Cellulose Nanofiber/Carbon Nanotube Conductive Nano-Network as a Reinforcement Template for Polydimethylsiloxane Nanocomposite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
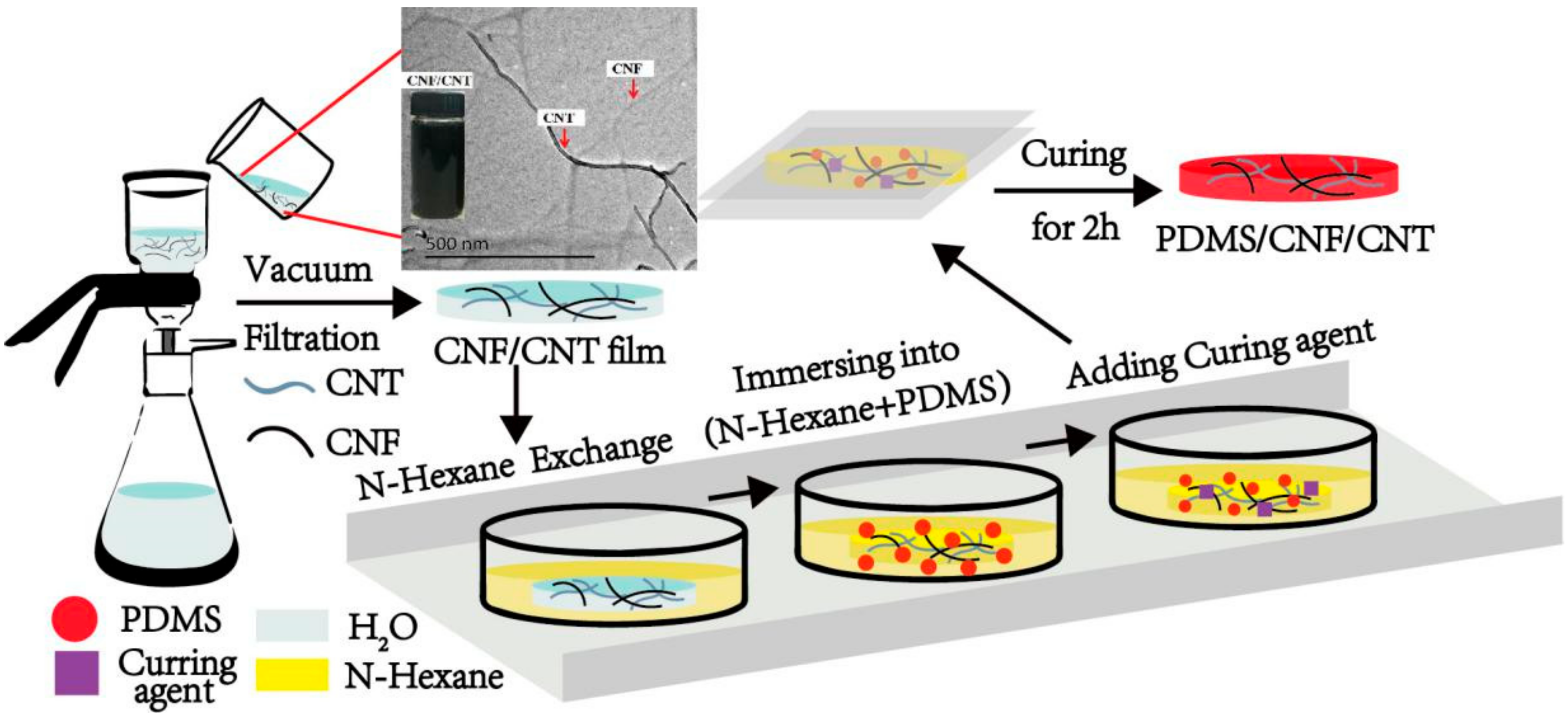
2.2. Preparation of CNF/MWCNT Film
2.3. Preparation of PDMS/CNF/MWCNT Film
2.4. Characterization
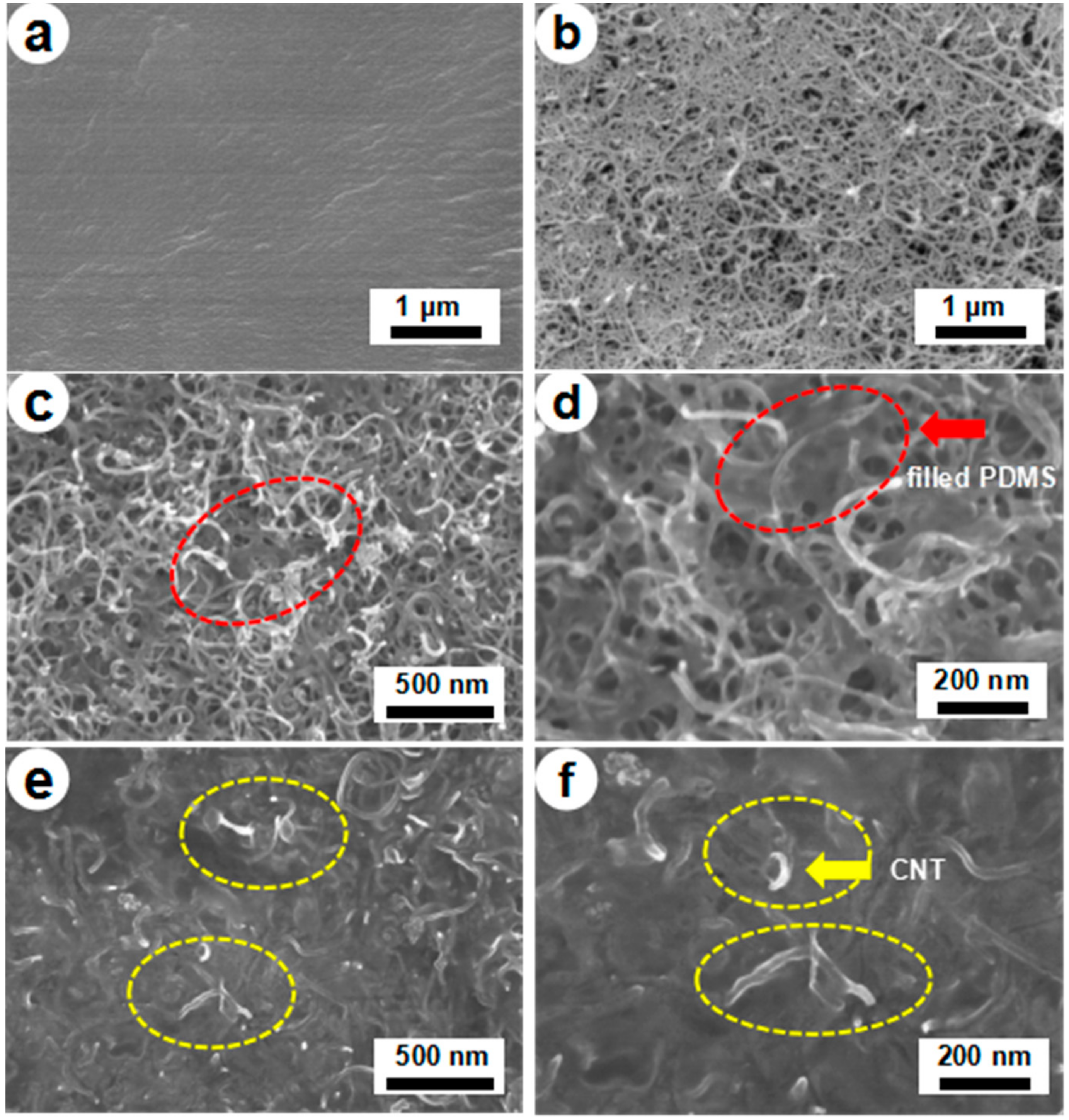
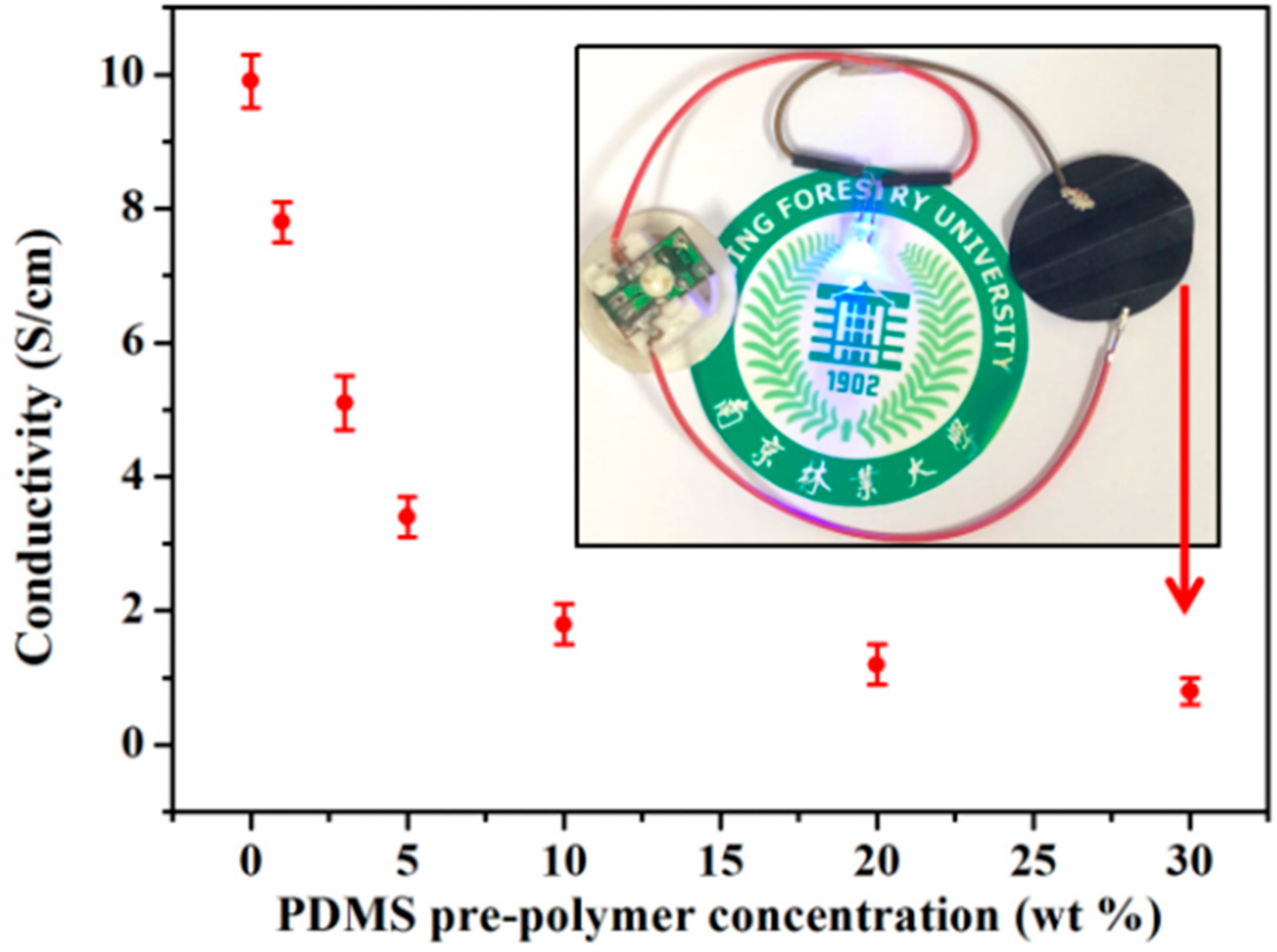
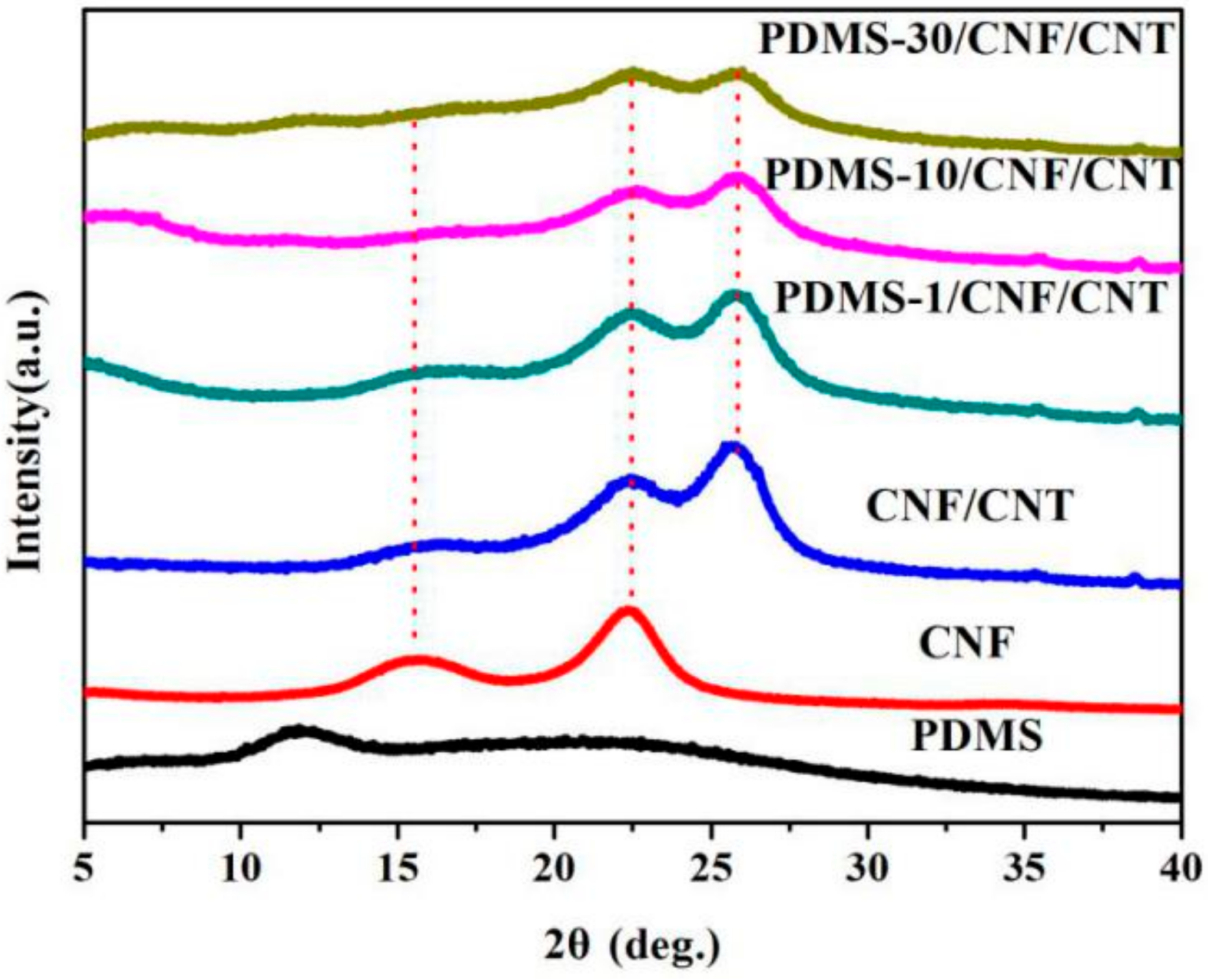
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Osicka, J.; Mrlik, M.; Ilcikova, M.; Hanulikova, B.; Urbanek, P.; Sedlacik, M.; Mosnacek, J. Reversible Actuation Ability upon Light Stimulation of the Smart Systems with Controllably Grafted Graphene Oxide with Poly (Glycidyl Methacrylate) and PDMS Elastomer: Effect of Compatibility and Graphene Oxide Reduction on the Photo-Actuation Performance. Polymers 2018, 10, 832. [Google Scholar] [CrossRef]
- Liang, H.W.; Guan, Q.F.; Zhu, Z.; Song, L.T.; Yao, H.B.; Lei, X. Highly conductive and stretchable conductors fabricated from bacterial cellulose. Npg. Asia. Mater. 2012, 4, e19. [Google Scholar] [CrossRef]
- González-Rivera, J.; Iglio, R.; Barillaro, G.; Duce, C.; Tinè, M.R. Structural and Thermoanalytical Characterization of 3D Porous PDMS Foam Materials: The Effect of Impurities Derived from a Sugar Templating Process. Polymers 2018, 10, 616. [Google Scholar] [CrossRef]
- Moretti, G.; Righi, M.; Vertechy, R.; Fontana, M. Fabrication and Test of an Inflated Circular Diaphragm Dielectric Elastomer Generator Based on PDMS Rubber Composite. Polymers 2017, 9, 283. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, B.; Yoon, J.; Kim, Y.; Park, J.; Kim, H.J.; Kim, D.H.; Kim, D.M.; Kim, S.; Choi, S.J. Highly transparent tactile sensor based on a percolated carbon nanotube network. AIP Adv. 2018, 8, 065109. [Google Scholar] [CrossRef]
- Iglio, R.; Mariani, S.; Robbiano, V.; Strambini, L.; Barillaro, G. Flexible Polydimethylsiloxane Foams Decorated with Multiwalled Carbon Nanotubes Enable Unprecedented Detection of Ultralow Strain and Pressure Coupled with a Large Working Range. ACS Appl. Mater. Interface 2018, 10, 13877–13885. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Park, J.Y.; Lee, C.S.; Kwon, O.S.; Park, S.H.; Bae, J. Surface engineered poly(dimethylsiloxane)/carbon nanotube nanocomposite pad as a flexible platform for chemical sensors. Compos. Part A Appl. Sci. Manuf. 2018, 107, 55–60. [Google Scholar] [CrossRef]
- Rasel, M.S.; Maharjan, P.; Salauddin, M.; Rahman, M.T.; Cho, H.O.; Kim, J.W.; Park, J.Y. An impedance tunable and highly efficient triboelectric nanogenerator for large-scale, ultra-sensitive pressure sensing applications. Nano Energy 2018, 49, 603–613. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, J.; Shi, Z.; Xiang, S.; Xiong, C. Recent progress of nanocellulose-based electroconductive materials and their applications as electronic devices. J. For. Eng. 2018, 3, 1–11. [Google Scholar]
- Li, M.-C.; Wu, Q.; Song, K.; Lee, S.; Qing, Y.; Wu, Y. Cellulose Nanoparticles: Structure–Morphology–Rheology Relationships. ACS Sustain. Chem. Eng. 2015, 3, 821–832. [Google Scholar] [CrossRef]
- Wang, H.; Song, Y.; Zhou, J.; Xu, X.; Hong, W.; Yan, J.; Xue, R.; Zhao, H.; Liu, Y.; Gao, J. High-performance supercapacitor materials based on polypyrrole composites embedded with core-sheath polypyrrole@MnMoO4 nanorods. Electrochim. Acta 2016, 212, 775–783. [Google Scholar] [CrossRef]
- Lee, D.; Cho, Y.G.; Song, H.K.; Chun, S.J.; Park, S.B.; Choi, D.H.; Lee, S.Y.; Yoo, J.T.; Lee, S.Y. Coffee-driven green activation of cellulose and its use for all-paper flexible supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 22568–22577. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yuan, L.; Tang, C. Sustainable Elastomers from Renewable Biomass. Acc. Chem Res. 2017, 50, 1762–1773. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Luo, H.; Zhou, H.; Zhou, X.; Zhang, X.X.; Lin, W. Dramatically enhanced strain- and moisture-sensitivity of bioinspired fragmentized carbon architectures regulated by cellulose nanocrystals. Chem. Eng. J. 2018, 34, 452–461. [Google Scholar] [CrossRef]
- Zhai, T.; Zheng, Q.; Cai, Z.; Turng, L.S.; Xia, H.; Gong, S. Poly(vinyl alcohol)/cellulose nanofibril hybrid aerogels with an aligned microtubular porous structure and their composites with polydimethylsiloxane. ACS Appl. Mater. Interfaces 2015, 7, 7436–7444. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Wei, J.; Aitomäki, Y.; Noël, M.; Oksman, K. Well-dispersed cellulose nanocrystals in hydrophobic polymers by in situ polymerization for synthesizing highly reinforced bio-nanocomposites. Nanoscale 2018, 10, 11797–11807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.Y.; Li, J.Y. Enhanced Swelling, Mechanical and Thermal Properties of Cellulose Nanofibrils (CNF)/Poly(vinyl alcohol) (PVA) Hydrogels with Controlled Porous Structure. J. Nanosci. Nanotechnol. 2018, 18, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xue, X.; Wang, S.; Zhang, Y. Preparing nanocellulose whisker reinforced ABS composites by liquid mixing method. J. For. Eng. 2016, 1, 91–95. [Google Scholar]
- Chen, C.; Haiying, W.; Suiyi, L.; Fang, L.; Li, D. Reinforcement of cellulose nanofibers in polyacrylamide gels. Cellulose 2017, 24, 5487–5493. [Google Scholar] [CrossRef]
- Zhang, Z.; Tingaut, P.; Rentsch, D.; Zimmermann, T.; Sebe, G. Controlled silylation of nanofibrillated cellulose in water: Reinforcement of a model polydimethylsiloxane network. ChemSusChem 2015, 8, 2681–2690. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, A.; Teramoto, Y. Recent Advances in Nanocellulose Composites with Polymers: A Guide for Choosing Partners and How to Incorporate Them. Polymers 2018, 10, 517. [Google Scholar] [CrossRef]
- Liu, C.X.; Choi, J.W. Improved Dispersion of Carbon Nanotubes in Polymers at High Concentrations. Nanomaterials 2012, 2, 329–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, F.; Berglund, L.A. Toward Semistructural Cellulose Nanocomposites: The Need for Scalable Processing and Interface Tailoring. Biomacromolecules 2018, 19, 2341–2350. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Huang, J.; Liu, C.; Ding, B.; Kuga, S.; Cai, J.; Zhang, L. Three-Dimensional Nanoporous Cellulose Gels as a Flexible Reinforcement Matrix for Polymer Nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 22990–22998. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Yuwen, H.; Lin, N. Self-bonding sandwiched membranes from PDMS and cellulose nanocrystals by engineering strategy of layer-by-layer curing. Compos. Sci. Technol. 2018, 161, 8–15. [Google Scholar] [CrossRef]
- Cha, J.E.; Kim, S.Y.; Lee, S.H. Effect of Continuous Multi-Walled Carbon Nanotubes on Thermal and Mechanical Properties of Flexible Composite Film. Nanomaterials 2016, 6, 182. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Mo, M.; Chen, W.; Pan, M.; Xu, Z.; Wang, H.; Li, D. Highly conductive nanocomposites based on cellulose nanofiber networks via NaOH treatments. Compos. Sci. Technol. 2018, 156, 103–108. [Google Scholar]
- Abe, K.; Iwamoto, S.; Yano, H. Obtaining cellulose nanofibers with a uniform width of 15 nm from wood. Biomacromolecules 2007, 8, 3276–3278. [Google Scholar] [CrossRef] [PubMed]
- Koga, H.; Saito, T.; Kitaoka, T.; Nogi, M.; Suganuma, K.; Isogai, A. Transparent, conductive, and printable composites consisting of tempo-oxidized nanocellulose and carbon nanotube. Biomacromolecules 2013, 14, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Niu, R.; Gong, J.; Xu, D.; Tang, T.; Sun, Z.-Y. Influence of molecular weight of polymer matrix on the structure and rheological properties of graphene oxide/polydimethylsiloxane composites. Polymer 2014, 55, 5445–5453. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Zhu, H.; Zhou, L.; Zheng, X.; Li, X.; Chen, Z.; Wang, Y.; Zhang, D.; Pan, D. Preparation of nitrogen-doped biomass-derived carbon nanofibers/graphene aerogel as a binder-free electrode for high performance supercapacitors. Appl. Surf. Sci. 2017, 426, 99–106. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.; Yang, S.; Luo, B.; Zhou, C. Liquid Crystalline Behaviors of Chitin Nanocrystals and Their Reinforcing Effect on Natural Rubber. ACS Sustain. Chem. Eng. 2017, 6, 325–336. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, X.; Tan, S.; Wu, W.; Shi, J.; Zhou, H.; Chen, P. Preparation and characterisation of CNF/MWCNT carbon aerogel as efficient adsorbents. IET Nanobiotechnol. 2018, 12, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Ceseracciu, L.; Heredia-Guerrero, J.A.; Dante, S.; Athanassiou, A.; Bayer, I.S. Robust and biodegradable elastomers based on corn starch and polydimethylsiloxane (PDMS). ACS Appl. Mater. Interface 2015, 7, 3742–3753. [Google Scholar] [CrossRef] [PubMed]
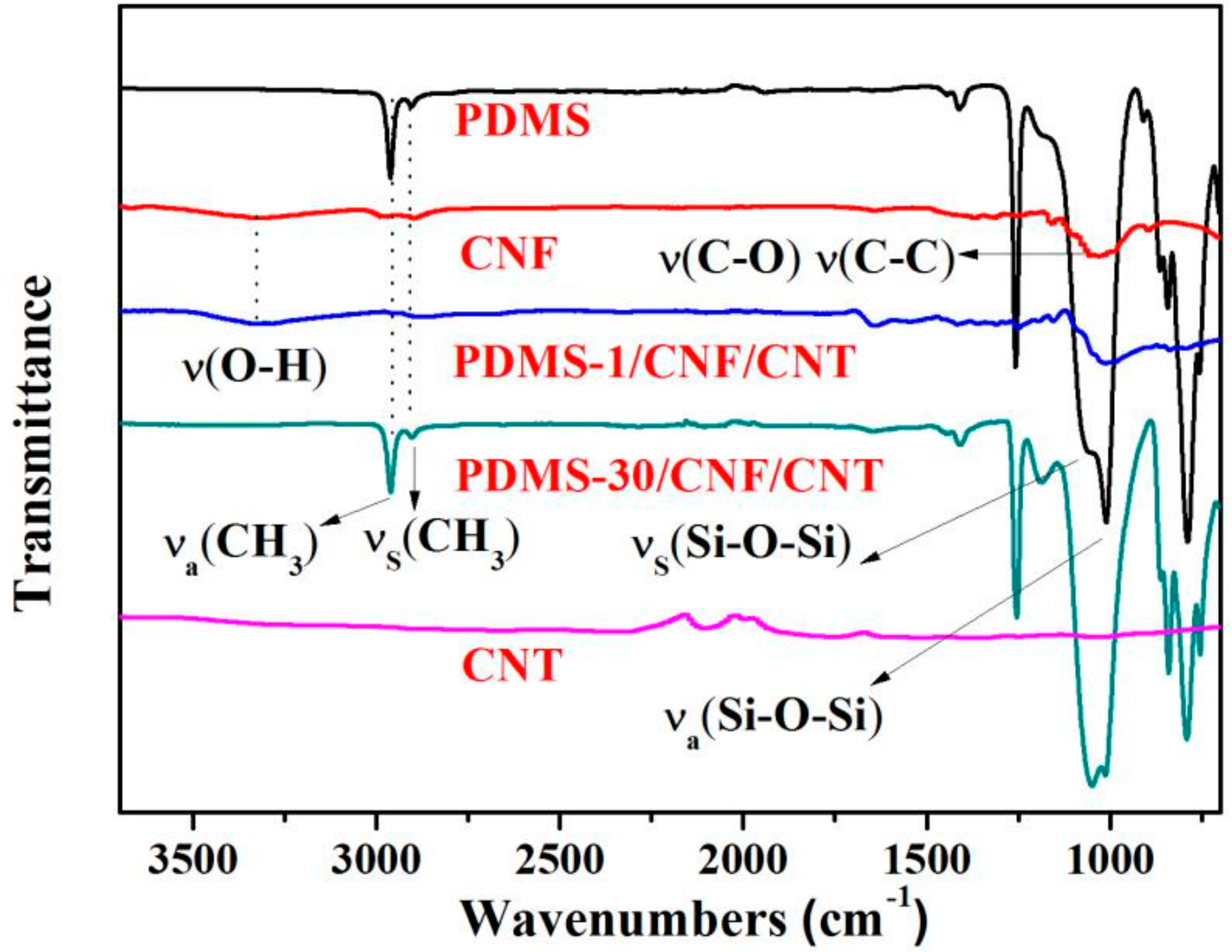
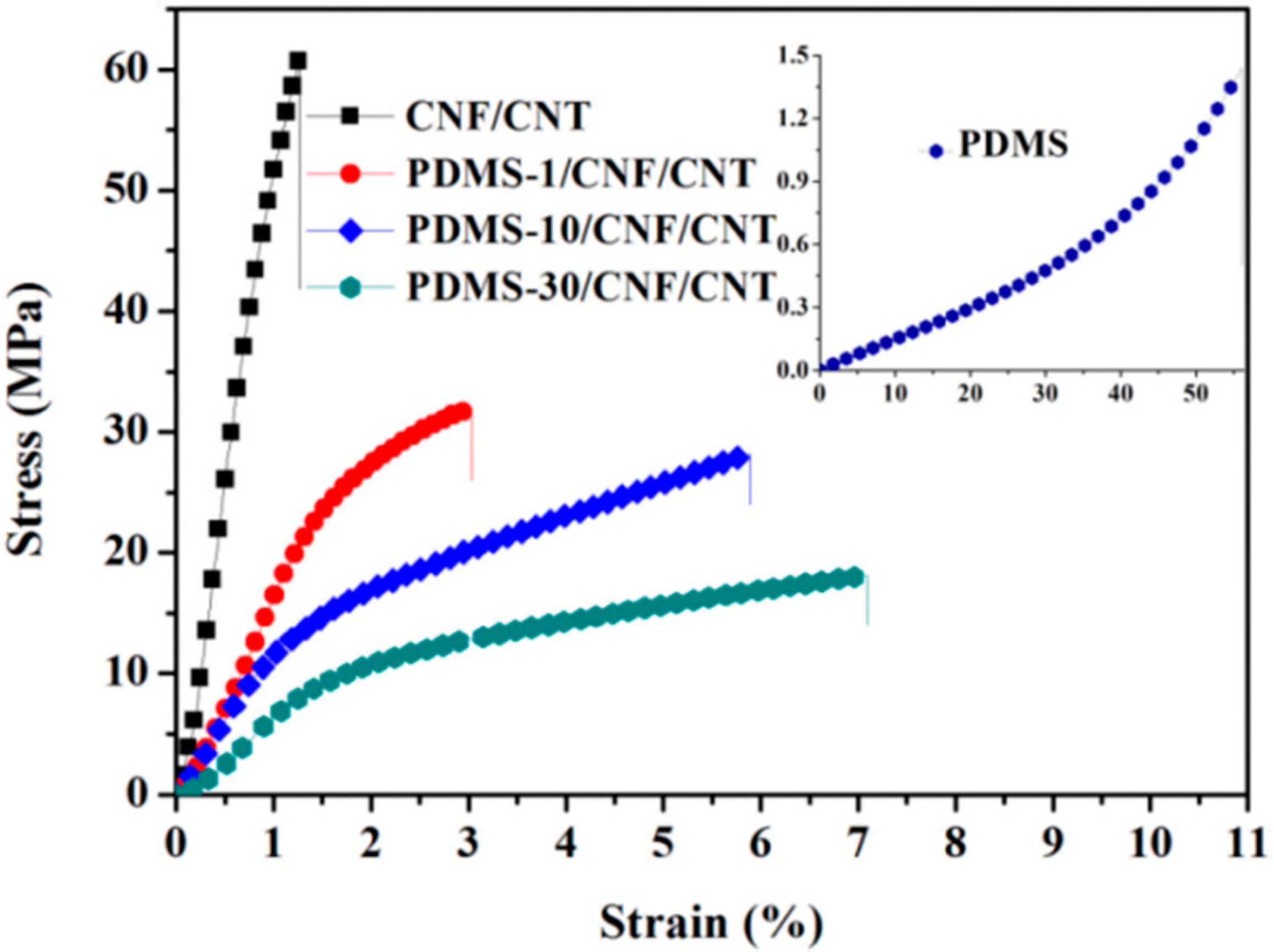
| Sample Data | PDMS-1/CNF/CNT | PDMS-10/CNF/CNT | PDMS-30/CNF/CNT | CNF/CNT | PDMS |
|---|---|---|---|---|---|
| Thickness (μm) | 60 ± 3 | 74 ± 4 | 96 ± 4 | 41 ± 3 | 61 ± 2 |
| PDMS (%) | 23.7 ± 2.1 | 50.5 ± 2.2 | 71.3 ± 3.1 | 0.0 | 100.0 |
| Stress (MPa) | 32.6 ± 3.7 | 28.1 ± 1.6 | 18.3 ± 2.5 | 61.7 ± 3.1 | 1.5 ± 0.2 |
| Strain (%) | 3.1 ± 0.2 | 5.8 ± 0.5 | 7.3 ± 0.4 | 1.1 ± 0.2 | 51 ± 3 |
| Young’s modulus (MPa) | 1616 ± 114 | 1322 ± 107 | 805 ± 53 | 5132 ± 308 | 1.4 ± 0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Bu, X.; Feng, Q.; Li, D. Cellulose Nanofiber/Carbon Nanotube Conductive Nano-Network as a Reinforcement Template for Polydimethylsiloxane Nanocomposite. Polymers 2018, 10, 1000. https://doi.org/10.3390/polym10091000
Chen C, Bu X, Feng Q, Li D. Cellulose Nanofiber/Carbon Nanotube Conductive Nano-Network as a Reinforcement Template for Polydimethylsiloxane Nanocomposite. Polymers. 2018; 10(9):1000. https://doi.org/10.3390/polym10091000
Chicago/Turabian StyleChen, Chuchu, Xiangting Bu, Qian Feng, and Dagang Li. 2018. "Cellulose Nanofiber/Carbon Nanotube Conductive Nano-Network as a Reinforcement Template for Polydimethylsiloxane Nanocomposite" Polymers 10, no. 9: 1000. https://doi.org/10.3390/polym10091000




