A Geometry Effect of Carbon Nanomaterials on Flame Retardancy and Mechanical Properties of Ethylene-Vinyl Acetate/Magnesium Hydroxide Composites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Graphene
2.3. Preparation of EVA Composites
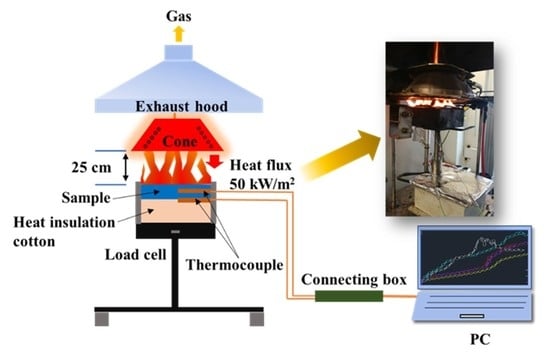
2.4. Characterization and Measurement
3. Results and Discussion
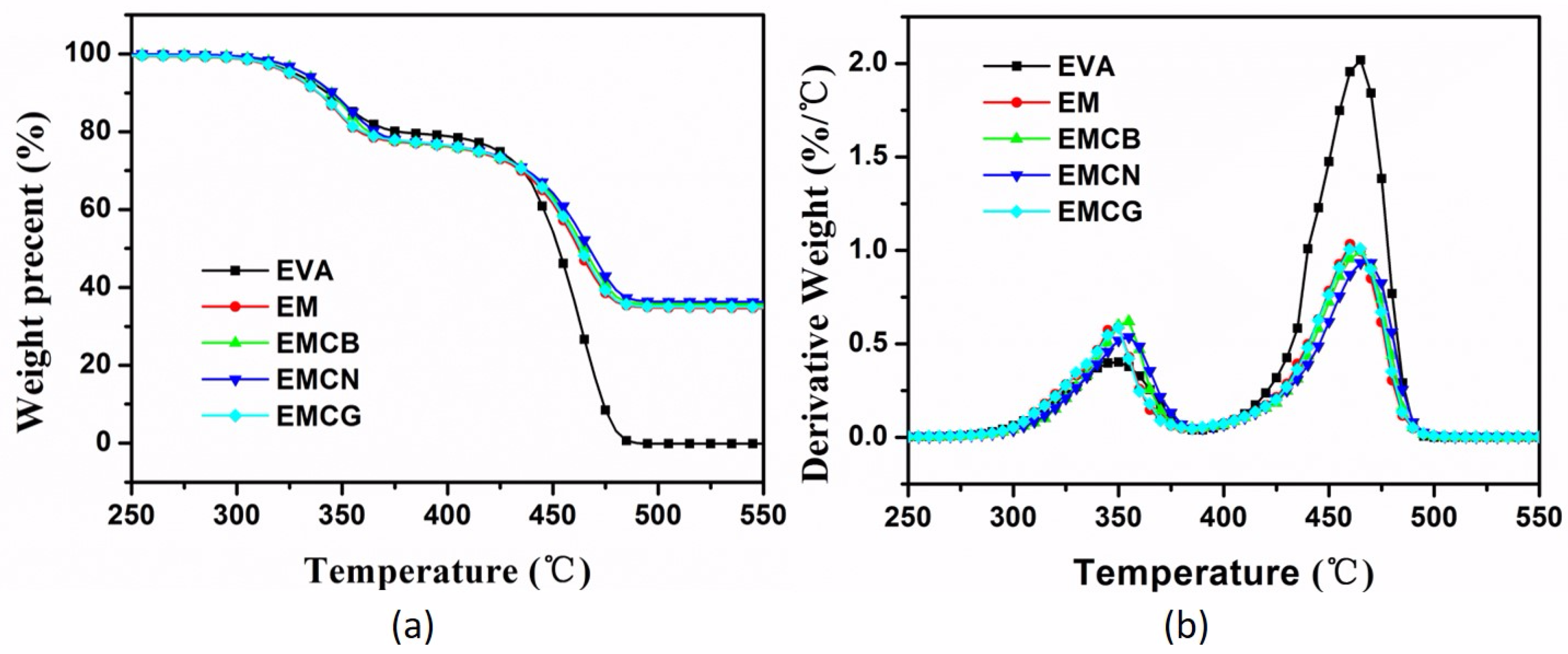
3.1. Thermal Stability
3.2. LOI and UL-94
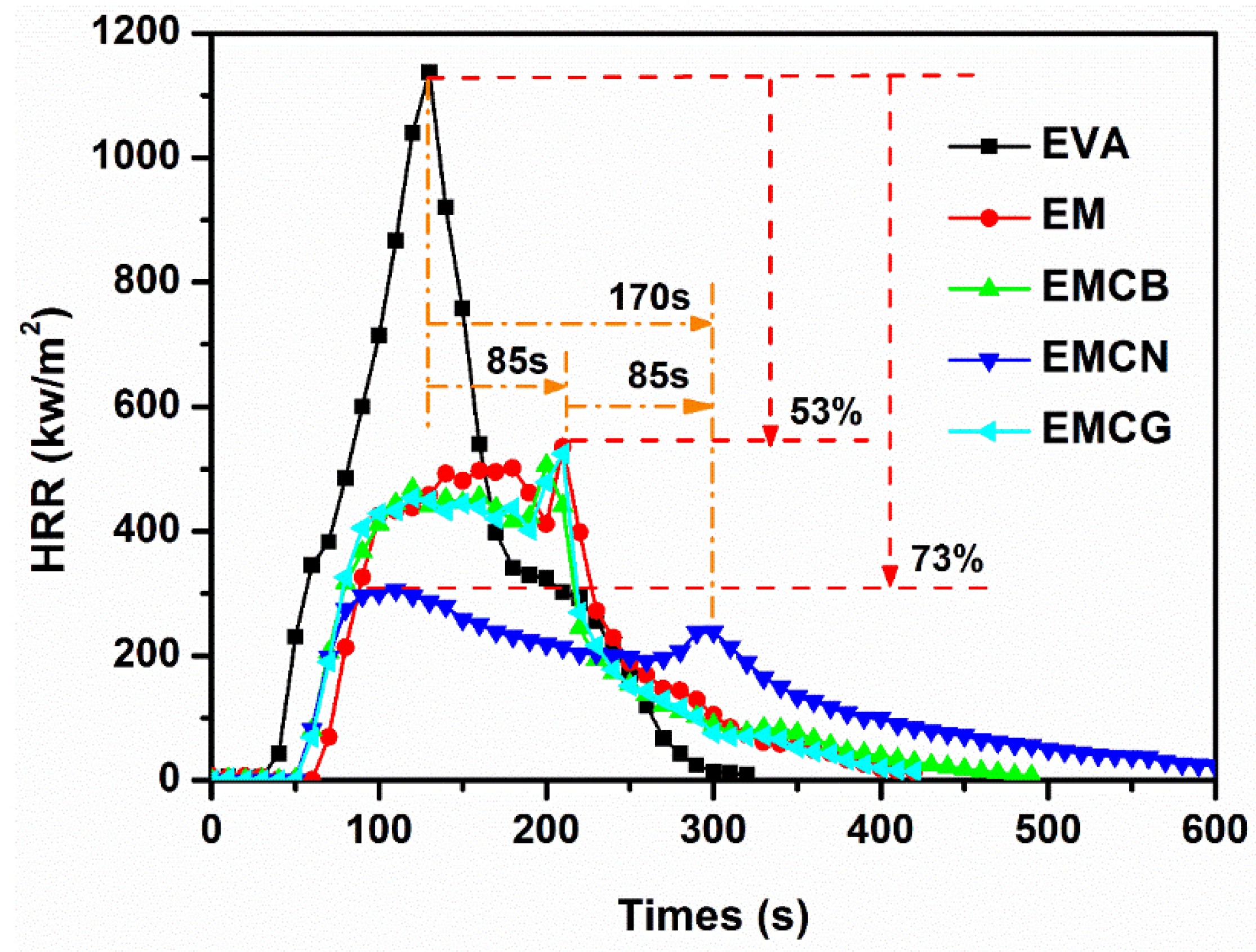
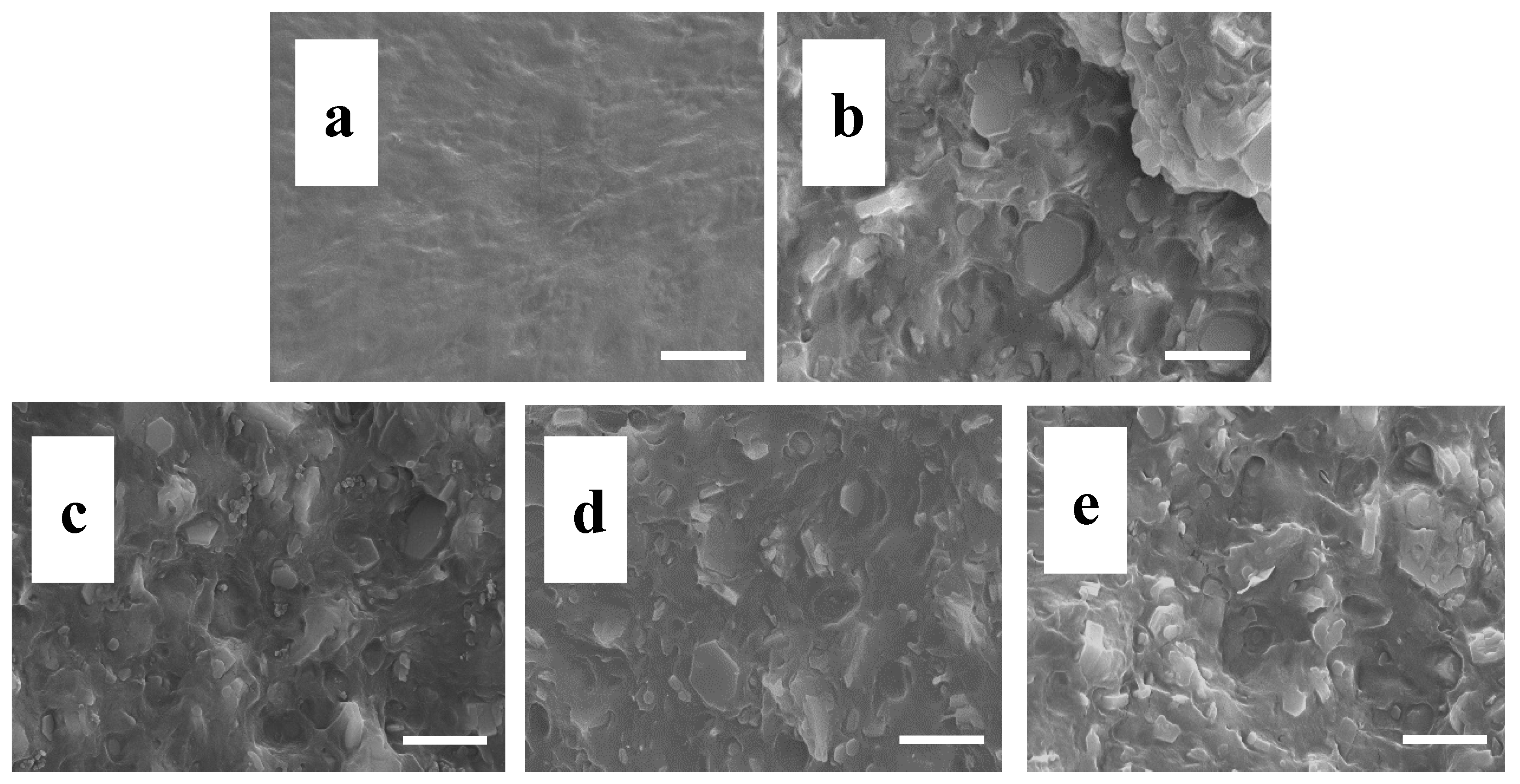
3.3. Cone Calorimeter Test
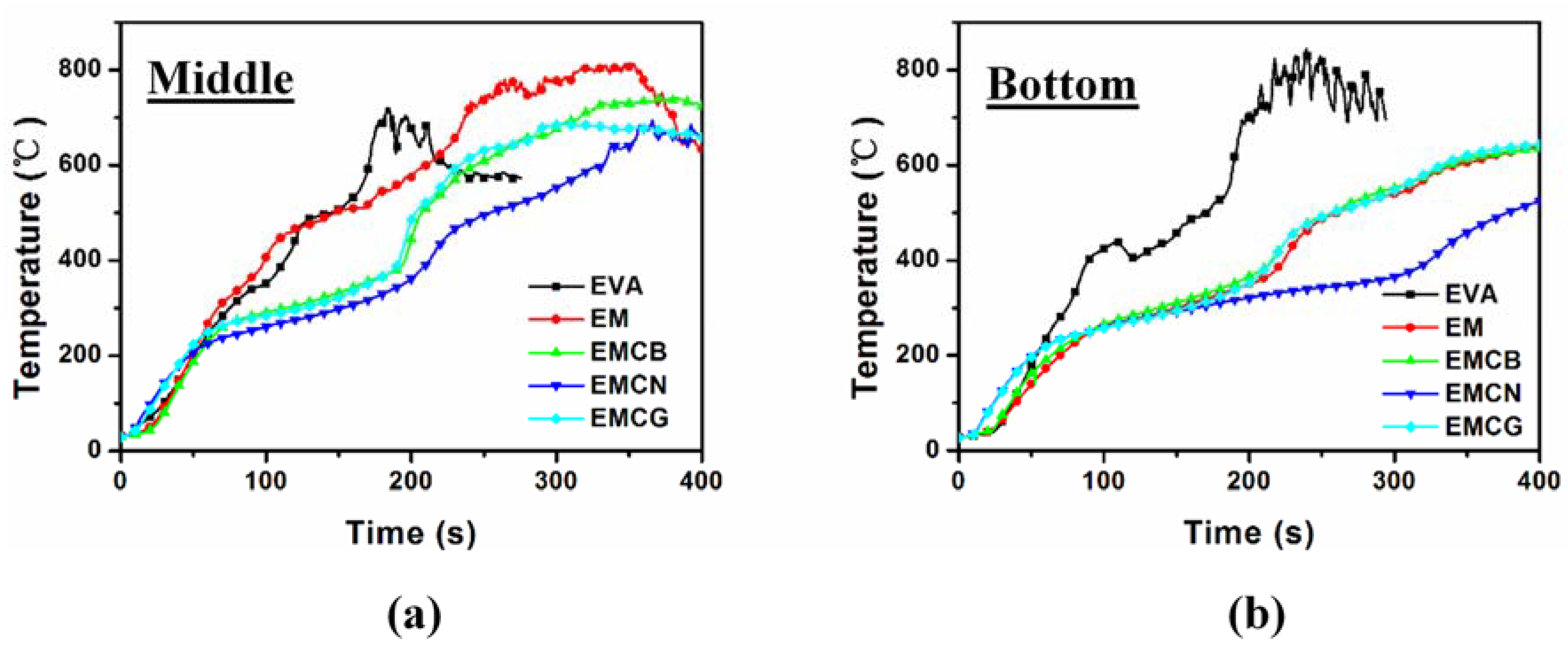
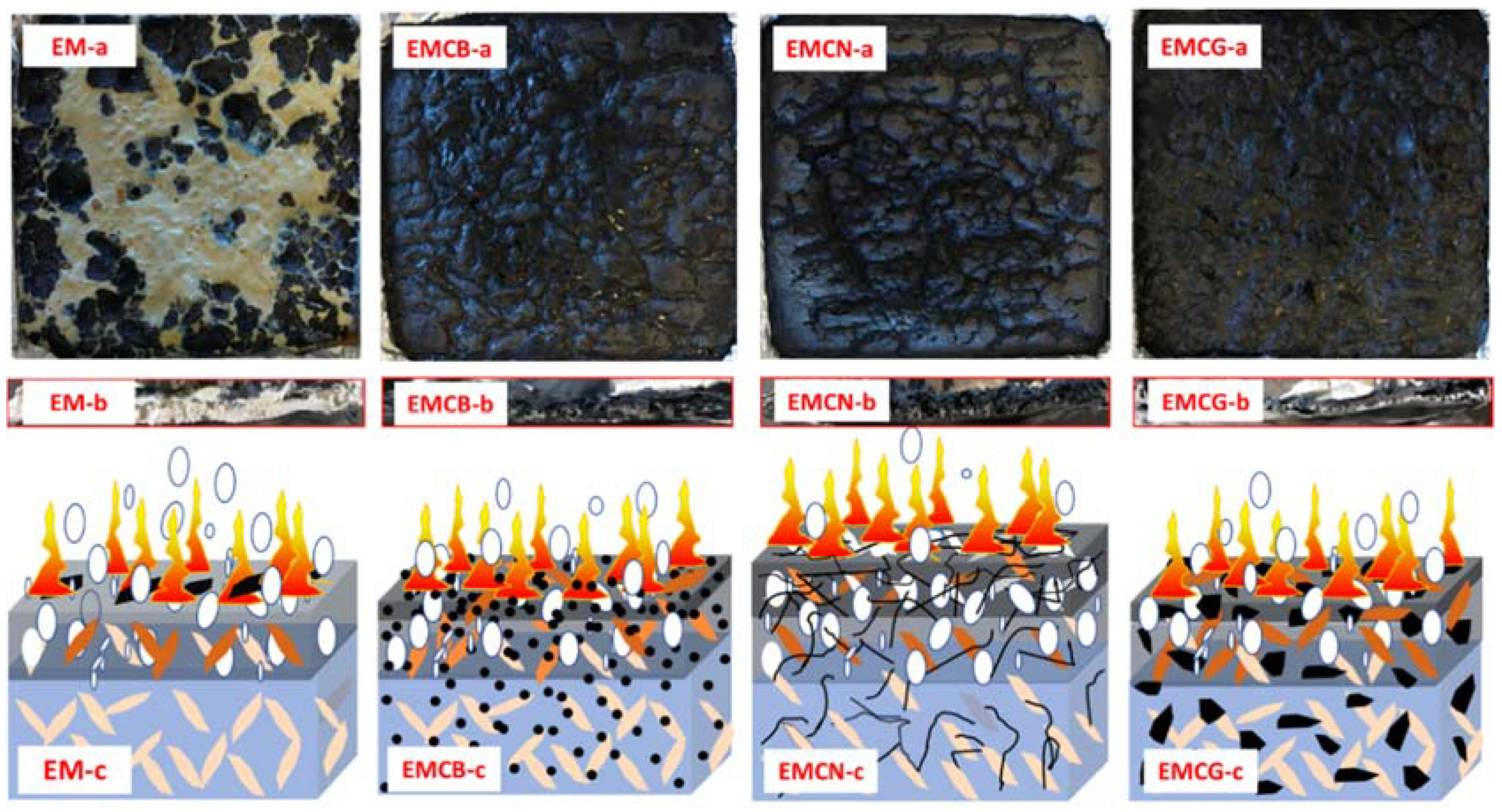
3.4. Flame-Retardant Mechanism
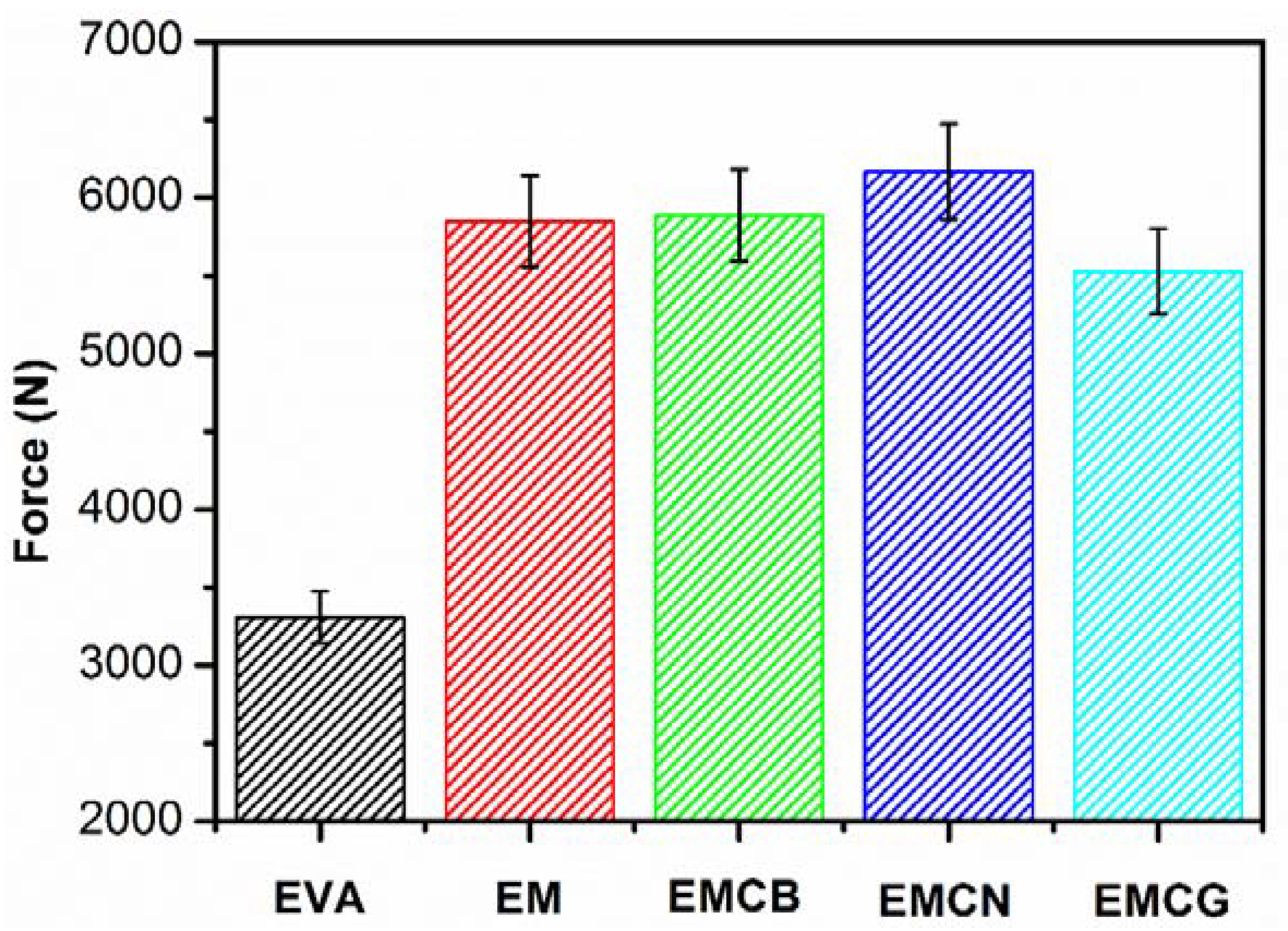
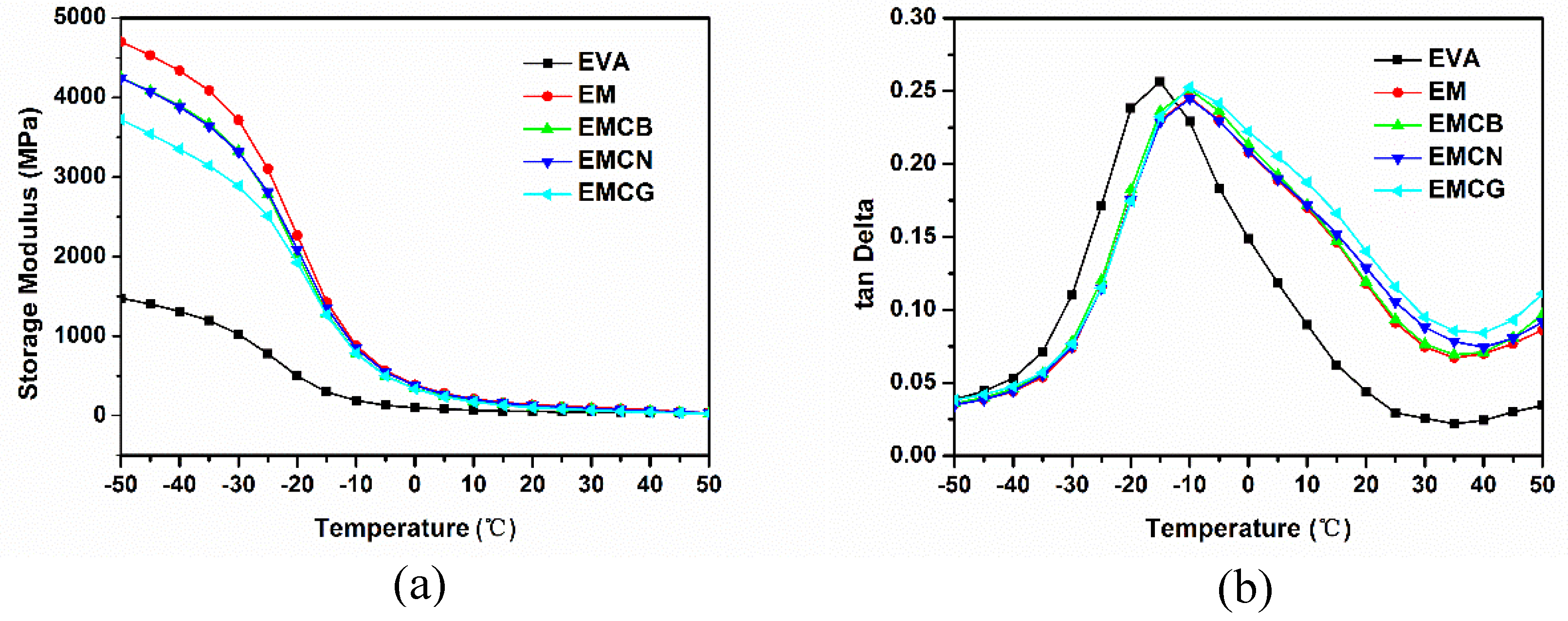
3.5. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fu, M.Z.; Qu, B.J. Synergistic flame retardant mechanism of fumed silica in ethylene-vinyl acetate/magnesium hydroxide blends. Polym. Degrad. Stab. 2004, 85, 633–639. [Google Scholar] [CrossRef]
- Isitman, N.A.; Kaynak, C. Nanoclay and carbon nanotubes as potential synergists of an organophosphorus flame-retardant in poly(methyl methacrylate). Polym. Degrad. Stab. 2010, 95, 1523–1532. [Google Scholar] [CrossRef]
- Presti, C.; Ferry, L.; Alauzun, J.G.; Dumazert, L.; Gallard, B.; Quantin, J.C.; Lopez Cuesta, J.M.; Mutin, P.H. Functionalized nanodiamond as potential synergist in flame-retardant ethylene vinyl acetate. Diam. Relat. Mater. 2017, 76, 141–149. [Google Scholar] [CrossRef]
- Liu, L.; Hu, J.; Zhuo, J.L.; Jiao, C.M.; Chen, X.L.; Li, S.X. Synergistic flame retardant effects between hollow glass microspheres and magnesium hydroxide in ethylene-vinyl acetate composites. Polym. Degrad. Stab. 2014, 104, 87–94. [Google Scholar] [CrossRef]
- Wu, X.F.; Wang, L.C.; Wu, C.; Yu, J.H.; Xie, L.Y.; Wang, G.L.; Jiang, P.K. Influence of char residues on flammability of EVA/EG, EVA/NG and EVA/GO composites. Polym. Degrad. Stab. 2012, 97, 54–63. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Tomaszewska, J.; Skórczewska, K.; Jesionowski, T. Preparation and Characterization of Eco-Friendly Mg(OH)2/Lignin Hybrid Material and Its Use as a Functional Filler for Poly(Vinyl Chloride). Polymers 2017, 9, 258. [Google Scholar] [CrossRef]
- Maira, B.; Chammingkwan, P.; Terano, M.; Taniike, T. New Reactor Granule Technology for Highly Filled Nanocomposites: Effective Flame Retardation of Polypropylene/Magnesium Hydroxide Nanocomposites. Macromol. Mater. Eng. 2015, 300, 679–683. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.; Zaghloul, M.M.Y. Influence of flame retardant magnesium hydroxide on the mechanical properties of high density polyethylene composites. J. Reinf. Plast. Compos. 2017, 36, 1802–1816. [Google Scholar] [CrossRef]
- Suihkonen, R.; Nevalainen, K.; Orell, O.; Honkanen, M.; Tang, L.C.; Zhang, H.; Zhang, Z.; Vuorinen, J. Performance of epoxy filled with nano- and micro-sized Magnesium hydroxide. J. Mater. Sci. 2012, 47, 1480–1488. [Google Scholar] [CrossRef]
- Liu, J.C.; Peng, S.G.; Zhang, Y.B.; Chang, H.B.; Yu, Z.L.; Pan, B.L.; Lu, C.; Ma, J.Y.; Niu, Q.S. Influence of microencapsulated red phosphorus on the flame retardancy of high impact polystyrene/magnesium hydroxide composite and its mode of action. Polym. Degrad. Stab. 2015, 121, 208–221. [Google Scholar] [CrossRef]
- Durin, F.A.; Ferry, L.; Lopez, C.J.M.; Crespy, A. Magnesium hydroxide/zinc borate/talc compositions as flame-retardants in EVA copolymer. Polym. Int. 2000, 49, 1101–1105. [Google Scholar] [CrossRef]
- Yen, Y.Y.; Wang, H.T.; Guo, W.J. Synergistic flame retardant effect of metal hydroxide and nanoclay in EVA composites. Polym. Degrad. Stab. 2012, 97, 863–869. [Google Scholar] [CrossRef]
- Riva, A.; Zanetti, M.; Braglia, M.; Camino, G.; Falqui, L. Thermal degradation and rheological behaviour of EVA/montmorillonite nanocomposites. Polym. Degrad. Stab. 2002, 77, 299–304. [Google Scholar] [CrossRef]
- Zhang, G.B.; Ding, P.; Zhang, M.; Qu, B.J. Synergistic effects of layered double hydroxide with hyperfine magnesium hydroxide in halogen-free flame retardant EVA/HFMH/LDH nanocomposites. Polym. Degrad. Stab. 2007, 92, 1715–1720. [Google Scholar] [CrossRef]
- Dittrich, B.; Wartig, K.A.; Mülhaupt, R.; Schartel, B. Flame-Retardancy Properties of Intumescent Ammonium Poly(Phosphate) and Mineral Filler Magnesium Hydroxide in Combination with Graphene. Polymers 2014, 6, 2875–2895. [Google Scholar] [CrossRef] [Green Version]
- Dittrich, B.; Wartig, K.A.; Hofmann, D.; Mülhaupt, R.; Schartel, B. Flame retardancy through carbon nanomaterials: Carbon black, multiwall nanotubes, expanded graphite, multi-layer graphene and graphene in polypropylene. Polym. Degrad. Stab. 2013, 98, 1495–1505. [Google Scholar] [CrossRef]
- Wang, X.; Kalali, E.N.; Wan, J.T.; Wang, D.Y. Carbon-family materials for flame retardant polymeric materials. Prog. Polym. Sci. 2017, 69, 22–46. [Google Scholar] [CrossRef]
- Mamani, A.; Ebrahimi, M.; Ataeefard, M. A study on mechanical, thermal and flame retardant properties of epoxy/expandable graphite composites. Pigment Resin Technol. 2017, 46, 131–138. [Google Scholar] [CrossRef]
- Caschera, D.; Toro, R.G.; Federici, F.; Riccucci, C.; Ingo, G.M.; Gigli, G.; Cortese, B. Flame retardant properties of plasma pre-treated/diamond-like carbon (DLC) coated cotton fabrics. Cellulose 2015, 22, 2797–2809. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Grulke, E.; Hilding, J.; Harris, R.; Awad, W.; Douglas, J. Thermal Degradation and Flammability Properties of Poly(propylene)/Carbon Nanotube Composites. Macromol. Rapid Commun. 2002, 23, 761–765. [Google Scholar] [CrossRef]
- Wen, X.; Wang, Y.J.; Gong, J.; Liu, J.; Tian, N.N.; Wang, Y.H.; Jiang, Z.W.; Qiu, J.; Tang, T. Thermal and flammability properties of polypropylene/carbon black nanocomposites. Polym. Degrad. Stab. 2012, 97, 793–801. [Google Scholar] [CrossRef]
- Yang, H.F.; Gong, J.; Wen, X.; Xue, J.; Chen, Q.; Jiang, Z.W.; Tian, N.N.; Tang, T. Effect of carbon black on improving thermal stability, flame retardancy and electrical conductivity of polypropylene/carbon fiber composites. Compos. Sci. Technol. 2015, 113, 31–37. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang,, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Fang, Z.P.; Yan, H.Q.; Chevali, V.S. Synergistic flame retardancy effect of graphene nanosheets and traditional retardants on epoxy resin. Compos. Part A Appl. Sci. Manuf. 2016, 89, 26–32. [Google Scholar] [CrossRef]
- Liu, S.; Yan, H.Q.; Fang, Z.P.; Wang, H. Effect of graphene nanosheets on morphology, thermal stability and flame retardancy of epoxy resin. Compos. Sci. Technol. 2014, 90, 40–47. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.W.; Li, C.; Shi, G.Q. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Li, Z.; González, A.J.; Heeralal, V.B.; Wang, D.Y. Covalent assembly of MCM-41 nanospheres on graphene oxide for improving fire retardancy and mechanical property of epoxy resin. Compos. Part B Eng. 2018, 138, 101–112. [Google Scholar] [CrossRef]
- Yu, L.; Chen, L.; Dong, L.P.; Li, L.J.; Wang, Y.Z. Organic-inorganic hybrid flame retardant: Preparation, characterization and application in EVA. RSC Adv. 2014, 4, 17812–17821. [Google Scholar] [CrossRef]
- Zhou, K.Q.; Tang, G.; Jiang, S.H.; Gui, Z.; Hu, Y. Combination effect of MoS2 with aluminum hypophosphite in flame retardant ethylene-vinyl acetate composites. RSC Adv. 2016, 6, 37672–37680. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Du, F.M.; Douglas, J.F.; Winey, K.I.; Harris, R.H., Jr.; Shields, J.R. Nanoparticle networks reduce the flammability of polymer nanocomposites. Nat. Mater. 2005, 4, 928–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Liu, L.J.; Jimenez Gonzalez, A.; Wang, D.Y. Bioinspired polydopamine-induced assembly of ultrafine Fe(OH)3 nanoparticles on halloysite toward highly efficient fire retardancy of epoxy resin via an action of interfacial catalysis. Polym. Chem. 2017, 8, 3926–3936. [Google Scholar] [CrossRef]
- Jian, R.K.; Wang, P.; Xia, L.; Yu, X.Q.; Zheng, X.L.; Shao, Z.B. Low-flammability epoxy resins with improved mechanical properties using a Lewis base based on phosphaphenanthrene and 2-aminothiazole. J. Mater. Sci. 2017, 52, 9907–9921. [Google Scholar] [CrossRef]
- Jian, R.K.; Wang, P.; Duan, W.S.; Wang, J.S.; Zheng, X.L.; Weng, J.B. Synthesis of a Novel P/N/S-Containing Flame Retardant and Its Application in Epoxy Resin: Thermal Property, Flame Retardance, and Pyrolysis Behavior. Ind. Eng. Chem. Res. 2016, 55, 11520–11527. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Mu, M.F.; Winey, K.; Cipriano, B.; Raghavan, S.R.; Pack, S.; Rafailovich, M.; Yang, Y.; Grulke, E.; Shields, J.; et al. Relation between the viscoelastic and flammability properties of polymer nanocomposites. Polymer 2008, 49, 4358–4368. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Fagan, J.; Douglas, J.F.; Yamamoto, K.; Heckert, A.N.; Leigh, S.D.; Obrzut, J.; Du, F.M.; Lin-Gibson, S.; Mu, M.F.; et al. Relationship between dispersion metric and properties of PMMA/SWNT nanocomposites. Polymer 2007, 48, 4855–4866. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, J.; Jiménez-Morales, A.; Torralba, J.M. Torque rheology of zircon feedstocks for powder injection moulding. J. Eur. Ceram. Soc. 2012, 32, 4063–4072. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Wu, Q.H.; Qu, B.J. Synergistic effects and mechanism of multiwalled carbon nanotubes with magnesium hydroxide in halogen-free flame retardant EVA/MH/MWNT nanocomposites. Polym. Degrad. Stab. 2009, 94, 751–756. [Google Scholar] [CrossRef]
- Wang, D.; Song, L.; Zhou, K.Q.; Yu, X.J.; Hu, Y.; Wang, J. Anomalous nano-barrier effects of ultrathin molybdenum disulfide nanosheets for improving the flame retardance of polymer nanocomposites. J. Mater. Chem. A 2015, 3, 14307–14317. [Google Scholar] [CrossRef]



| Sample | EVA (wt %) | MH (wt %) | CB (wt %) | CN (wt %) | CG (wt %) |
|---|---|---|---|---|---|
| EVA | 100 | 0 | 0 | 0 | 0 |
| EM | 50 | 50 | 0 | 0 | 0 |
| EMCB | 50 | 49 | 1 | 0 | 0 |
| EMCN | 50 | 49 | 0 | 1 | 0 |
| EMCG | 50 | 49 | 0 | 0 | 1 |
| Sample | T−5%a (°C) | T−50%b (°C) | Tmax1c (°C) | Tmax2d (°C) | Char e (%) |
|---|---|---|---|---|---|
| EVA | 327 | 452 | 348 | 466 | 0 |
| EM | 324 | 462 | 348 | 461 | 34.5 |
| EMCB | 331 | 464 | 353 | 464 | 35.5 |
| EMCN | 332 | 467 | 354 | 468 | 36.1 |
| EMCG | 325 | 463 | 349 | 463 | 35.0 |
| Samples | LOI (%) | UL-94 | ||||
|---|---|---|---|---|---|---|
| t1 (s) | t2 (s) | Dripping | Igniting the Cotton | Rating | ||
| EVA | 18.5 ± 0.2 | / | / | Yes | Yes | Fail |
| EM | 25.8 ± 0.2 | 2 | 9 | Yes | No | V-1 |
| EMCB | 28.2 ± 0.2 | 1 | 2 | No | No | V-0 |
| EMCN | 33.3 ± 0.2 | 1 | 1 | No | No | V-0 |
| EMCG | 27.6 ± 0.2 | 1 | 3 | No | No | V-0 |
| Sample | tign (s) | PHRR (kW/m2) | THR (MJ/m2) | SPR (m2/s) | TSP (m2/kg) | esidue (wt %) |
|---|---|---|---|---|---|---|
| EVA | 36 ± 2 | 1139 ± 50 | 110 ± 5 | 0.084 ± 0.004 | 10.0 ± 0.5 | 0.0 |
| EM | 66 ± 2 | 536 ± 20 | 85 ± 5 | 0.058 ± 0.002 | 5.9 ± 0.2 | 40.4 ± 1.0 |
| EMCB | 55 ± 1 | 506 ± 20 | 84 ± 5 | 0.052 ± 0.002 | 5.3 ± 0.2 | 41.6 ± 1.5 |
| EMCN | 50 ± 2 | 308 ± 15 | 83 ± 4 | 0.029 ± 0.001 | 6.4 ± 0.3 | 48.7 ± 2.0 |
| EMCG | 54 ± 1 | 564 ± 20 | 82 ± 4 | 0.053 ± 0.002 | 5.5 ± 0.2 | 42.9 ± 1.0 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| EVA | 23.9 ± 0.5 | 1286 ± 50 |
| EM | 10.5 ± 0.3 | 753 ± 30 |
| EMCB | 10.6 ± 0.2 | 758 ± 25 |
| EMCN | 9.8 ± 0.2 | 612 ± 25 |
| EMCG | 10.7 ± 0.3 | 634 ± 25 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.-Q.; Li, Z.; Yang, Y.-X.; Zhang, Y.-L.; Wen, X.; Li, N.; Fu, C.; Jian, R.-K.; Li, L.-J.; Wang, D.-Y. A Geometry Effect of Carbon Nanomaterials on Flame Retardancy and Mechanical Properties of Ethylene-Vinyl Acetate/Magnesium Hydroxide Composites. Polymers 2018, 10, 1028. https://doi.org/10.3390/polym10091028
Liu Z-Q, Li Z, Yang Y-X, Zhang Y-L, Wen X, Li N, Fu C, Jian R-K, Li L-J, Wang D-Y. A Geometry Effect of Carbon Nanomaterials on Flame Retardancy and Mechanical Properties of Ethylene-Vinyl Acetate/Magnesium Hydroxide Composites. Polymers. 2018; 10(9):1028. https://doi.org/10.3390/polym10091028
Chicago/Turabian StyleLiu, Zhi-Qi, Zhi Li, Yun-Xian Yang, Yan-Ling Zhang, Xin Wen, Na Li, Can Fu, Rong-Kun Jian, Li-Juan Li, and De-Yi Wang. 2018. "A Geometry Effect of Carbon Nanomaterials on Flame Retardancy and Mechanical Properties of Ethylene-Vinyl Acetate/Magnesium Hydroxide Composites" Polymers 10, no. 9: 1028. https://doi.org/10.3390/polym10091028