Mechanism of Yellowing: Carbonyl Formation during Hygrothermal Aging in a Common Amine Epoxy
Abstract
:1. Introduction
2. Theory
2.1. Irreversible Degradation Mechanisms
- Hydrolysis (involves chain scission)
- Thermo-oxidation (might involve chain scission, backbone modifications or crosslinking)
- Photo-oxidation (might involve chain scission, backbone modifications or crosslinking)
- Residual curing (additional crosslinking)
- Leaching (initially present additives, impurities or degradation products)
2.2. Thermo-Oxidation and Leaching
3. Materials and Methods
3.1. Materials
3.2. Experimental Methods
3.2.1. Overview
3.2.2. Conditioning of Resin Samples in Distilled Water
3.2.3. Drying of Conditioned Resin Samples
3.2.4. Optical Microscopy
3.2.5. SEM and EDX
3.2.6. FT-NIR
3.2.7. ATR-FT-IR
3.2.8. HR-ICP-MS
3.2.9. pH Measurements
3.2.10. DMTA
4. Results
4.1. Discoloration and Changes in Morphology
4.2. Changes in Chemical Composition
5. Discussion
5.1. Logic of the Investigation
5.2. Leaching
5.3. Thermo-Oxidation
5.4. The Cause of Yellowing
5.5. Increasing Epoxy Service Life
5.6. On the Similarity of Yellowing and Thermo-Oxidation Kinetics
6. Conclusions
- Yellowing occurred due to the thermo-oxidative carbonyl formation in the epoxy carbon-carbon backbone via nucleophilic radical attack. The change in color was irreversible. Morphology was found to be unaffected.
- No chain scission (hydrolysis or oxidation-induced) was present, whilst thermo-oxidation and leaching occurred.
- Compounds involved in leaching were identified to be epichlorohydrin and inorganic impurities but were unrelated to yellowing.
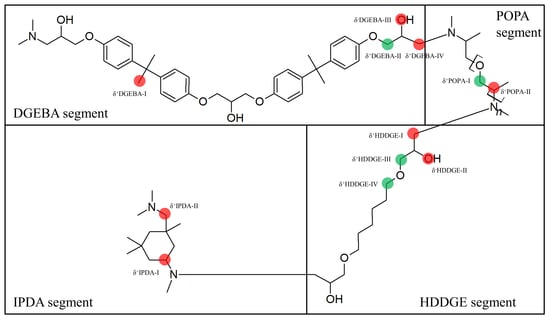
- Four unique reactive sites responsible for thermo-oxidation were found. One reactive site was involved in minor thermo-oxidative crosslinking of the HDDGE segments, while three other sites were linked to carbonyl formation. Noteworthy that all three sites involved in carbonyl formation had similar structures, containing highly reactive polyoxypropylene and i-propanol moieties. Respective reactions were proposed.
- It is speculated that yellowing could be prevented or delayed by adding phenolic antioxidants, such as hindered phenols.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maggana, C.; Pissis, P. Water sorption and diffusion studies in an epoxy resin system. J. Polym. Sci. Part B 1999, 37, 1165–1182. [Google Scholar] [CrossRef]
- Popineau, S.; Rondeau-Mouro, C.; Sulpice-Gaillet, C.; Shanahan, M.E.R. Free/Bound water absorption in an epoxy adhesive. Polymer 2005, 46, 10733–10740. [Google Scholar] [CrossRef]
- Chiang, C.-L.; Ma, C.-C.M.; Wang, F.-Y.; Kuan, H.-C. Thermo-oxidative degradation of novel epoxy containing silicon and phosphorous nanocomposites. Eur. Polym. J. 2003, 39, 825–830. [Google Scholar] [CrossRef]
- Ernault, E.; Richaud, E.; Fayolle, B. Thermal oxidation of epoxies: Influence of diamine hardener. Polym. Degrad. Stab. 2016, 134, 76–86. [Google Scholar] [CrossRef] [Green Version]
- Down, J.L. The Yellowing of Epoxy Resin Adhesives: Report on High-Intensity Light Aging. Stud. Conserv. 1986, 31, 159–170. [Google Scholar] [CrossRef]
- Down, J.L. The yellowing of epoxy resin adhesives: Report on natural dark aging. Stud. Conserv. 1984, 29, 63–76. [Google Scholar]
- Coutinho, I.; Ramos, A.M.; Lima, A.M.; Fernandes, F.B. Studies of the degradation of epoxy resins used for the conservation of glass. In Proceedings of the Holding it all together, Ancient and Modern Approaches to Joining, Repair and Consolidation, London, UK, 21–22 February 2008. [Google Scholar] [CrossRef]
- Tennent, N.H. Clear and Pigmented Epoxy Resins for Stained Glass Conservation: Light Ageing Studies. Stud. Conserv. 1979, 24, 153–164. [Google Scholar]
- Ginell, W.S.; Coffman, R. Epoxy resin-consolidated stone: Appearance change on aging. Stud. Conserv. 1998, 43, 242–248. [Google Scholar]
- Mailhot, B.; Morlat-Thérias, S.; Ouahioune, M.; Gardette, J.-L. Study of the Degradation of an Epoxy/Amine Resin, 1 Photo- and Thermo-Chemical Mechanisms. Macromol. Chem. Phys. 2005, 206, 575–584. [Google Scholar] [CrossRef]
- Ernault, E.; Richaud, E.; Fayolle, B. Origin of epoxies embrittlement during oxidative ageing. Polym. Test. 2017, 63, 448–454. [Google Scholar] [CrossRef] [Green Version]
- Pham, H.Q.; Marks, M.J. Epoxy resins. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2005; pp. 155–244. [Google Scholar]
- Rocha, I.B.C.M.; Raijmaekers, S.; Nijssen, R.P.L.; van der Meer, F.P.; Sluys, L.J. Hygrothermal ageing behaviour of a glass/epoxy composite used in wind turbine blades. J. Compos. Struct. 2017, 174, 110–122. [Google Scholar] [CrossRef]
- Krauklis, A.E.; Gagani, A.I.; Echtermeyer, A.T. Hygrothermal Aging of Amine Epoxy: Reversible Static and Fatigue Properties. Open Eng. 2018. under review. [Google Scholar]
- Startsev, V.O.; Lebedev, M.P.; Khrulev, K.A.; Molokov, M.V.; Frolov, A.S.; Nizina, T.A. Effect of outdoor exposure on the moisture diffusion and mechanical properties of epoxy polymers. Polym. Test. 2018, 65, 281–296. [Google Scholar] [CrossRef]
- Toscano, A.; Pitarresi, G.; Scafidi, M.; Di Filippo, M.; Spadaro, G.; Alessi, S. Water diffusion and swelling stresses in highly crosslinked epoxy matrices. Polym. Degrad. Stab. 2016, 133, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Allen, N.S.; Robinson, P.J.; White, N.J.; Swales, D.W. Photo-oxidative Stability of Electron Beam and UV Cured Acrylated Epoxy and Urethane Acrylate Resin Films. Polym. Degrad. Stab. 1987, 19, 147–160. [Google Scholar] [CrossRef]
- Buch, X.; Shanahan, M.E.R. Thermal and thermo-oxidative ageing of an epoxy adhesive. Polym. Degrad. Stab. 2000, 68, 403–411. [Google Scholar] [CrossRef]
- Wang, M. The hygrothermal aging process and mechanism of the novolac epoxy resin. Compos. Part B 2016, 107, 1–8. [Google Scholar] [CrossRef]
- Clancy, T.C.; Frankland, S.J.V.; Hinkley, J.A.; Gates, T.S. Molecular modeling for calculation of mechanical properties of epoxies with moisture ingress. Polymer 2009, 50, 2736–2742. [Google Scholar] [CrossRef] [Green Version]
- López-Ballester, E.; Doménech-Carbó, M.T.; Gimeno-Adelantado, J.V.; Bosch-Reig, F. Study of FT-IR spectroscopy of ageing of adhesives used in restoration of archaeological glass objects. J. Mol. Struct. 1999, 482, 525–531. [Google Scholar] [CrossRef]
- Xiao, G.Z.; Shanahan, M.E.R. Irreversible effects of hygrothermal aging on DGEBA/DDA epoxy resin. J. Appl. Polym. Sci. 1998, 69, 363–369. [Google Scholar] [CrossRef]
- Belec, L.; Nguyen, T.H.; Nguyen, D.L.; Chailan, J.F. Comparative effects of humid tropical weathering and artificial ageing on a model composite properties from nano- to macro-scale. Compos. Part A Appl. Sci. Manuf. 2015, 68, 235–241. [Google Scholar] [CrossRef]
- Heinze, S.; NTNU, Trondheim, Norway. Personal communication, 2017.
- Celina, M.C.; Dayile, A.R.; Quintana, A. A perspective on the inherent oxidation sensitivity of epoxy materials. Polymer 2013, 54, 3290–3296. [Google Scholar] [CrossRef]
- Colin, X.; Verdu, J. Thermal ageing and lifetime prediction for organic matrix composites. Plast. Rubber Compos. 2003, 32, 349–356. [Google Scholar] [CrossRef]
- Ernault, E.; Richaud, E.; Fayolle, B. Thermal-oxidation of epoxy/amine followed by glass transition temperature changes. Polym. Degrad. Stab. 2017, 138, 82–90. [Google Scholar] [CrossRef]
- Galant, C.; Fayolle, B.; Kuntz, M.; Verdu, J. Thermal and radio-oxidation of epoxy coatings. Prog. Org. Coat. 2010, 69, 322–329. [Google Scholar] [CrossRef]
- Bellenger, V.; Verdu, J.; Francilette, J.; Hoarau, P.; Morel, E. Infra-red study of hydrogen bonding in amine-crosslinked epoxies. Polymer 1987, 28, 1079–1086. [Google Scholar] [CrossRef]
- Bruchet, A.; Elyasmino, N.; Decottignies, V.; Noyon, N. Leaching of bisphenol A and F from new and old epoxy coatings: Laboratory and field studies. Water Sci. Technol. 2014, 14, 383–389. [Google Scholar] [CrossRef]
- Rajasärkkä, J.; Pernica, M.; Kuta, J.; Lašňák, J.; Šimek, Z.; Bláha, L. Drinking water contaminants from epoxy resin-coated pipes: A field study. Water Res. 2016, 103, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Lipke, U.; Haverkamp, J.B.; Zapf, T.; Lipperheide, C. Matrix effect on leaching of Bisphenol A diglycidyl ether (BADGE) from epoxy resin based inner lacquer of aluminium tubes into semi-solid dosage forms. Eur. J. Pharm. Biopharm. 2016, 101, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vermeirssen, E.L.M.; Dietschweiler, C.; Werner, I.; Burkhardt, M. Corrosion protection products as a source of bisphenol A and toxicity to the aquatic environment. Water Res. 2017, 123, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.Z.; Delamar, M.; Shanahan, M.E.R. Irreversible interactions between water and DGEBA/DDA epoxy resin during hygrothermal aging. J. Appl. Polym. Sci. 1997, 65, 449–458. [Google Scholar] [CrossRef]
- Plastics in the Determination of Dynamic Mechanical Properties–Part 11: Glass Transition Temperature; International Standard ISO 6721-11:2012(E); ISO: Geneva, Switzerland, 2012.
- Celina, M.C. Review of polymer oxidation and its relationship with materials performance and lifetime prediction. Polym. Degrad. Stab. 2013, 98, 2419–2429. [Google Scholar] [CrossRef]
- Krauklis, A.E.; Gagani, A.I.; Echtermeyer, A.T. Near-infrared spectroscopic method for monitoring water content in epoxy resins and fiber-reinforced composites. Materials 2018, 11, 586. [Google Scholar] [CrossRef] [PubMed]
- Lyon, R.E.; Chike, K.E.; Angel, S.M. In situ Cure Monitoring of Epoxy Resins Using Fiber-Optic Raman Spectroscopy. J. Appl. Polym. Sci. 1994, 53, 1805–1812. [Google Scholar] [CrossRef]
- De’Néve, B.; Shanahan, M.E.R. Water absorption by an epoxy resin and its effect on the mechanical properties and infra-red spectra. Polymer 1993, 34, 5099–5105. [Google Scholar] [CrossRef]
- Larché, J.-F.; Bussiére, P.-O.; Thérias, S.; Gardette, J.-L. Photooxidation of polymers: Relating material properties to chemical changes. Polym. Degrad. Stab. 2012, 97, 25–34. [Google Scholar] [CrossRef]
- Zahra, Y.; Djouani, F.; Fayolle, B.; Kuntz, M.; Verdu, J. Thermo-oxidative aging of epoxy coating systems. Prog. Org. Coat. 2014, 77, 380–387. [Google Scholar] [CrossRef] [Green Version]
- Hexion Technical Data Sheet: EPIKOTE RIMR 135 and EPIKURE RIMH 134–137. 2006. Available online: http://www.hexion.com/en-us/chemistry/epoxy-resins-curing-agents-modifiers/epoxy-tds (accessed on 1 August 2018).
- Deng, T.; Liu, Y.; Cui, X.; Yang, Y.; Jia, S.; Wang, Y.; Lu, C.; Li, D.; Cai, R.; Hou, X. Cleavage of C–N bonds in carbon fiber/epoxy resin composites. Green Chem. 2015, 17, 2141–2145. [Google Scholar] [CrossRef]
- Unnikrishnan, K.P. Studies on the Toughening of Epoxy Resins. Ph.D. Thesis, Cochin University of Science and Technology, Kochi, India, 2006. [Google Scholar]
- Rasoldier, N.; Colin, X.; Verdu, J.; Bocquet, M.; Olivier, L.; Chocinski-Arnault, L.; Lafarie-Frenot, M.C. Model systems for thermo-oxidised epoxy composite matrices. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1522–1529. [Google Scholar] [CrossRef]
- Li, K.; Wang, K.; Zhan, M.-S.; Xu, W. The change of thermal-mechanical properties and chemical structure of ambient cured DGEBA/TEPA under accelerated thermo-oxidative aging. Polym. Degrad. Stab. 2013, 98, 2340–2346. [Google Scholar] [CrossRef]
- Sauvant-Moynot, V.; Duval, S.; Grenier, J. Innovative pipe coating material and process for high temperature fields. Oil Gas Sci. Technol. 2002, 57, 269–279. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene: Review. SpringerPlus 2013, 2, 398–429. [Google Scholar] [CrossRef] [PubMed]
- Rosu, D.; Rosu, L.; Cascaval, C.N. IR-change and yellowing of polyurethane as a result of UV irradiation. Polym. Degrad. Stab. 2009, 94, 591–596. [Google Scholar] [CrossRef]
- Lin, M.-S.; Chiu, C.-C. Protection of epoxy resin against thermo-oxidation via co-curing epoxy/resole (I). Polym. Degrad. Stab. 2000, 69, 251–253. [Google Scholar] [CrossRef]
- Lin, M.-S.; Chiu, C.-C. Protection of epoxy resin against thermo-oxidation via co-curing epoxy/resole (II). Polym. Degrad. Stab. 2001, 71, 327–329. [Google Scholar] [CrossRef]













© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krauklis, A.E.; Echtermeyer, A.T. Mechanism of Yellowing: Carbonyl Formation during Hygrothermal Aging in a Common Amine Epoxy. Polymers 2018, 10, 1017. https://doi.org/10.3390/polym10091017
Krauklis AE, Echtermeyer AT. Mechanism of Yellowing: Carbonyl Formation during Hygrothermal Aging in a Common Amine Epoxy. Polymers. 2018; 10(9):1017. https://doi.org/10.3390/polym10091017
Chicago/Turabian StyleKrauklis, Andrey E., and Andreas T. Echtermeyer. 2018. "Mechanism of Yellowing: Carbonyl Formation during Hygrothermal Aging in a Common Amine Epoxy" Polymers 10, no. 9: 1017. https://doi.org/10.3390/polym10091017