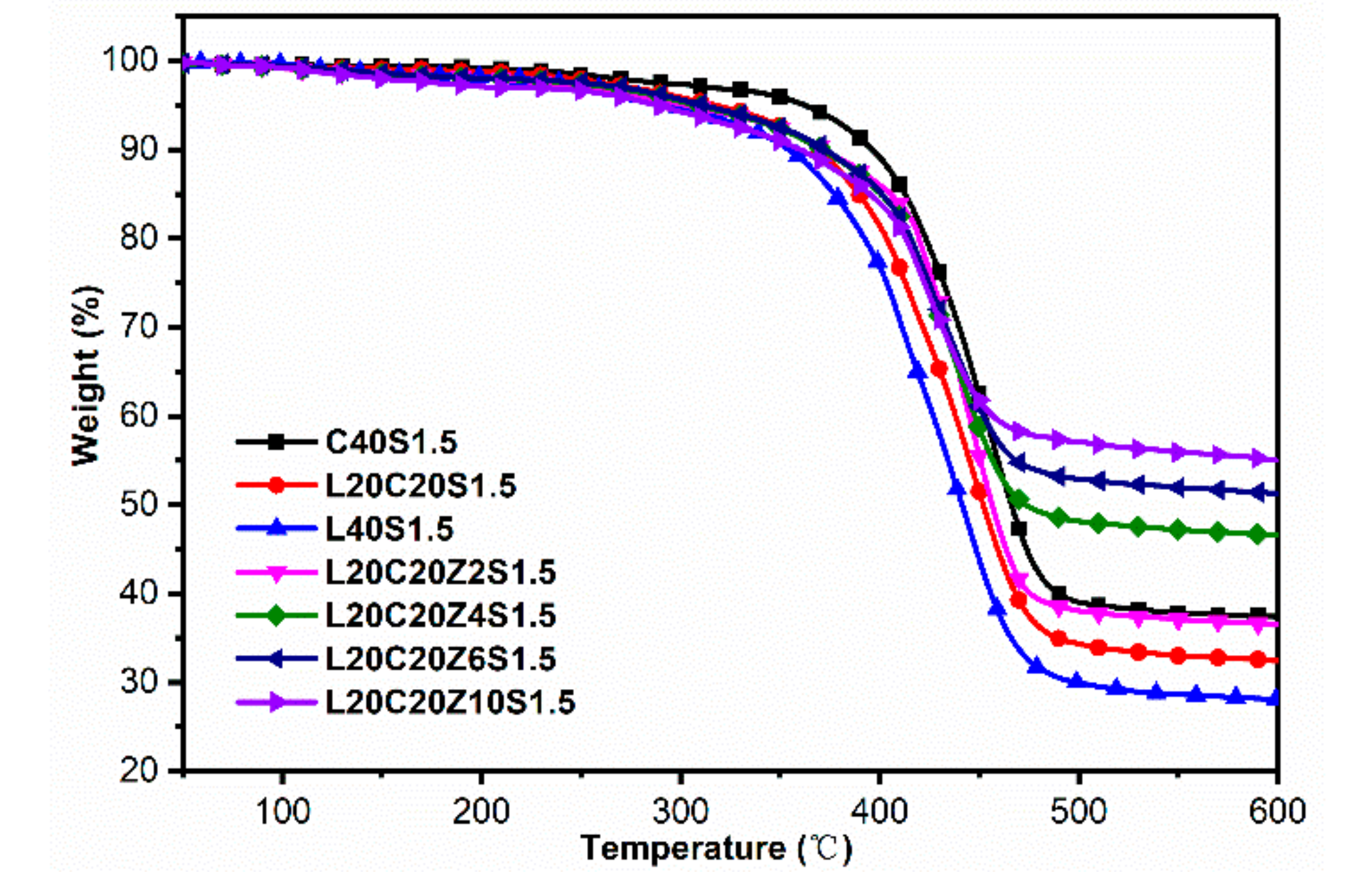
3.5. Thermal Behaviors of Lignin/CB/NBR Elastomers
3.6. Oil Resistance of Lignin/CB/NBR Elastomers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schmitt, C.N.Z.; Politi, Y.; Reinecke, A.; Harrington, M.J. Role of sacrificial protein-metal bond exchange in mussel byssal thread self-healing. Biomacromolecules 2015, 16, 2852–2861. [Google Scholar] [CrossRef] [PubMed]
- Byette, F.; Laventure, A.; Marcotte, I.; Pellerin, C. Metal-ligand interactions and salt bridges as sacrificial bonds in mussel byssus-derived materials. Biomacromolecules 2016, 17, 3277–3286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Sun, T.L.; Zhang, A.K.; Ikura, Y.; Nakajima, T.; Nonoyama, T.; Kurokawa, T.; Ito, O.; Ishitobi, H.; Gong, J.P. Tough physical double-network hydrogels based on amphiphilic triblock copolymers. Adv. Mater. 2016, 28, 4884–4890. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-Y.; Zhao, X.; Llleperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Moonet, D.J.; Viassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ducrot, E.; Chen, Y.; Bulters, M.; Sijbesma, R.P.; Creton, C. Toughening elastomers with sacrificial bonds and watching them break. Science 2014, 344, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Huang, J.; Guo, B.; Zhang, L.; Liu, F. Bioinspired engineering of sacrificial metal-ligand bonds into elastomers with supramechanical performance and adaptive recovery. Macromolecules 2016, 49, 1781–1789. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Tang, Z.; Huang, J.; Guo, B.; Huang, G. Bioinspired engineering of two different types of sacrificial bonds into chemically cross-linked cis-1,4-polyisoprene toward a high-performance elastomer. Macromolecules 2016, 49, 8593–8604. [Google Scholar] [CrossRef]
- Huang, J.; Tang, Z.; Yang, Z.; Guo, B. Bioinspired interface engineering in elastomer/graphene composites by constructing sacrificial metal-ligand bonds. Macromol. Rapid Commun. 2016, 37, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Praveen, S.; Chattopadhyay, P.K.; Albert, P.; Dalvi, V.G.; Chakraborty, B.C.; Chattopadhyay, S. Synergistic effect of carbon black and nanoclay fillers in styrene butadiene rubber matrix: Development of dual structure. Compos. Part A 2009, 40, 309–316. [Google Scholar] [CrossRef]
- Jiang, T.D. The existence of lignin. In Lignin, 2nd ed.; Chemical Industry Press: Beijing, China, 2001; pp. 2–3. [Google Scholar]
- Qian, Y.; Qiu, X.; Zhu, S. Lignin: A nature-inspired sun blocker for broad-spectrum sunscreens. Green Chem. 2015, 17, 320–324. [Google Scholar] [CrossRef]
- Frigerio, P. Biopolymers in Elastomers: Lignin as Biofiller for Tyre Compound. Ph.D. Thesis, University of Milan Bicocca, Milan, Italy, 2014. [Google Scholar]
- Miao, C.; Hamad, W.Y. Controlling lignin particle size for polymer blend applications. J. Appl. Polym. Sci. 2017, 134, 44669–44679. [Google Scholar] [CrossRef]
- Bahl, K.; Miyoshi, T.; Jana, S.C. Hybrid fillers of lignin and carbon black for lowering of viscoelastic loss in rubber compounds. Polymer 2014, 55, 3825–3835. [Google Scholar] [CrossRef]
- Tran, C.D.; Chen, J.; Keum, J.K.; Naskar, A.K. A new class of renewable thermoplastics with extraordinary performance from nanostructured lignin-elastomers. Adv. Funct. Mater. 2016, 26, 2677–2685. [Google Scholar] [CrossRef]
- Bova, T.; Tran, C.D.; Balakshin, M.Y.; Chen, J.; Capanemac, E.A.; Naskar, A.K. An approach towards tailoring interfacial structures and properties of multiphase renewable thermoplastics from lignin-nitrile rubber. Green Chem. 2016, 18, 5423–5437. [Google Scholar] [CrossRef]
- Ikeda, Y.; Phakkeeree, T.; Junkong, P.; Yokohama, H.; Phinyocheep, P.; Kitanod, R.; Kato, A. Reinforcing biofiller “lignin” for high-performance green natural rubber nanocomposites. RSC Adv. 2017, 7, 5222–5231. [Google Scholar] [CrossRef]
- Bahl, K.; Swanson, N.; Pugh, C.; Jana, S.C. Polybutadiene-g-polypentafluorostyrene as a coupling agent for lignin-filled rubber compounds. Polymer 2014, 55, 6754–6763. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J.J. Statistical mechanics of cross-linked polymer networks I. rubberlike elasticity. J. Chem. Phys. 1943, 11, 512–520. [Google Scholar] [CrossRef]
- Bristow, G.M.; Watson, W.F. Cohesive energy densities of polymers. Part 1.—Cohesive energy densities of rubbers by swelling measurements. Trans. Faraday Soc. 1958, 54, 1731–1741. [Google Scholar] [CrossRef]
- Antony, P.; Bandyopadhyay, S.; De, S.K. Synergism in properties of ionomeric polyblends based on zinc salts of carboxylated nitrile rubber and poly(ethylene-co-acrylic acid). Polymer 2000, 41, 787–793. [Google Scholar] [CrossRef]
- Li, M.-F.; Sun, S.-N.; Xu, F.; Sun, R.-C. Mild acetosolv process to fractionate bamboo for the biorefinery: Structural and antioxidant properties of the dissolved lignin. J. Agric. Food Chem. 2012, 60, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; He, H.; Jiang, H.; Ma, L.; Jia, D.M. Nano-lignin filled natural rubber composites: Preparation and characterization. eXPRESS Polym. Lett. 2013, 7, 480–493. [Google Scholar] [CrossRef] [Green Version]
- Yount, W.C.; Loveless, D.M.; Craig, S.L. Small-molecule dynamics and mechanisms underlying the macroscopic mechanical properties of coordinatively cross-linked polymer networks. J. Am. Chem. Soc. 2005, 127, 14488–14496. [Google Scholar] [CrossRef] [PubMed]
- Mozhdehi, D.; Ayala, S.; Cromwell, O.R.; Guan, Z.B. Self-healing multiphase polymers via dynamic metal-ligand interactions. J. Am. Chem. Soc. 2014, 136, 16128–16131. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qiu, X.; Liu, W.; Yang, D. Facile preparation of well-combined lignin-based carbon/ZnO hybrid composite with excellent photocatalytic activity. Appl. Surf. Sci. 2017, 426, 206–216. [Google Scholar] [CrossRef]





| Sample | Lignin | CB | ZnCl2 | S |
|---|---|---|---|---|
| L0C0S1.5 | 0 | 0 | 0 | 1.5 |
| L0C0Z10S1.5 | 0 | 0 | 10 | 1.5 |
| C40S1.5 | 0 | 40 | 0 | 1.5 |
| L20C20S1.5 | 20 | 20 | 0 | 1.5 |
| L40S1.5 | 40 | 0 | 0 | 1.5 |
| L20C20Z2S1.5 | 20 | 20 | 2 | 1.5 |
| L20C20Z4S1.5 | 20 | 20 | 4 | 1.5 |
| L20C20Z6S1.5 | 20 | 20 | 6 | 1.5 |
| L20C20Z10S1.5 | 20 | 20 | 10 | 1.5 |
| L20C20Z4S1.0 | 20 | 20 | 4 | 1.0 |
| L20C20Z4S0.5 | 20 | 20 | 4 | 0.5 |
| Sample | TS (min) | T90 (min) | ML (dN.m) | MH (dN.m) | ΔM (dN.m) | CRI (min−1) | μ (×10−4 mol/c |
|---|---|---|---|---|---|---|---|
| C40S1.5 | 0.73 | 15.02 | 1.57 | 17.26 | 15.69 | 6.99 | 3.28 |
| L20C20S1.5 | 1.98 | 17.32 | 1.11 | 15.78 | 14.67 | 6.52 | 1.80 |
| L40S1.5 | 2.32 | 18.55 | 0.66 | 13.19 | 12.53 | 6.16 | 1.33 |
| L20C20Z2S1.5 | 1.22 | 9.96 | 1.17 | 20.61 | 19.44 | 11.44 | 2.18 |
| L20C20Z4S1.5 | 1.20 | 9.78 | 1.35 | 22.18 | 20.85 | 11.64 | 2.67 |
| L20C20Z6S1.5 | 1.32 | 9.50 | 2.25 | 23.41 | 21.16 | 12.21 | 2.93 |
| L20C20Z10S1.5 | 1.12 | 8.08 | 2.88 | 26.42 | 23.54 | 14.35 | 3.36 |
| L20C20Z4S1.0 | 1.47 | 9.97 | 1.41 | 17.93 | 16.18 | 11.75 | 1.35 |
| L20C20Z4S0.5 | 2.02 | 12.98 | 1.65 | 12.67 | 11.02 | 9.12 | 0.43 |
| Sample | Elongation at Break (%) | Tensile Strength (MPa) | Young Modulus (MPa) | Energy Dissipation (MJ·m−3) | Elastic Recovery (%) | Hardness (Shore A) |
| C40S1.5 | 408 (±11) | 22.6 (±0.7) | 9.3 (±0.2) | 41.4 | 99.3 (±0.2) | 67 |
| L20C20S1.5 | 560 (±9) | 19.4 (±0.4) | 9.0 (±0.1) | 44.0 | 98.9 (±0.1) | 65 |
| L40S1.5 | 702 (±23) | 12.8 (±0.2) | 7.8 (±0.2) | 33.8 | 98.7 (±0.2) | 63 |
| L20C20Z2S1.5 | 418 (±6) | 23.1 (±0.1) | 11.7 (±0.3) | 44.6 | 98.9 (±0.2) | 70 |
| L20C20Z4S1.5 | 326 (±5) | 21.7 (±0.4) | 12.1 (±0.2) | 35.3 | 99.0 (±0.3) | 71 |
| L20C20Z6S1.5 | 336 (±5) | 24.2 (±0.7) | 14.7 (±0.3) | 39.1 | 98.9 (±0.1) | 73 |
| L20C20Z10S1.5 | 332 (±16) | 25.9 (±0.4) | 13.4 (±0.4) | 41.0 | 98.9 (±0.1) | 75 |
| L20C20Z4S1.0 | 442 (±15) | 18.8 (±0.6) | 9.8 (±0.1) | 37.3 | 99.2 (±0.2) | 69 |
| L20C20Z4S0.5 | 579 (±8) | 19.5 (±0.3) | 9.2 (±0.2) | 46.3 | 99.2 (±0.3) | 66 |
| Sample | Tg (°C) from DSC | Tg (°C) from DMA | T10%1 (°C) | T30%1 (°C) | T50%1 (°C) | Residue at 600 °C (%) |
|---|---|---|---|---|---|---|
| C40S1.5 | −10.0 | −4.4 | 396.4 | 439.9 | 466.1 | 37.4 |
| L20C20S1.5 | −8.6 | −4.1 | 368.6 | 421.8 | 452.0 | 32.4 |
| L40S1.5 | −8.5 | −4.7 | 354.6 | 411.3 | 441.4 | 28.1 |
| L20C20Z2S1.5 | −8.4 | −4.0 | 372.2 | 433.4 | 456.4 | 36.5 |
| L20C20Z4S1.5 | −5.9 | −4.0 | 371.2 | 432.1 | 473.3 | 46.6 |
| L20C20Z6S1.5 | −4.3 | 1.9 | 373.0 | 433.5 | -- | 51.3 |
| L20C20Z10S1.5 | −0.9 | 9.5 | 360.2 | 431.5 | -- | 55.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Liu, W.; Huang, J.; Yang, D.; Qiu, X. Bioinspired Engineering towards Tailoring Advanced Lignin/Rubber Elastomers. Polymers 2018, 10, 1033. https://doi.org/10.3390/polym10091033
Wang H, Liu W, Huang J, Yang D, Qiu X. Bioinspired Engineering towards Tailoring Advanced Lignin/Rubber Elastomers. Polymers. 2018; 10(9):1033. https://doi.org/10.3390/polym10091033
Chicago/Turabian StyleWang, Haixu, Weifeng Liu, Jinhao Huang, Dongjie Yang, and Xueqing Qiu. 2018. "Bioinspired Engineering towards Tailoring Advanced Lignin/Rubber Elastomers" Polymers 10, no. 9: 1033. https://doi.org/10.3390/polym10091033