New Polyhedral Oligomeric Silsesquioxanes-Based Fluorescent Ionic Liquids: Synthesis, Self-Assembly and Application in Sensors for Detecting Nitroaromatic Explosives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization and Measurements
2.3. General Procedures to Synthesize the IL-POSSs
3. Results
3.1. Synthesis and Characterization
3.2. Thermal Properties
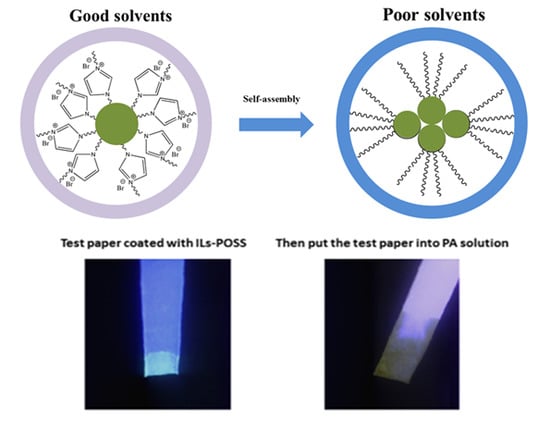
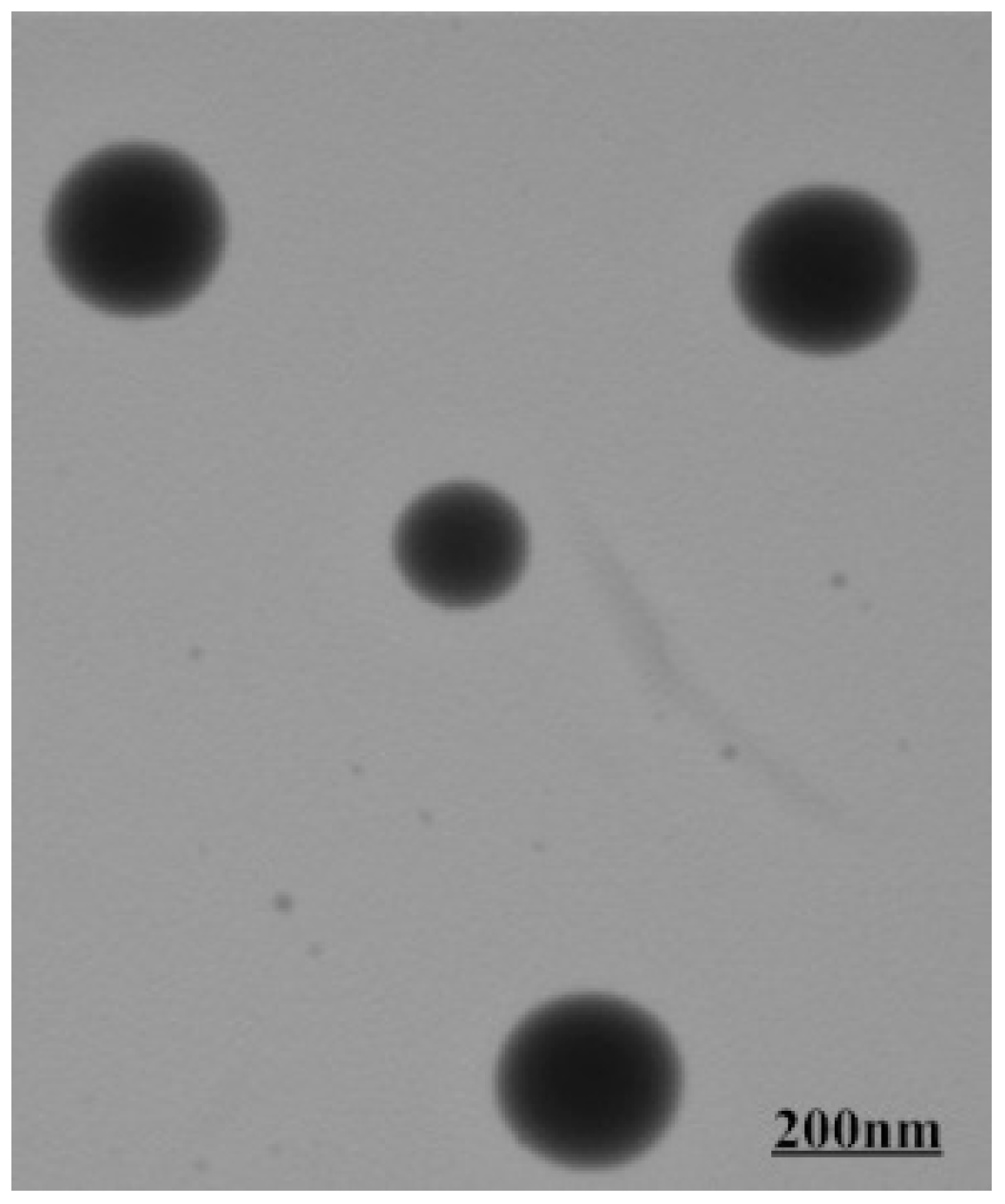
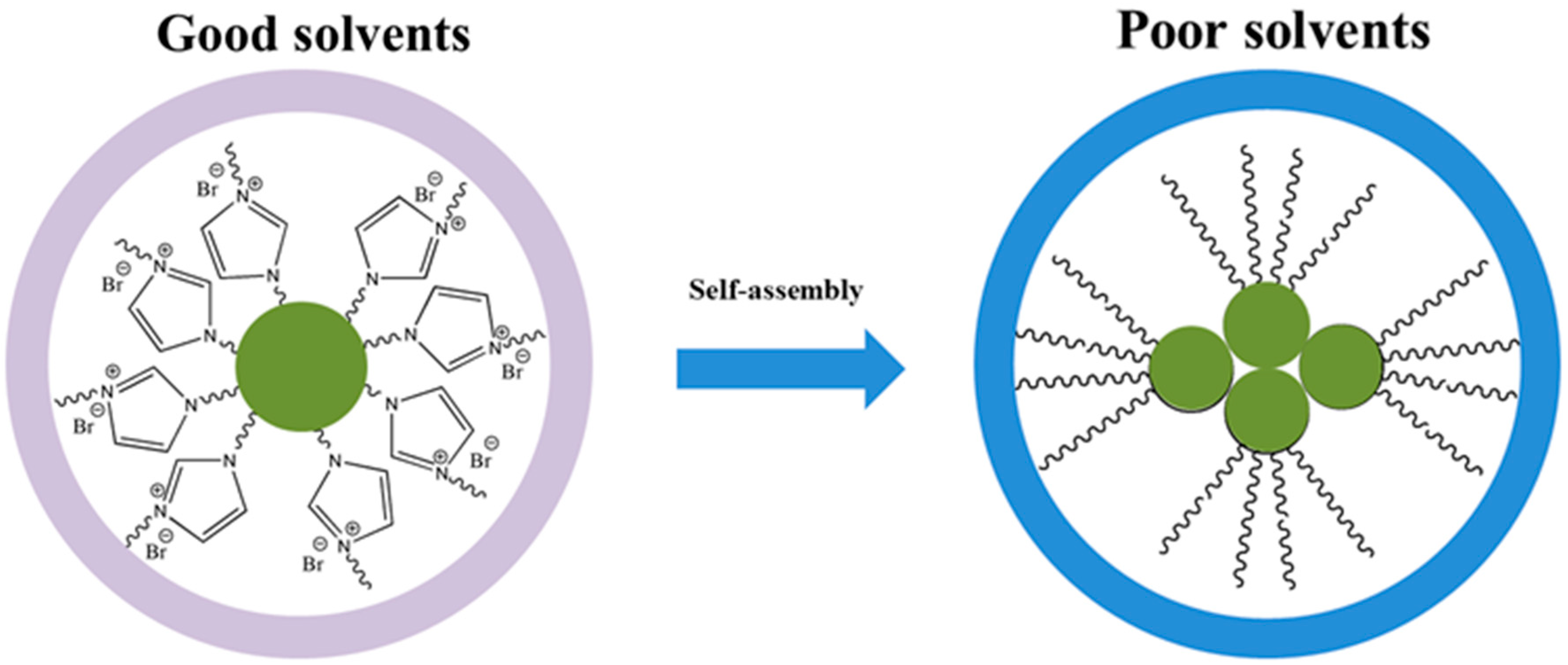
3.3. Self-Assembly Behaviors
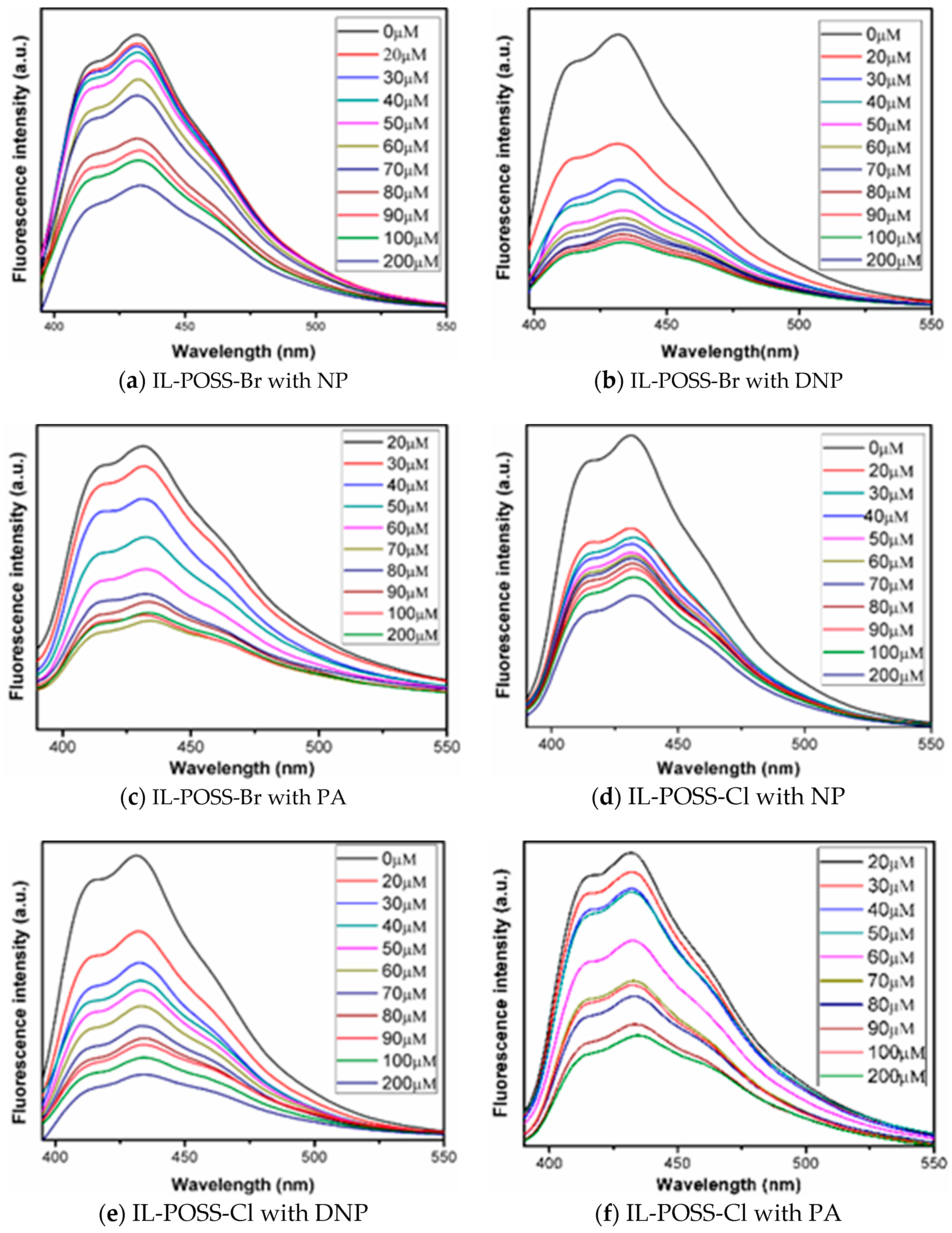
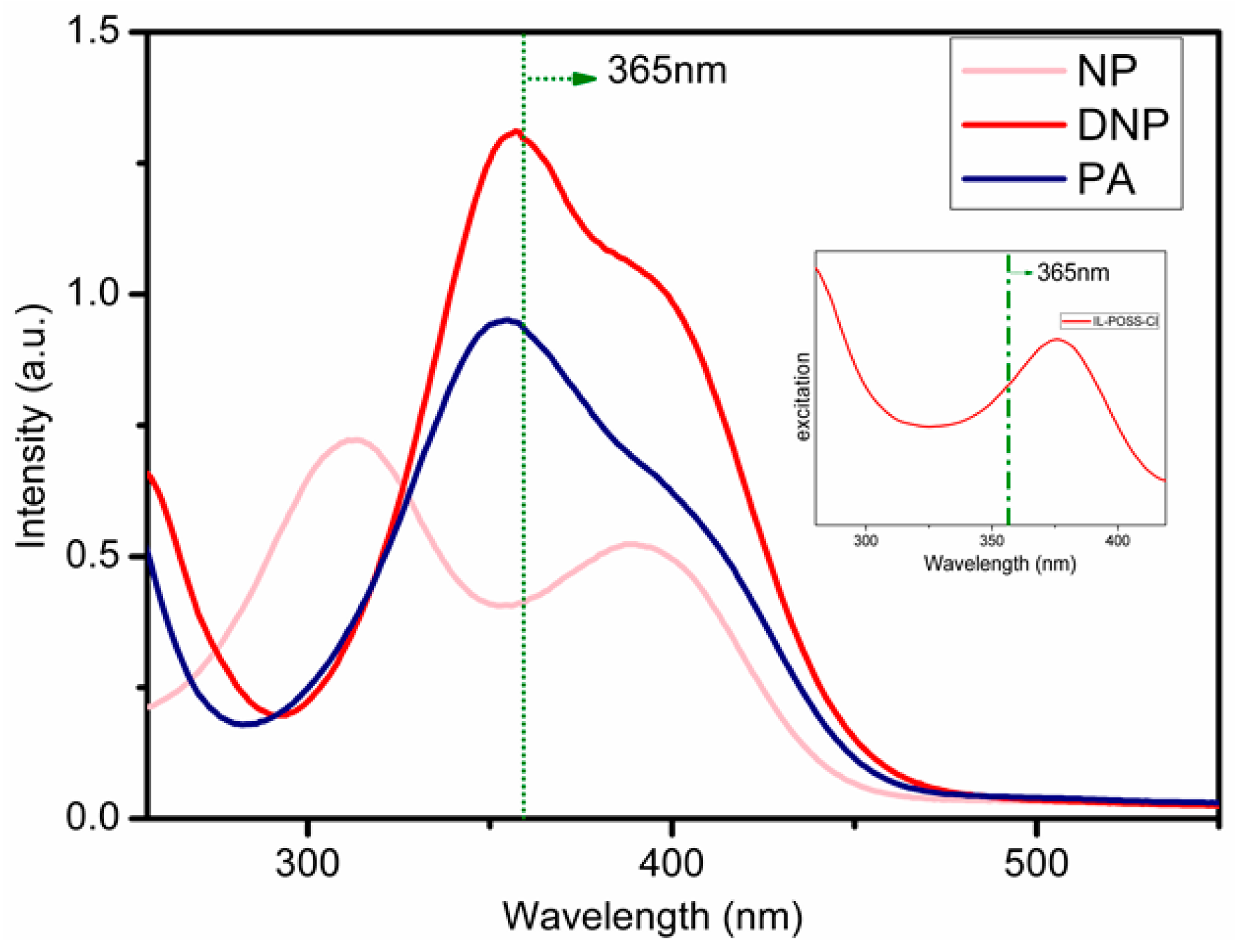
3.4. Optical Properties
4. Discussion
4.1. IL-POSSs in the Detection of Nitroaromatic Explosives
4.2. Simple and Efficient Testing of IL-POSSs as Sensors for Detecting Nitroaromatic Explosives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Yao, L.; Zhang, B.; Jiang, H.; Zhang, L.; Zhu, X. Poly(ionic liquid): A new phase in a thermoregulated phase separated catalysis and catalyst recycling system of transition metal-mediated atrp. Polymers 2018, 10, 347. [Google Scholar] [CrossRef]
- Zheng, X.; Lin, Q.; Jiang, P.; Li, Y.; Li, J. Ionic liquids incorporating polyamide 6: Miscibility and physical properties. Polymers 2018, 10, 562. [Google Scholar] [CrossRef]
- Fu, J.; Lu, Q.; Shang, D.; Chen, L.; Jiang, Y.; Xu, Y.; Yin, J.; Dong, X.; Deng, W.; Yuan, S. A novel room temperature POSS ionic liquid-based solid polymer electrolyte. J. Mater. Sci. 2018, 53, 8420–8435. [Google Scholar] [CrossRef]
- Topal, S.Z.; Ertekin, K.; Topkaya, D.; Alp, S.; Yenigul, B. Emission based oxygen sensing approach with tris(2,2′-bipyridyl)ruthenium(ii) chloride in green chemistry reagents: Room temperature ionic liquids. Microchim. Acta 2008, 161, 209–216. [Google Scholar] [CrossRef]
- Shang, D.; Fu, J.; Lu, Q.; Chen, L.; Yin, J.; Dong, X.; Xu, Y.; Jia, R.; Yuan, S.; Chen, Y.; et al. A novel polyhedral oligomeric silsesquioxane based ionic liquids (POSS-ILs) polymer electrolytes for lithium ion batteries. Solid State Ionics 2018, 319, 247–255. [Google Scholar] [CrossRef]
- Tanaka, K.; Ishiguro, F.; Jeon, J.-H.; Hiraoka, T.; Chujo, Y. POSS ionic liquid crystals. NPG Asia Mater. 2015, 7, 174. [Google Scholar] [CrossRef]
- Manickam, S.; Cardiano, P.; Mineo, P.G.; Lo Schiavo, S. Star-shaped quaternary alkylammonium polyhedral oligomeric silsesquioxane ionic liquids. Eur. J. Inorg. Chem. 2014, 2014, 2704–2710. [Google Scholar] [CrossRef]
- Na, W.; Lee, A.S.; Lee, J.H.; Hong, S.M.; Kim, E.; Koo, C.M. Hybrid ionogel electrolytes with POSS epoxy networks for high temperature lithium ion capacitors. Solid State Ionics 2017, 309, 27–32. [Google Scholar] [CrossRef]
- Sun, J.K.; Antonietti, M.; Yuan, J. Nanoporous ionic organic networks: From synthesis to materials applications. Chem. Soc. Rev. 2016, 45, 6627–6656. [Google Scholar] [CrossRef] [PubMed]
- Daver, F.; Kajtaz, M.; Brandt, M.; Shanks, R. Creep and recovery behavior of polyolefin-rubber nanocomposites developed for additive manufacturing. Polymers 2016, 8, 437. [Google Scholar] [CrossRef]
- Tan, J.; Ma, D.; Sun, X.; Feng, S.; Zhang, C. Synthesis and characterization of an octaimidazolium-based polyhedral oligomeric silsesquioxanes ionic liquid by an ion-exchange reaction. Dalton Trans. 2013, 42, 4337–4339. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, O.; Fina, A.; Cavallo, D.; Gioffredi, E.; Delprato, G. On a novel method to synthesize POSS-based hybrids: An example of the preparation of TPU based system. Express Polym. Lett. 2013, 7, 966–973. [Google Scholar] [CrossRef] [Green Version]
- McCusker, C.; Carroll, J.B.; Rotello, V.M. Cationic polyhedral oligomeric silsesquioxane (POSS) units as carriers for drug delivery processes. Chem. Commun. 2005, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Saez, I.M.; Goodby, J.W. Chiral nematic octasilsesquioxanes. J. Mater. Chem. 2001, 11, 2845–2851. [Google Scholar] [CrossRef]
- Pan, Q.; Chen, X.; Fan, X.; Shen, Z.; Zhou, Q. Organic–inorganic hybrid bent-core liquid crystals with cubic silsesquioxane cores. J. Mater. Chem. 2008, 18, 3481–3488. [Google Scholar] [CrossRef]
- Ye, S.-H.; Li, L.; Zhang, M.; Zhou, Z.; Quan, M.-H.; Guo, L.-F.; Wang, Y.; Yang, M.; Lai, W.-Y.; Huang, W. Pyridine linked fluorene hybrid bipolar host for blue, green, and orange phosphorescent organic light-emitting diodes toward solution processing. J. Mater. Chem. C 2017, 5, 11937–11946. [Google Scholar] [CrossRef]
- Li, L.; Liu, H. Rapid preparation of silsesquioxane-based ionic liquids. Chemistry 2016, 22, 4713–4716. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.; Nischang, I. Tailor-made hybrid organicinorganic porous materials based on polyhedral oligomeric silsesquioxanes (POSS) by the stepgrowth mechanism of thiolene ‘click’ chemistry. Chem. Eur. J. 2013, 19, 17310–17313. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.; Scholder, P.; Nischang, I. Conceptual design of large surface area porous polymeric hybrid media based on polyhedral oligomeric silsesquioxane precursors: Preparation, tailoring of porous properties, and internal surface functionalization. ACS Appl. Mater. Interfaces 2013, 5, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Qu, Y.; Zhai, Y.; Liu, Y.; Sun, Z.; Yang, J.; Jin, L. {MSU/PDDA}n LBL assembled modified sensor for electrochemical detection of ultratrace explosive nitroaromatic compounds. Electrochem. Commun. 2007, 9, 1719–1724. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Sun, Z.; Sun, T.; Xie, Z.; Huang, Y.; Jing, X. Light-induced synthesis of cross-linked polymers and their application in explosive detection. Eur. Polym. J. 2015, 63, 149–155. [Google Scholar] [CrossRef]
- Yu, R.; Li, Y.; Tao, F.; Cui, Y.; Song, W.; Li, T. A novel double-layer electrospun nanofibrous membrane sensor for detecting nitroaromatic compounds. J. Mater. Sci. 2016, 51, 10350–10360. [Google Scholar] [CrossRef]
- Qin, J.; Chen, L.; Zhao, C.; Lin, Q.; Chen, S. Cellulose nanofiber/cationic conjugated polymer hybrid aerogel sensor for nitroaromatic vapors detection. J. Mater. Sci. 2017, 52, 8455–8464. [Google Scholar] [CrossRef]
- Kumari, S.; Joshi, S.; Cordova-Sintjago, T.C.; Pant, D.D.; Sakhuja, R. Highly sensitive fluorescent imidazolium-based sensors for nanomolar detection of explosive picric acid in aqueous medium. Sens. Actuators B 2016, 229, 599–608. [Google Scholar] [CrossRef]
- Massera, E.; Castaldo, A.; Quercia, L.; Di Francia, G. Fabrication and characterization of polysilsesquioxanes nanocomposites based chemical sensor. Sens. Actuators B 2008, 129, 487–490. [Google Scholar] [CrossRef]
- Karthik, P.; Pandikumar, A.; Preeyanghaa, M.; Kowsalya, M.; Neppolian, B. Amino-functionalized mil-101(Fe) metal-organic framework as a viable fluorescent probe for nitroaromatic compounds. Microchim. Acta 2017, 184, 2265–2273. [Google Scholar] [CrossRef]
- Tian, X.; Qi, X.; Liu, X.; Zhang, Q. Selective detection of picric acid by a fluorescent ionic liquid chemosensor. Sens. Actuators B 2016, 229, 520–527. [Google Scholar] [CrossRef]
- Dai, J.; Dong, X.; Fidalgo de Cortalezzi, M. Molecularly imprinted polymers labeled with amino-functionalized carbon dots for fluorescent determination of 2,4-dinitrotoluene. Microchim. Acta 2017, 184, 1369–1377. [Google Scholar] [CrossRef]
- Xiong, J.F.; Li, J.X.; Mo, G.Z.; Huo, J.P.; Liu, J.Y.; Chen, X.Y.; Wang, Z.Y. Benzimidazole derivatives: Selective fluorescent chemosensors for the picogram detection of picric acid. J. Org. Chem. 2014, 79, 11619–11630. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Wang, Y.; Tang, W.; Yang, Y.; Xie, X. Progress in imidazolium ionic liquids assisted fabrication of carbon nanotube and graphene polymer composites. Polymers 2013, 5, 847–872. [Google Scholar] [CrossRef]
- Zuo, Y.; Gou, Z.; Li, Z.; Qi, J.; Feng, S. Unexpected self-assembly, photoluminescence behavior, and film-forming properties of polysiloxane-based imidazolium ionic liquids prepared by one-pot thiol–ene reaction. New J. Chem. 2017, 41, 14545–14550. [Google Scholar] [CrossRef]
- Cardiano, P.; Lazzara, G.; Manickam, S.; Mineo, P.; Milioto, S.; Lo Schiavo, S. POSS-tetraalkylammonium salts: A new class of ionic liquids. Eur. J. Inorg. Chem. 2012, 2012, 5668–5676. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, H.; Xu, J. Cubic polyhedral oligomeric silsesquioxane based functional materials: Synthesis, assembly, and applications. Chem. Asian J. 2016, 11, 1322–1337. [Google Scholar] [CrossRef] [PubMed]
- Cardiano, P.; Fazio, E.; Lazzara, G.; Manickam, S.; Milioto, S.; Neri, F.; Mineo, P.G.; Piperno, A.; Lo Schiavo, S. Highly untangled multiwalled carbon nanotube@polyhedral oligomeric silsesquioxane ionic hybrids: Synthesis, characterization and nonlinear optical properties. Carbon 2015, 86, 325–337. [Google Scholar] [CrossRef] [Green Version]
- Castriciano, M.A.; Leone, N.; Cardiano, P.; Manickam, S.; Scolaro, L.M.; Lo Schiavo, S. A new supramolecular polyhedral oligomeric silsesquioxanes (POSS)–porphyrin nanohybrid: Synthesis and spectroscopic characterization. J. Mater. Chem. C 2013, 1, 4746–4753. [Google Scholar] [CrossRef]
- Jeon, J.-H.; Tanaka, K.; Chujo, Y. Synthesis of sulfonic acid-containing POSS and its filler effects for enhancing thermal stabilities and lowering melting temperatures of ionic liquids. J. Mater. Chem. A 2014, 2, 624–630. [Google Scholar] [CrossRef]
- Zuo, Y.; Lu, H.; Xue, L.; Wang, X.; Wu, L.; Feng, S. Polysiloxane-based luminescent elastomers prepared by thiol-ene ‘click’ chemistry. Chemistry 2014, 20, 12924–12932. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Cao, J.; Feng, S. Sunlight-induced cross-linked luminescent films based on polysiloxanes and d-limonene via thiol-ene ‘click’ chemistry. Adv. Funct. Mater. 2015, 25, 2754–2762. [Google Scholar] [CrossRef]
- Xie, H.; Wang, H.; Xu, Z.; Qiao, R.; Wang, X.; Wang, X.; Wu, L.; Lu, H.; Feng, S. A silicon-cored fluoranthene derivative as a fluorescent probe for detecting nitroaromatic compounds. J. Mater. Chem. C 2014, 2, 9425–9430. [Google Scholar] [CrossRef]
- Sun, R.; Huo, X.; Lu, H.; Feng, S.; Wang, D.; Liu, H. Recyclable fluorescent paper sensor for visual detection of nitroaromatic explosives. Sens. Actuators B 2018, 265, 476–487. [Google Scholar] [CrossRef]
- Guo, L.; Zeng, X.; Lan, J.; Yun, J.; Cao, D. Absorption competition quenching mechanism of porous covalent organic polymer as luminescent sensor for selective sensing Fe3+. Chem. Select 2017, 2, 1041–1047. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Wang, D.; Han, D.; Sun, R.; Zhang, J.; Feng, S. New Polyhedral Oligomeric Silsesquioxanes-Based Fluorescent Ionic Liquids: Synthesis, Self-Assembly and Application in Sensors for Detecting Nitroaromatic Explosives. Polymers 2018, 10, 917. https://doi.org/10.3390/polym10080917
Li W, Wang D, Han D, Sun R, Zhang J, Feng S. New Polyhedral Oligomeric Silsesquioxanes-Based Fluorescent Ionic Liquids: Synthesis, Self-Assembly and Application in Sensors for Detecting Nitroaromatic Explosives. Polymers. 2018; 10(8):917. https://doi.org/10.3390/polym10080917
Chicago/Turabian StyleLi, Wensi, Dengxu Wang, Dongdong Han, Ruixue Sun, Jie Zhang, and Shengyu Feng. 2018. "New Polyhedral Oligomeric Silsesquioxanes-Based Fluorescent Ionic Liquids: Synthesis, Self-Assembly and Application in Sensors for Detecting Nitroaromatic Explosives" Polymers 10, no. 8: 917. https://doi.org/10.3390/polym10080917