Hyperbranched Liquid Crystals Modified with Sisal Cellulose Fibers for Reinforcement of Epoxy Composites
Abstract
:1. Introduction
2. Experiment Section
2.1. Materials
2.2. Preparation of Sisal Cellulose Fibers (SCFs)
2.3. The SCF-Grafted Hyperbranched Polyglycerol on the Surface (SCFs-HPG)
2.4. Preparation of 4,4-di (β-hydroxy ethoxy) Biphenyl (BP6)
2.5. Preparation of SCFs-HLP
2.6. Preparation of the SCFs-HLP/EP Composites
2.7. Characterizations
3. Results and Discussion
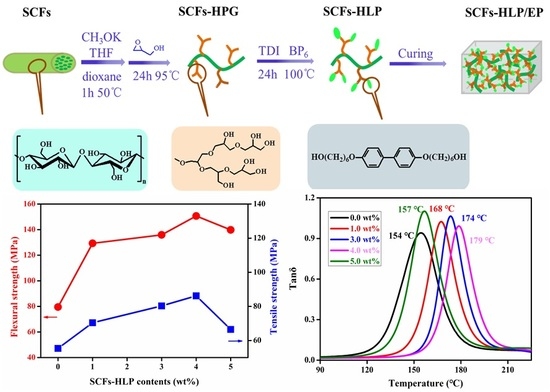
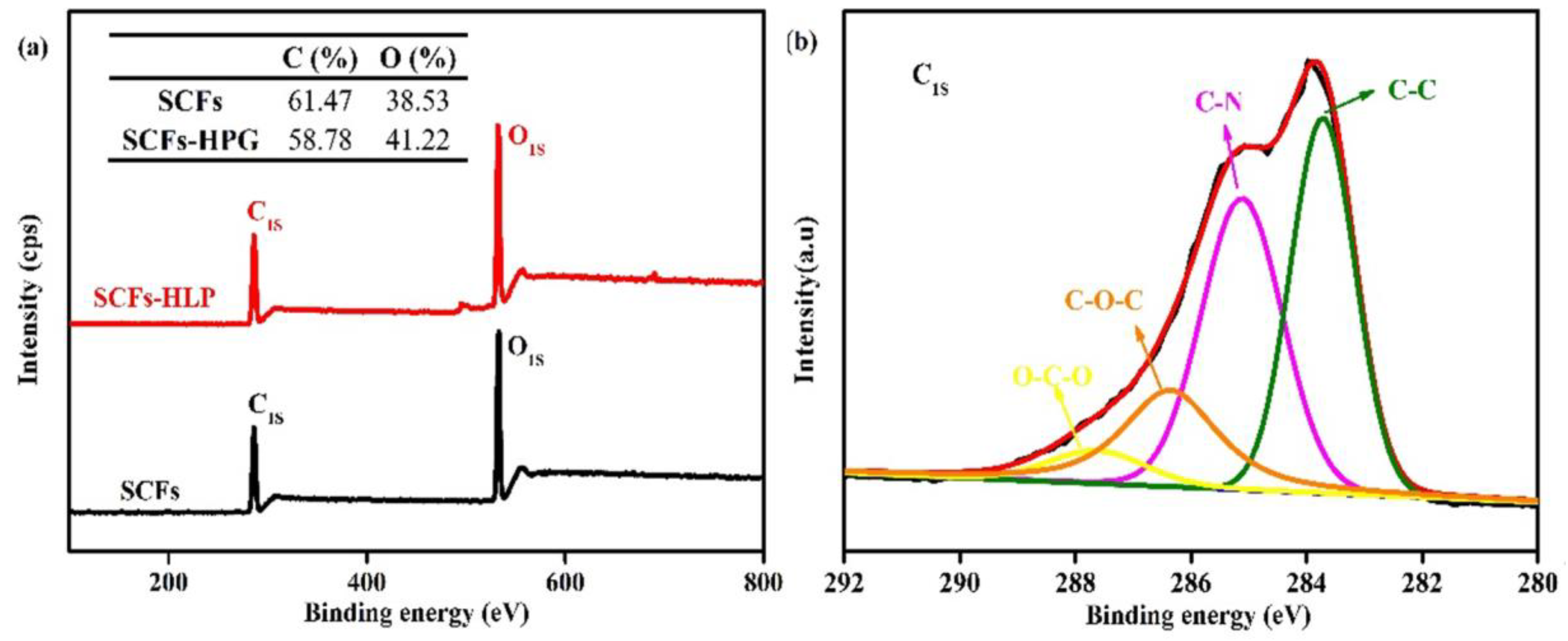
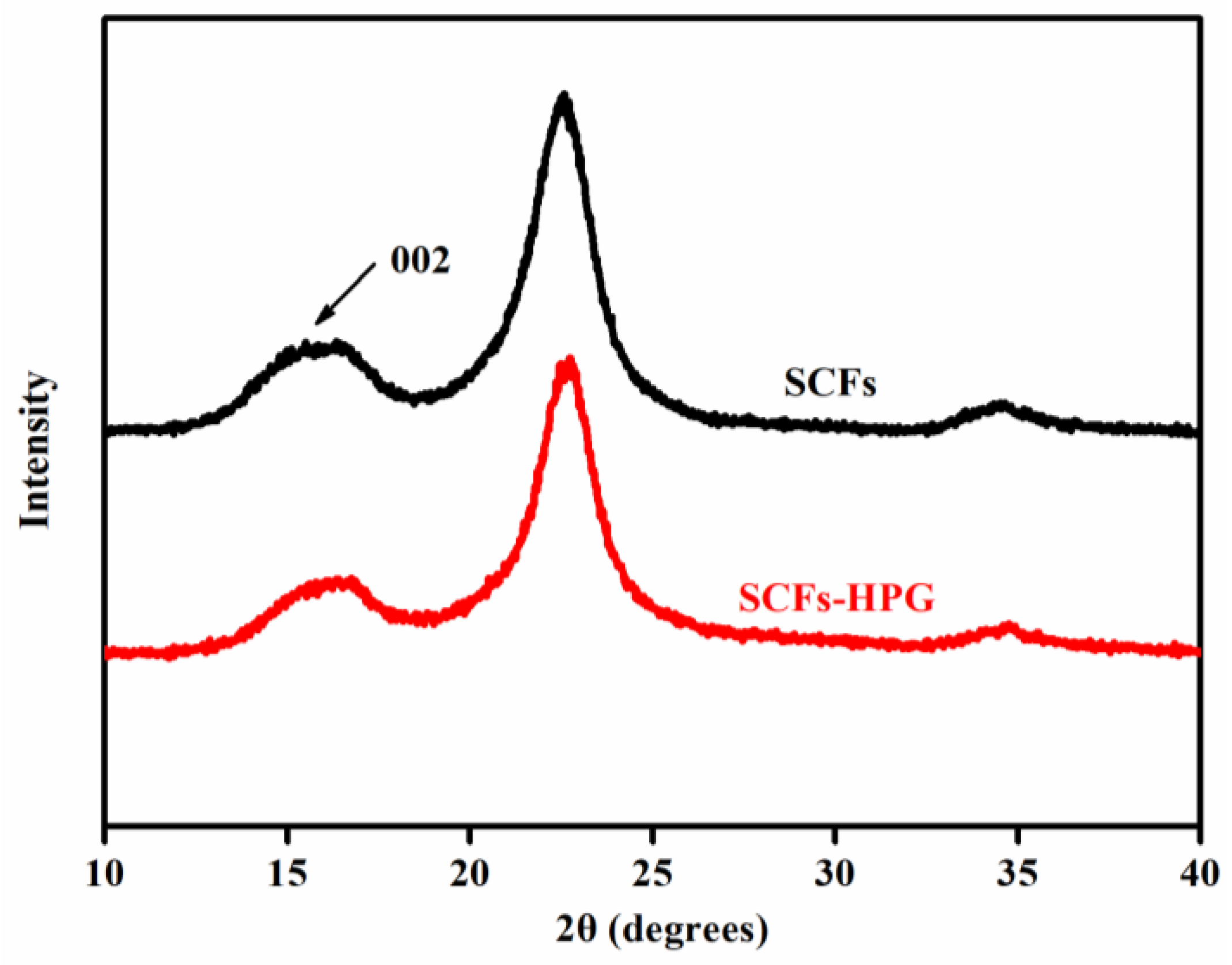
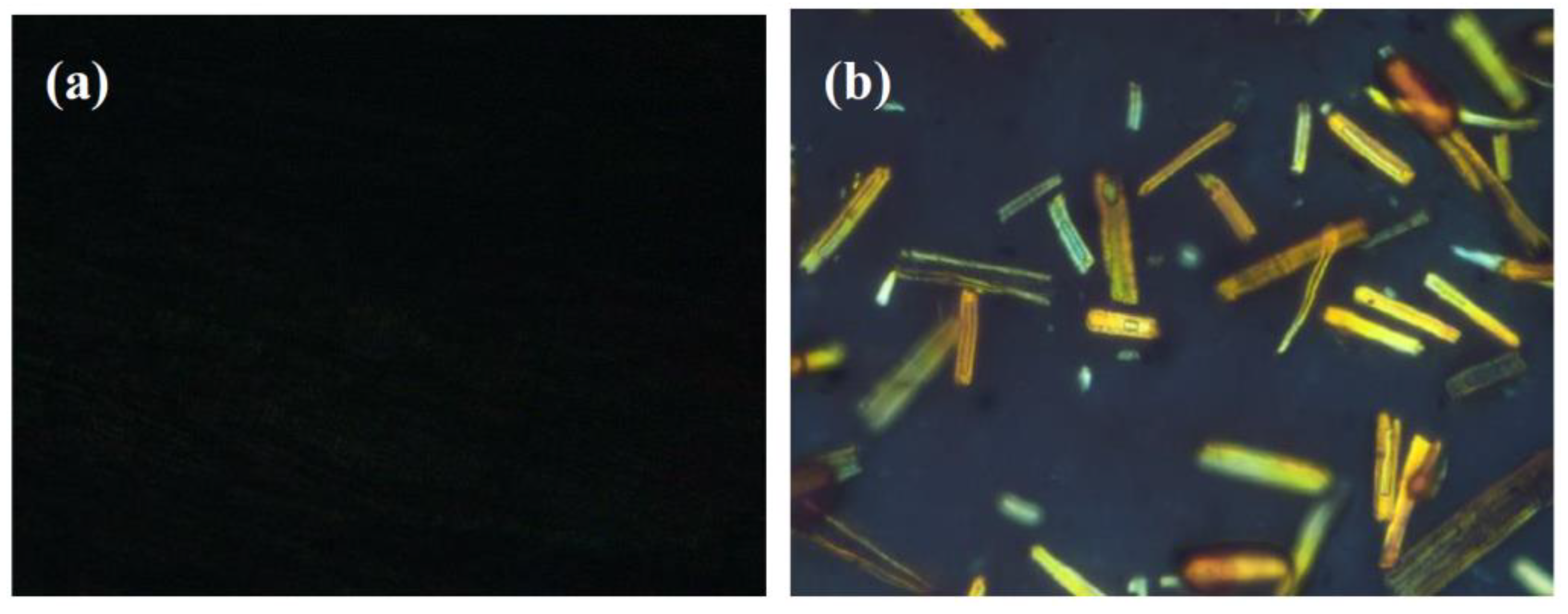
3.1. The Characterization of SCFs-HLP
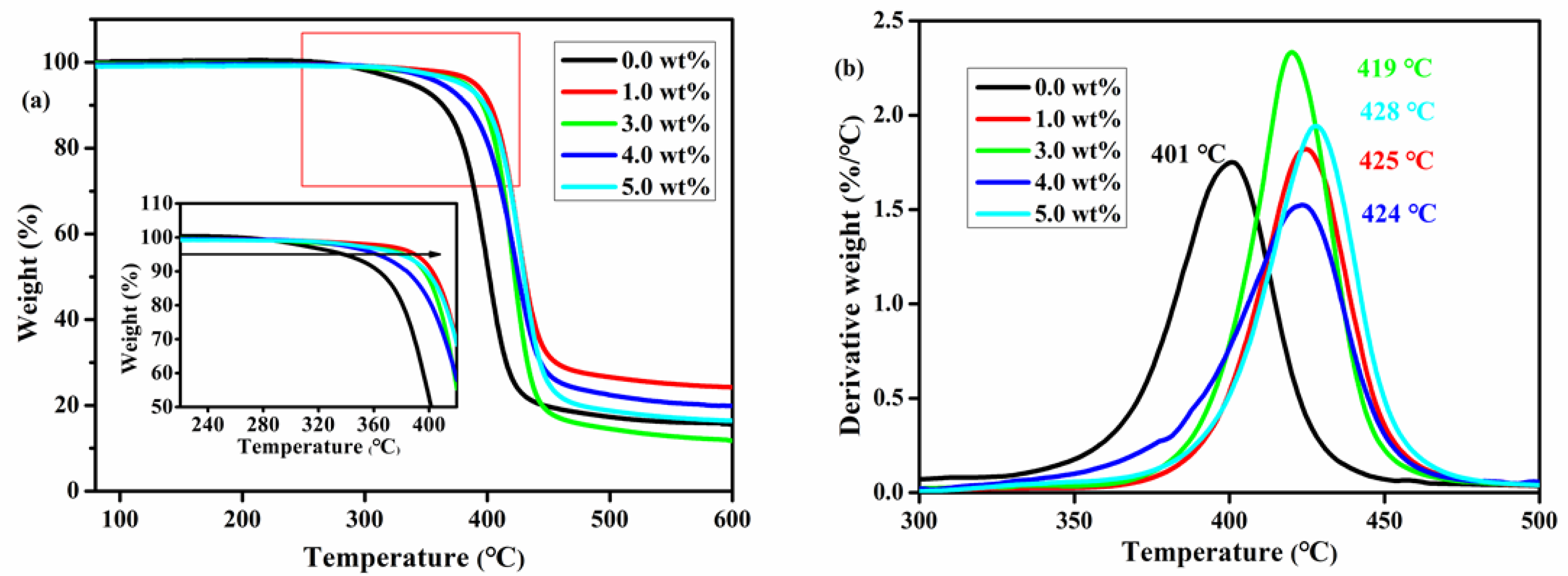
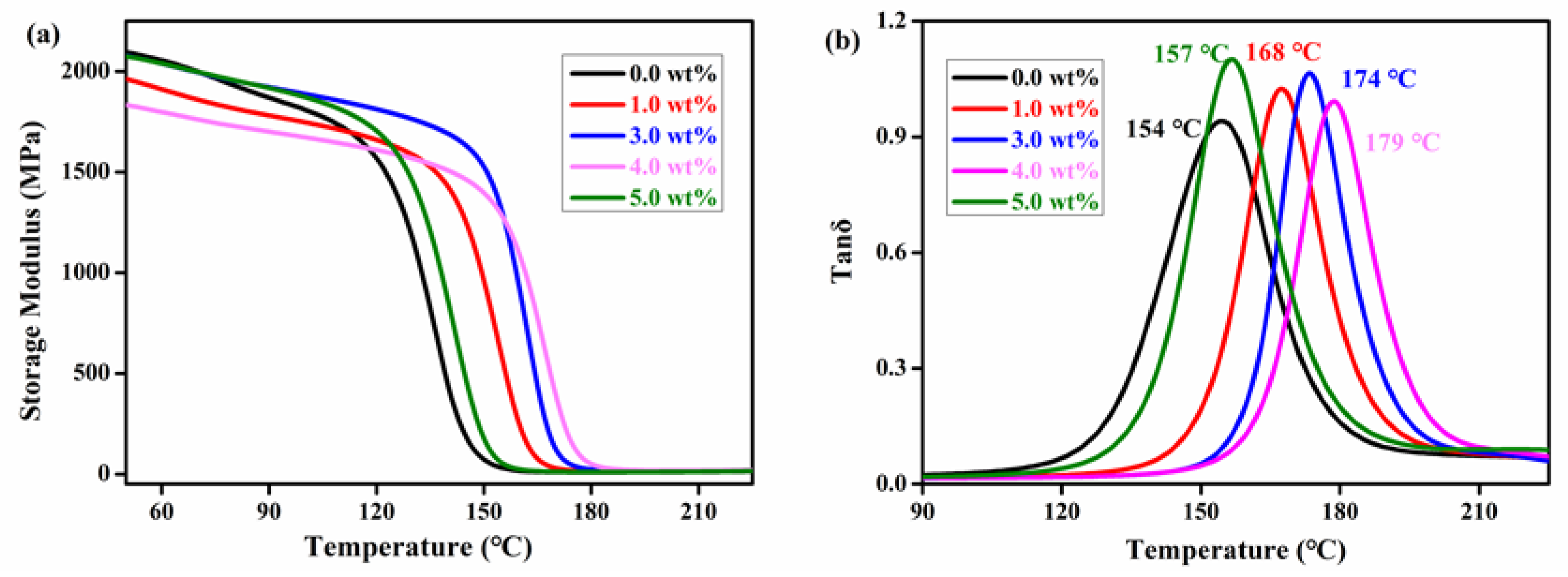
3.2. Thermal Properties of the Composites
3.3. Mechanical Properties of the Composites
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Joseph, K.; Varghese, S.; Kalaprasad, G.; Koshy, P.; Pavithranb, C. Influence of interfacial adhesion on the mechanical properties and fracture behaviour of short sisal fibre reinforced polymer composites. Eur. Polym. J. 1996, 32, 1243–1250. [Google Scholar] [CrossRef]
- Bodur, M.S.; Bakkal, M.; Sonmez, H.E. The effects of different chemical treatment methods on the mechanical and thermal properties of textile fiber reinforced polymer composites. J. Compos. Mater. 2016, 50, 3817–3830. [Google Scholar] [CrossRef]
- Geethamma, V.G.; Mathew, K.T.; Lakshminarayanan, R.; Thomas, S. Composite of short coir fibres and natural rubber: Effect of chemical modification, loading and orientation of fibre. Polymer 1998, 39, 1483–1491. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Bruni, G.P.; Lima, K.O.; Shanise, L.M.E.H.; Rosa, G.S.; Dias, A.R.; Zavareze, G.E.R. Cellulose fibers extracted from rice and oat husks and their application in hydrogel. Food. Chem. 2017, 221, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Dasan, Y.K.; Bhat, A.H.; Faiz, A. Development and material properties of poly(lactic acid)/poly(3-hydroxybutyrat-CO-3-hydroxyvalerate)-based nanocrystalline cellulose nanocomposites. J. Appl. Polym. Sci. 2016, 134, 44328. [Google Scholar]
- Wang, L.F.; Shankar, S.; Rhim, J.W. Properties of alginate-based films reinforced with cellulose fibers and cellulose nanowhiskers isolated from mulberry pulp. Food Hydrocoll. 2017, 63, 201–208. [Google Scholar] [CrossRef]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S. Review: Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1. [Google Scholar] [CrossRef]
- Constante, A.; Pillay, S.; Ning, H.; Vaidya, U.K. Utilization of algae blooms as a source of natural fibers for biocomposite materials: Study of morphology and mechanical performance of Lyngbya, fibers. Algal Res. 2015, 12, 412–420. [Google Scholar] [CrossRef]
- Pandey, J.K.; Ahn, S.H.; Lee, C.S.; Mohanty, A.K.; Misra, M. Recent advances in the application of natural fiber based composites. Macromol. Mater. Eng. 2010, 295, 975–989. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.K.; Fink, H.P.; Sain, M. Biocomposites reinforced with natural fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- Tajvidi, M.; Ebrahimi, G. Water uptake and mechanical characteristics of natural filler-polypropylene composites. J. Appl. Polym. Sci. 2010, 88, 941–946. [Google Scholar] [CrossRef]
- Li, Y.Q.; Gao, J.; Li, X.Y.; Xu, X.; Lu, S.R. High Mechanical and Thermal properties of epoxy composites with liquid crystalline polyurethane modified graphene. Polymers 2018, 10, 485. [Google Scholar] [CrossRef]
- Khan, M.A.; Ali, K.M.; Shafiurrahman, M. Swelling and thermal conductivity of wood and wood-plastic composite. Polym-Plast. Technol. Eng. 1997, 36, 179–187. [Google Scholar]
- Arteta, S.M.; Vera, R.; Pérez, L.D. Hydrophobic cellulose fibers via ATRP and their performance in the removal of pyrene from water. J. Appl. Polym. Sci. 2016, 134, 44482. [Google Scholar] [CrossRef]
- Chartrand, A.; Lavoie, J.M.; Huneault, A. Surface modification of microcrystalline cellulose (MCC) filler for CO2 capture. J. Appl. Polym. Sci. 2016, 133, 44348. [Google Scholar]
- Islam, J.M.; Hossan, M.A.; Alom, F.R.; Khan, M.I.H.; Khan, M.A. Extraction and characterization of crystalline cellulose from jute fiber and application as reinforcement in biocomposite: Effect of gamma radiation. J. Compos. Mater. 2016, 51, 31–38. [Google Scholar] [CrossRef]
- Kalia, S.; Kaith, B.S.; Kaur, I. Pretreatments of natural fibers and their application as reinforcing material in polymer composites-A review. Polym. Eng. Sci. 2009, 49, 1253–1272. [Google Scholar] [CrossRef] [Green Version]
- Carlmark, A.; Larsson, E.; Malmström, E. Grafting of cellulose by ring-opening polymerisation—A review. Eur. Polym. J. 2012, 48, 1646–1659. [Google Scholar] [CrossRef]
- Luo, Q.; Li, Y.Q.; Pan, L.L.; Song, L.F.; Yang, J.; Wu, L.Y.; Lu, S.R. Effective reinforcement of epoxy composites with hyperbranched liquid crystals grafted on microcrystalline cellulose fibers. Mater. Sci. 2016, 51, 1–12. [Google Scholar] [CrossRef]
- Sari, M.G.; Ramezanzadeh, B.; Pakdel, A.S.; Shahbazi, M. A physico-mechanical investigation of a novel hyperbranched polymer-modified clay/epoxy nanocomposite coating. Prog. Org. Coat. 2016, 99, 263–273. [Google Scholar] [CrossRef]
- Li, S.; Lin, Q.; Zhu, H.; Hou, H.; Li, Y.; Wu, Q.; Cui, C. Improved mechanical properties of epoxy-based composites with hyperbranched polymer grafting glass-fiber. Polym. Adv. Technol. 2016, 27, 898–904. [Google Scholar] [CrossRef]
- Thakur, S.; Karak, N. Ultratough, ductile, castor oil-Based, hyperbranched, polyurethane nanocomposite using functionalized reduced graphene oxide. ACS Sustain. Chem. Eng. 2014, 2, 1195–1202. [Google Scholar] [CrossRef]
- Abdollahi, A.; Rad, J.K.; Mahdavian, A.R. Stimuli-responsive cellulose modified by epoxy-functionalized polymer nanoparticles with photochromic and solvatochromic properties. Carbohyd. Polym. 2016, 150, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Chen, H.; Yang, J.; Zhou, X.; Zhang, F.; Gu, N. The preosteoblast response of electrospinning PLGA/PCL nanofibers: Effects of biomimetic architecture and collagen I. Int. J. Nanomed. 2016, 11, 4157. [Google Scholar]
- Blanco, I.; Cicala, G.; Faro, C.L.; Motta, O.; Recca, G. Thermomechanical and morphological properties of epoxy resins modified with functionalized hyperbranched polyester. Polym. Eng. Sci. 2006, 46, 1502–1511. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, X.; Kaschta, J.; Hao, X.; Liu, C.; Schubert, W. Viscoelastic and electrical behavior of poly(methyl methacrylate)/carbon black composites prior to and after annealing. Polymer 2017, 113, 34–38. [Google Scholar] [CrossRef]
- Lv, G.; Zhang, N.; Huang, M.; Shen, C.; Castro, J.; Tan, K.; Liu, X.; Liu, C. The remarkably enhanced particle erosion resistance and toughness properties of glass fiber/epoxy composites via thermoplastic polyurethane nonwoven fabric. Polym. Test. 2018, 69, 470–477. [Google Scholar] [CrossRef]
- Martínez-Hernández, A.L.; Velasco-Santos, C.; De-Icaza, M.; Castaño, V.M. Dynamical–mechanical and thermal analysis of polymeric composites reinforced with keratin biofibers from chicken feathers. Compos. Part B 2007, 38, 405–410. [Google Scholar] [CrossRef]
- Penoff, M.E.; Papagni, G.; Yañez, M.J.; Montemartini, P.E.; Oyanguren, P.A. Synthesis and characterization of an epoxy based thermoset containing a fluorinated thermoplastic. Polym. Sci. Part B 2010, 45, 2781–2792. [Google Scholar] [CrossRef]
- Sangermano, M.; Gianni, A.D.; Bongiovanni, R.; Priola, A.; Voit, B.; Pospiech, D. Synthesis of fluorinated hyperbranched polymers and their use as additives in cationic photopolymerization. Macromol. Mater. Eng. 2005, 290, 721–725. [Google Scholar] [CrossRef]
- Fiore, V.; Scalici, T.; Vitale, G.; Valenza, A. Static and dynamic mechanical properties of Arundo Donax, fillers-epoxy composites. Mater. Desig. 2014, 57, 456–464. [Google Scholar] [CrossRef]
- Liu, X.; Pan, Y.; Zheng, G.; Schubert, D.W. Rheological and electrical behavior of poly(methyl methacrylate)/carbon black composites as investigated by creep recovery in shear. Compos. Sci. Technol. 2016, 128, 1–7. [Google Scholar]
- Wu, L.Y.; Lu, S.R.; Pan, L.L.; Luo, Q.Y.; Yang, J.; Hou, L.R.; Li, Y.Q.; Yu, J.H. Effect of epoxidized soybean oil grafted poly(12-hydroxy stearate) on mechanical and thermal properties of microcrystalline cellulose fibers/polypropylene composites. Polym. Bull. 2017, 74, 911–930. [Google Scholar]





| SCFs-HLP (wt %) | Impact Strength (KJ m−2) | Tensile Strength (MPa) | Flexural Strength (MPa) |
|---|---|---|---|
| 0.0 | 17.5 ± 0.9 | 55.4 ± 1.3 | 79.5 ± 2.5 |
| 1.0 | 24.2 ± 3.8 | 70.4 ± 5.5 | 129.3 ± 4.2 |
| 3.0 | 32.5 ± 3.2 | 80.3 ± 6.1 | 135.9 ± 4.8 |
| 4.0 | 38.3 ± 2.8 | 86.2 ± 1.2 | 150.7 ± 4.2 |
| 5.0 | 27.0 ± 0.7 | 66.5 ± 4.3 | 139.8 ± 6.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Q.; Li, Y.; Ren, L.; Xu, X.; Lu, S. Hyperbranched Liquid Crystals Modified with Sisal Cellulose Fibers for Reinforcement of Epoxy Composites. Polymers 2018, 10, 1024. https://doi.org/10.3390/polym10091024
Luo Q, Li Y, Ren L, Xu X, Lu S. Hyperbranched Liquid Crystals Modified with Sisal Cellulose Fibers for Reinforcement of Epoxy Composites. Polymers. 2018; 10(9):1024. https://doi.org/10.3390/polym10091024
Chicago/Turabian StyleLuo, Qiyun, Yuqi Li, Li Ren, Xu Xu, and Shaorong Lu. 2018. "Hyperbranched Liquid Crystals Modified with Sisal Cellulose Fibers for Reinforcement of Epoxy Composites" Polymers 10, no. 9: 1024. https://doi.org/10.3390/polym10091024