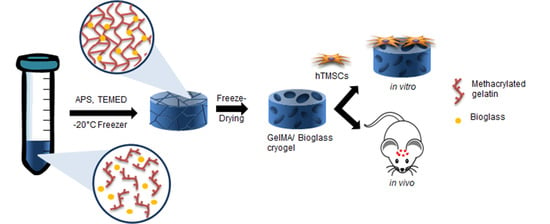
Bioglass-Incorporated Methacrylated Gelatin Cryogel for Regeneration of Bone Defects
Abstract
:1. Introduction
2. Materials and Methods
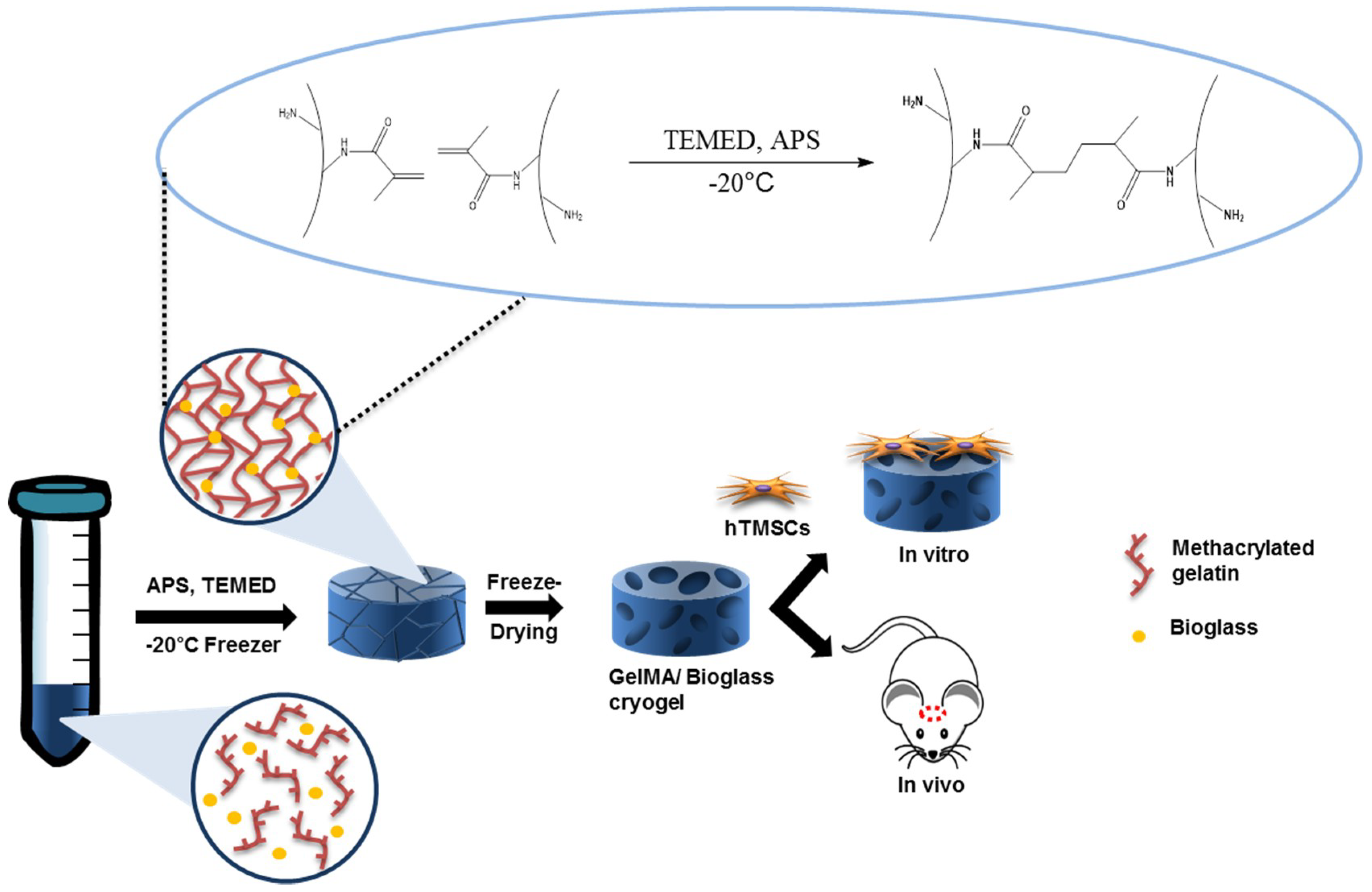
2.1. Synthesis of Methacrylated Gelatin
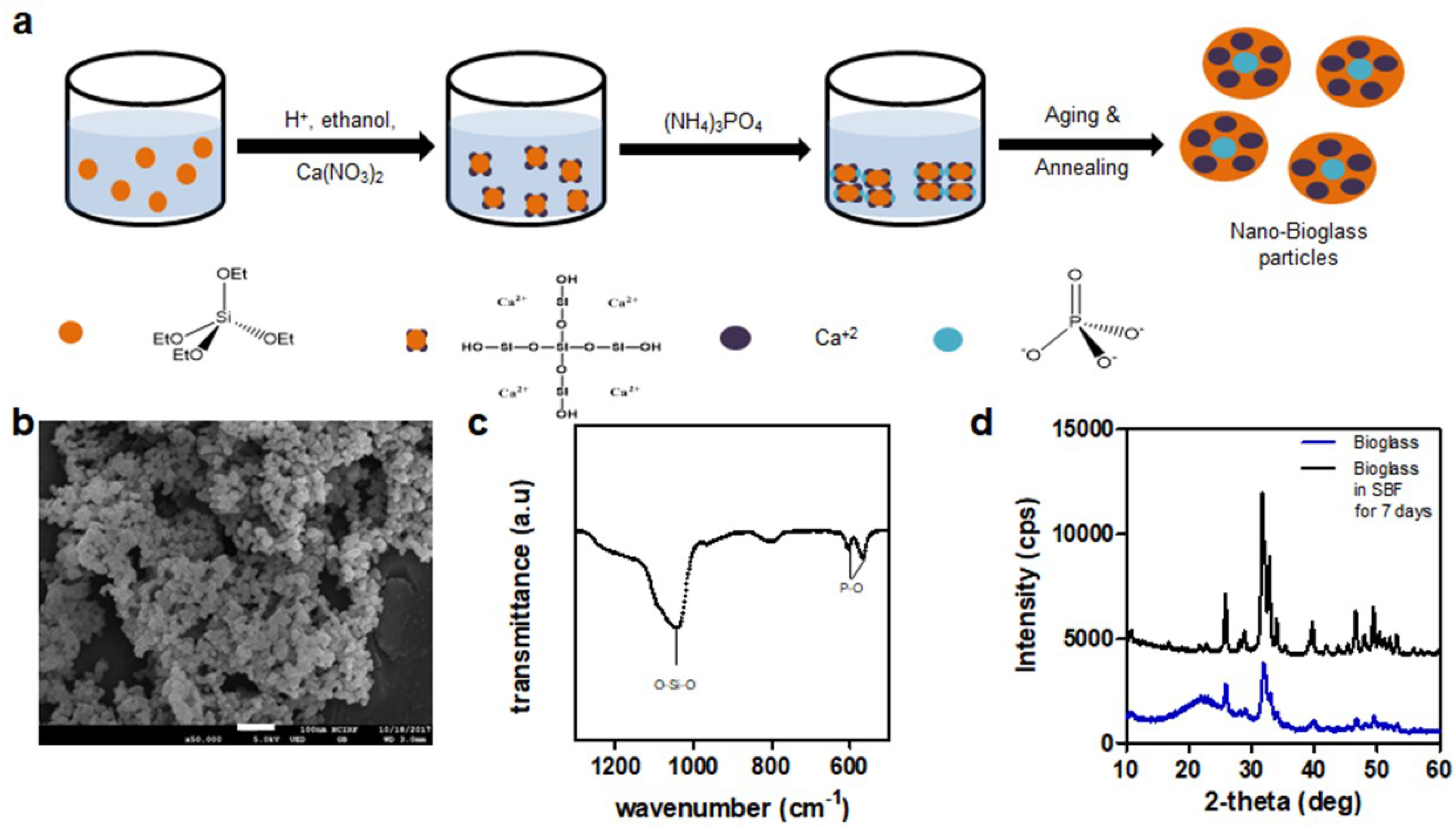
2.2. Preparation of Nanobioglass
2.3. Fabrication of GelMA-Bioglass Cryogel
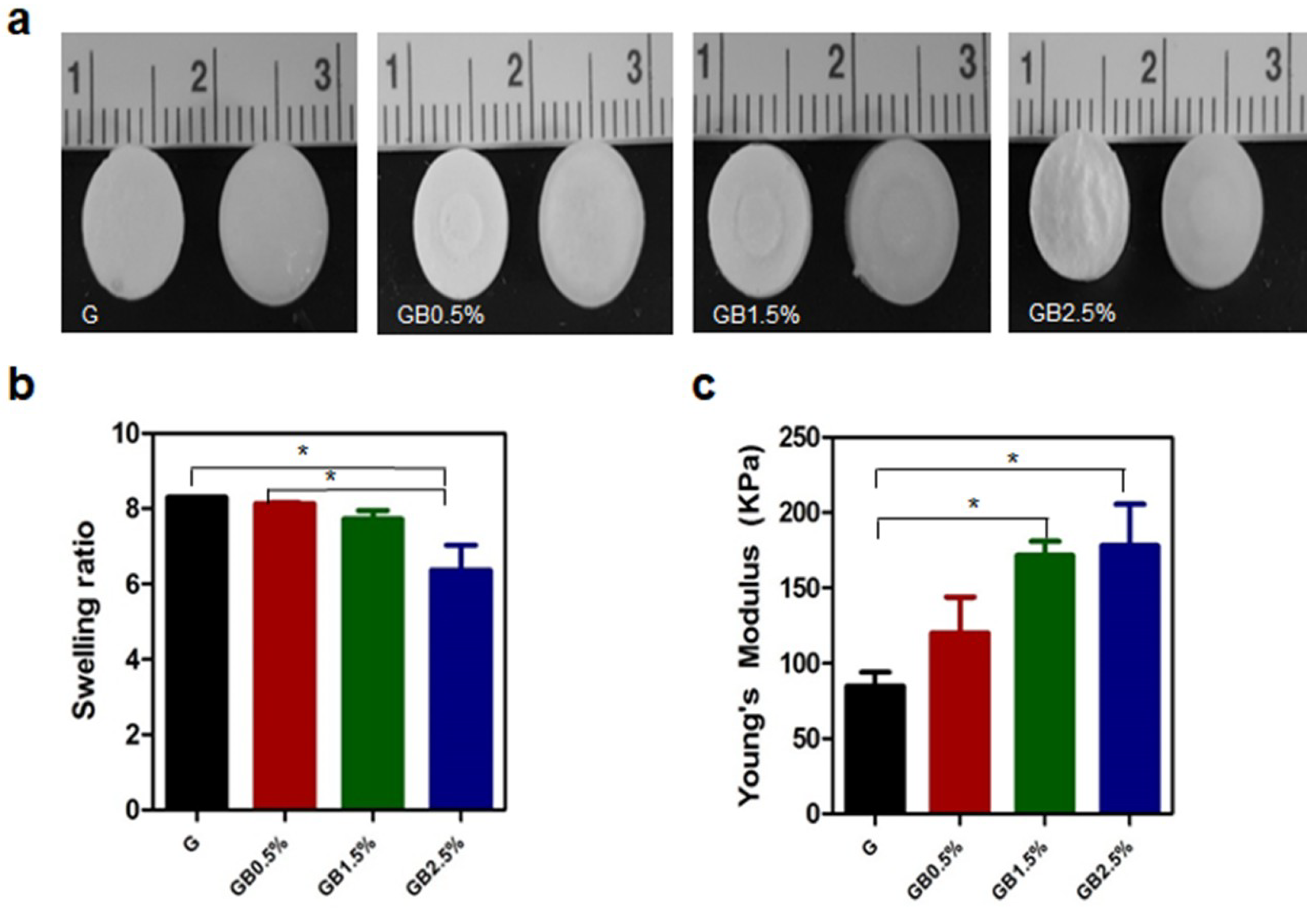
2.4. Swelling Ratio and Mechanical Property of Scaffold
2.5. Degradation by Collagenase
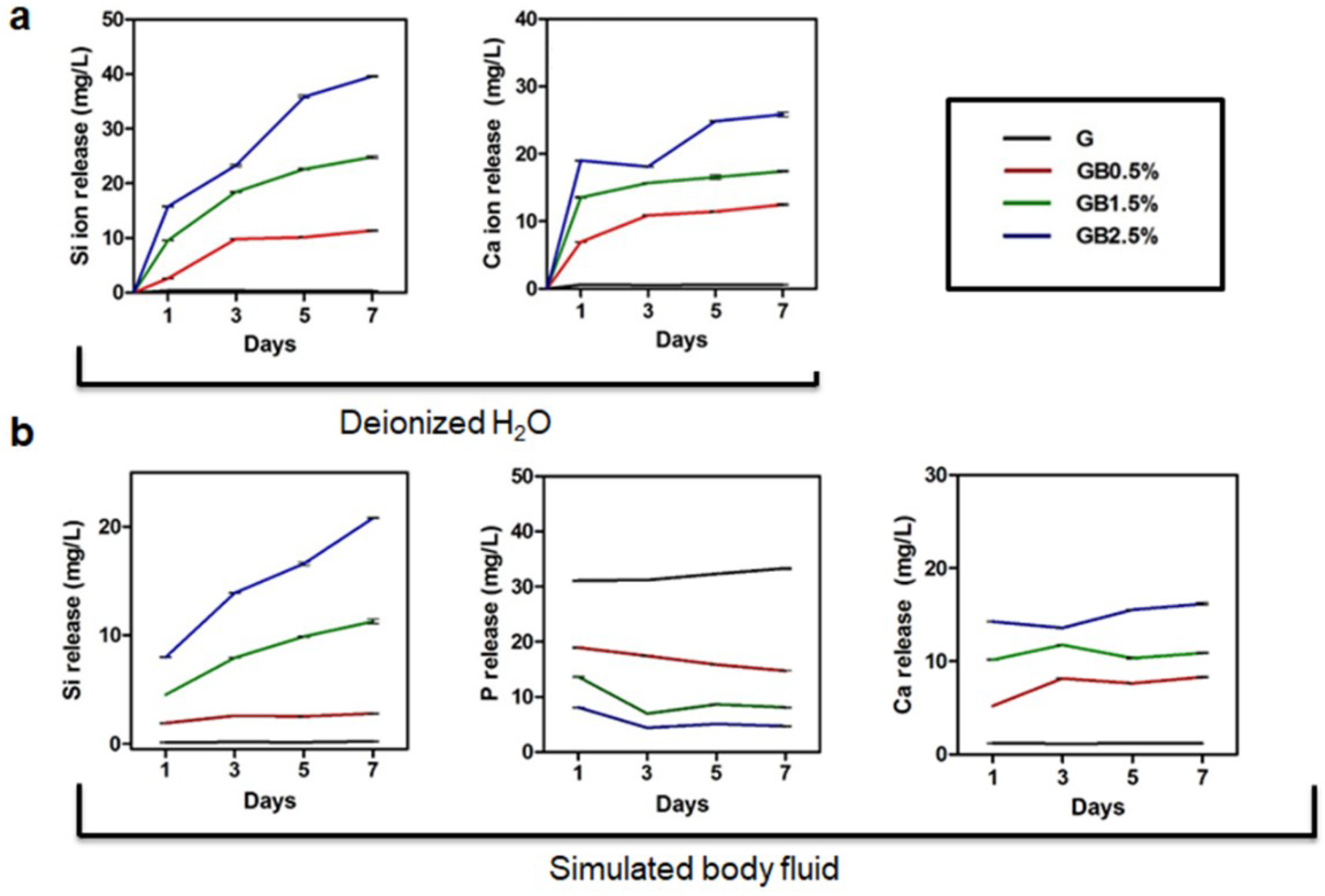
2.6. Ion Release Analysis
2.7. Scanning Electron Microscopy
2.8. Cell Cultures
2.9. Cell Viability
2.10. In Vitro Osteogenic Differentiation
2.11. Real Time-PCR
2.12. Alizarin Red Staining
2.13. Calvarial Defect Surgical Procedure
2.14. Microcomputed Tomography Analysis
2.15. Histological Analysis
2.16. Statistical Analysis
3. Results
3.1. Synthesis of Methacrylated Gelatin and Bioglass
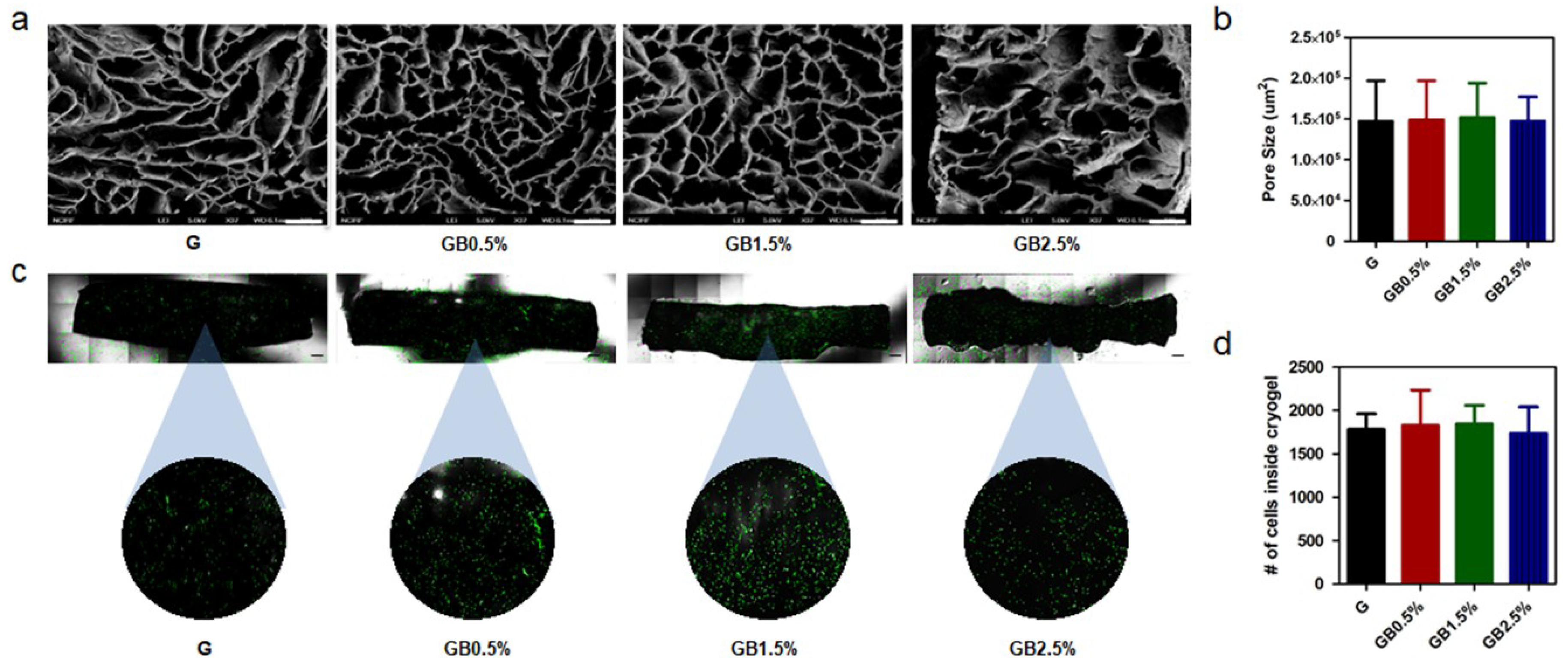
3.2. Characterization of Bioglass-Embedded Methacrylated Gelatin Cryogel
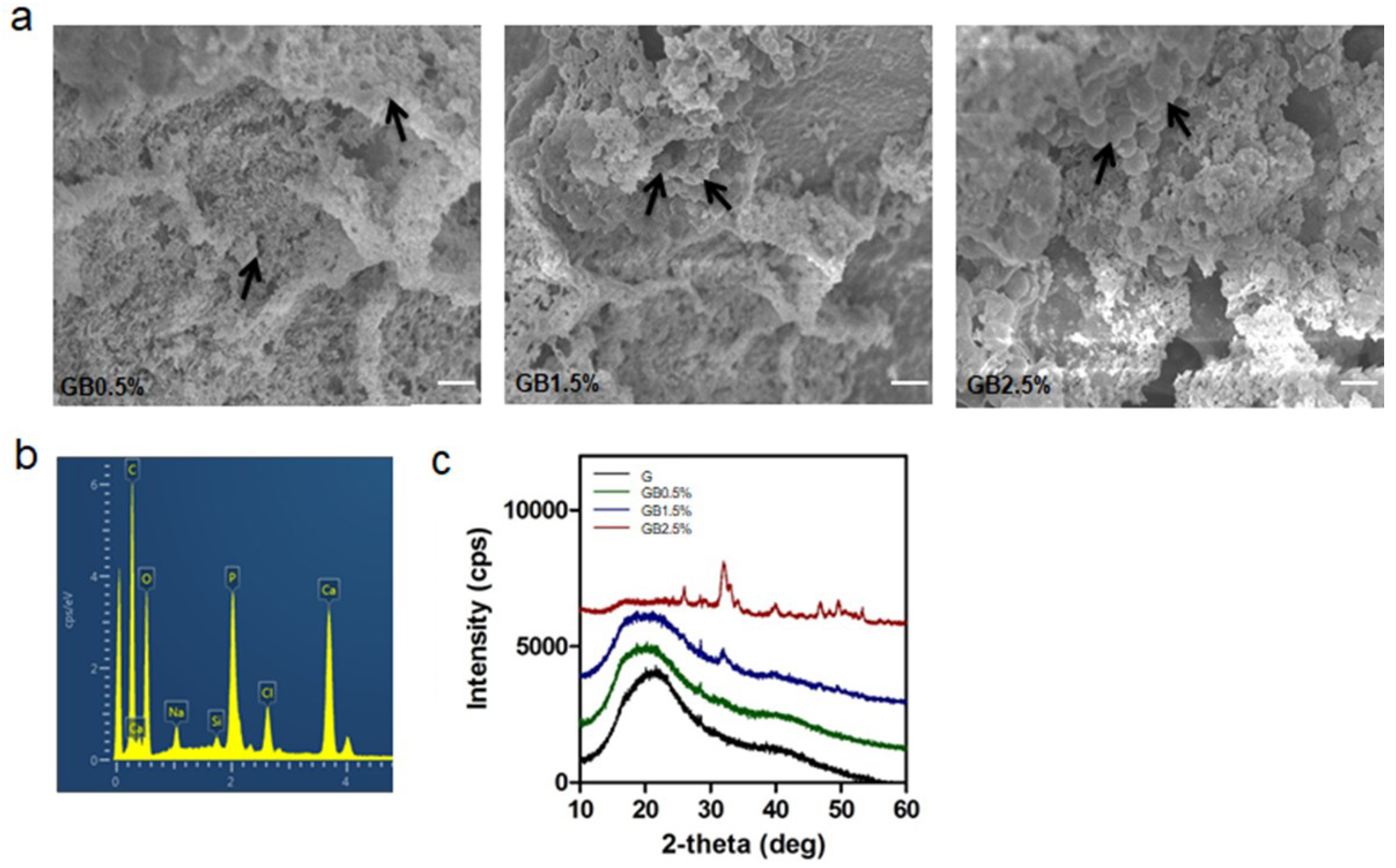
3.3. Hydroxyapatite Formation in SBF Solution
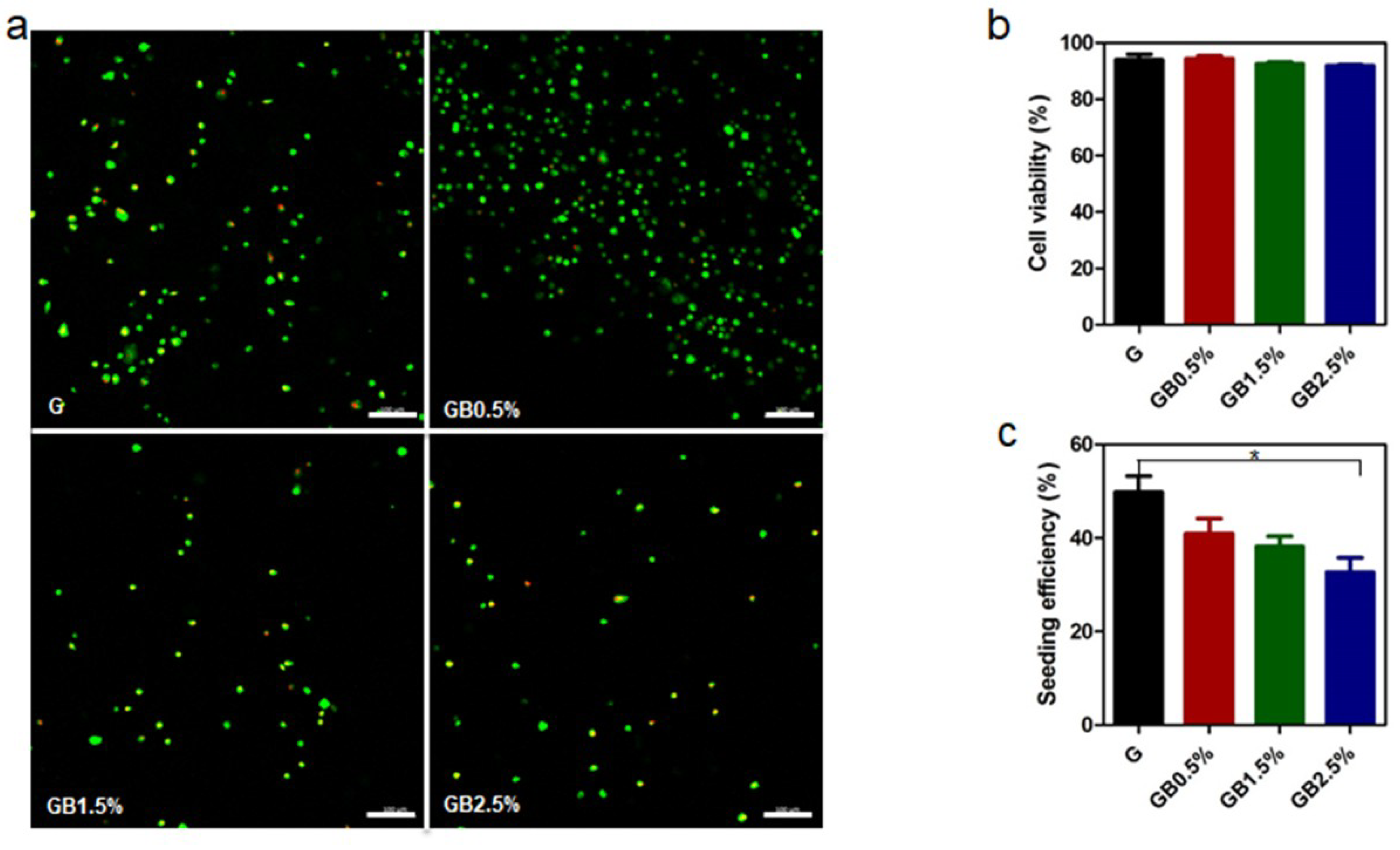
3.4. Cell Viability Analysis
3.5. Enhanced Osteogenic Responses on hTMSCs on GelMA-Bioglass cryogel
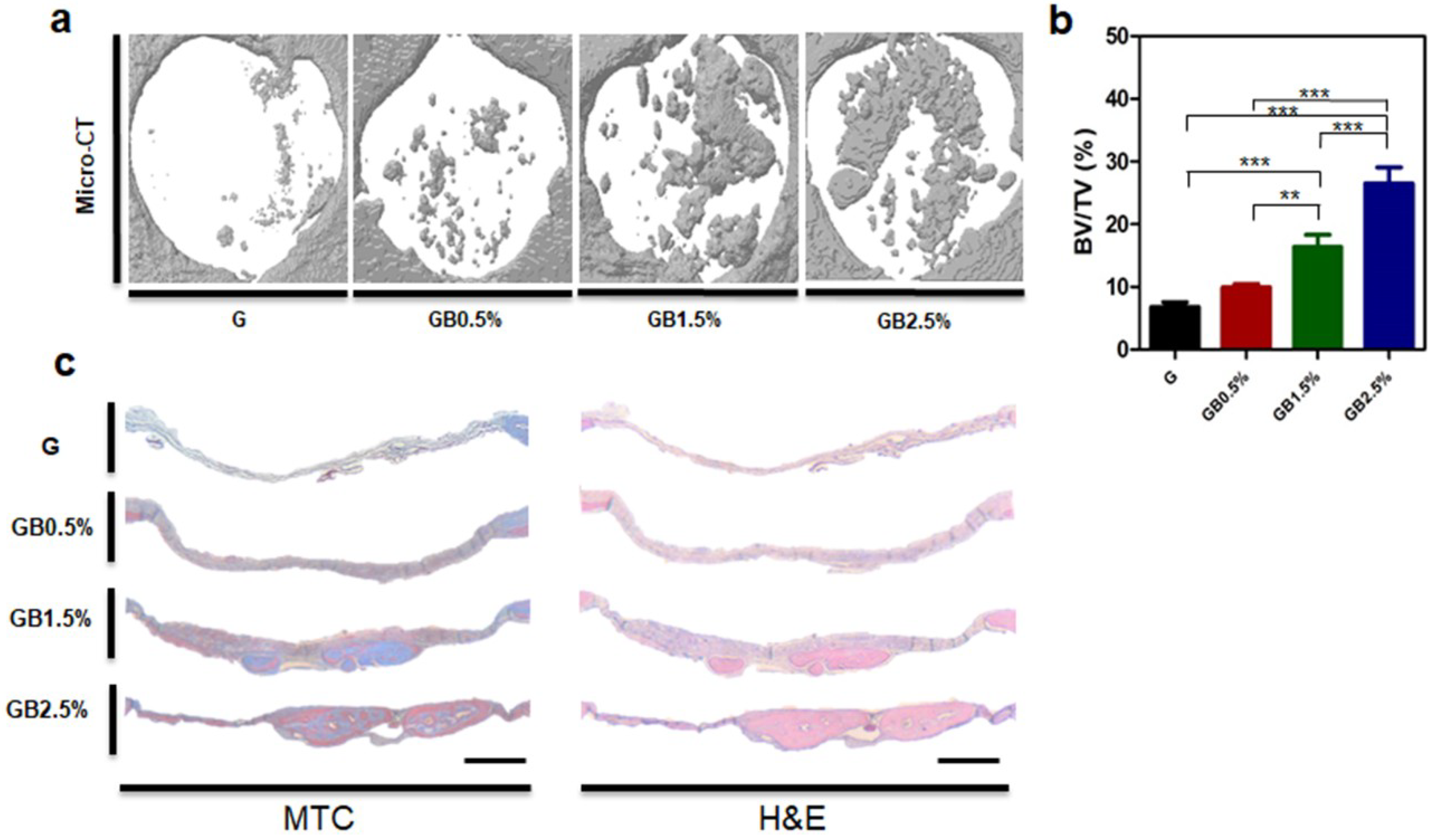
3.6. In Vivo Bone Regeneration after Implanting Bioglass-Embedded GelMA Cryogel
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fillingham, Y.; Jacobs, J. Bone grafts and their substitutes. Bone Jt. J. 2016, 98-B, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, M.H.; Delmonte, R.J.; Scripps, M.L. Autogenous bone grafting. J. Foot Ankle Surg. 1996, 35, 386–390. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Faour, O.; Goff, T.; Kanakaris, N.; Dimitriou, R. Masquelet technique for the treatment of bone defects: Tips-tricks and future directions. Injury 2011, 42, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Cox, G.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Reamer-irrigator-aspirator indications and clinical results: A systematic review. Int. Orthop. 2011, 35, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Hench, L.L. Materials perspective-biomedical materials for new millennium: Perspective on the future. Mater. Sci. Technol. 2001, 17, 891–900. [Google Scholar] [CrossRef]
- Betz, R.R. Limitations of autograft and allograft: New synthetic solutions. Orthopedics 2002, 25, s561–s570. [Google Scholar] [PubMed]
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-based nanocomposites for bone tissue engineering and regenerative medicine: A review. Adv. Mater. 2015, 27, 1143–1169. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.H.; Kim, S.K. Alginate composites for bone tissue engineering: A review. Int J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.C.; Thornby, J.A.; Gibbons, G.J.; Williams, M.A.; Mallick, K.K. 3D printing of porous hydroxyapatite scaffolds intended for use in bone tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 47, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Kim, H.D.; Park, J.; Lee, E.S.; Kim, E.; Lee, S.S.; Yang, J.K.; Lee, Y.S.; Hwang, N.S. Enhanced osteogenic commitment of murine mesenchymal stem cells on graphene oxide substrate. Biomater. Res. 2018, 22, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gwak, G.H.; Choi, A.J.; Bae, Y.S.; Choi, H.J.; Oh, J.M. Electrophoretically prepared hybrid materials for biopolymer hydrogel and layered ceramic nanoparticles. Biomater. Res. 2016, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Bang, S.; Cho, Y.; Lee, S.; Lee, I.; Zhang, S.; Noh, I. Research trends in biomimetic medical materials for tissue engineering: 3d bioprinting, surface modification, nano/micro-technology and clinical aspects in tissue engineering of cartilage and bone. Biomater. Res. 2016, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Kim, S.H.; Lee, H.; Hwang, N.S. Non-viral approaches for direct conversion into mesenchymal cell types: Potential application in tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Z.; Thompson, I.D.; Boccaccini, A.R. 45s5 bioglass-derived glass-ceramic scaffolds for bone tissue engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Lee, S.S.; Bae, S.; Lee, H.; Hwang, N.S. Heparin functionalized injectable cryogel with rapid shape-recovery property for neovascularization. Biomacromolecules 2018, 19, 2257–2269. [Google Scholar] [CrossRef] [PubMed]
- Kumari, J.; Kumar, A. Development of polymer based cryogel matrix for transportation and storage of mammalian cells. Sci. Rep. 2017, 7, 41551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savina, I.N.; Galaev, I.Y.; Mattiasson, B. Ion-exchange macroporous hydrophilic gel monolith with grafted polymer brushes. J. Mol. Recognit. 2006, 19, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Rahman, C.V.; Kuhn, G.; White, L.J.; Kirby, G.T.; Varghese, O.P.; McLaren, J.S.; Cox, H.C.; Rose, F.R.; Muller, R.; Hilborn, J.; et al. Plga/peg-hydrogel composite scaffolds with controllable mechanical properties. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.S.; Taite, L.J.; Moon, J.J.; Rowland, M.C.; Ruffino, K.A.; West, J.L. Photolithographic patterning of polyethylene glycol hydrogels. Biomaterials 2006, 27, 2519–2524. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Ikada, Y.; Tabata, Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. J. Biomater. Sci. Polym. Ed. 2001, 12, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Duan, B.; Lu, A.; Wang, Y.; Ye, Q.; Zhang, L. Biocompatible chitin/carbon nanotubes composite hydrogels as neuronal growth substrates. Carbohydr. Polym. 2017, 174, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Koshy, S.T.; Ferrante, T.C.; Lewin, S.A.; Mooney, D.J. Injectable, porous, and cell-responsive gelatin cryogels. Biomaterials 2014, 35, 2477–2487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of cell binding to collagen and gelatin: A study of the effect of 2d and 3d architecture and surface chemistry. J. Mater. Sci. Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef] [PubMed]
- Mazaki, T.; Shiozaki, Y.; Yamane, K.; Yoshida, A.; Nakamura, M.; Yoshida, Y.; Zhou, D.; Kitajima, T.; Tanaka, M.; Ito, Y.; et al. A novel, visible light-induced, rapidly cross-linkable gelatin scaffold for osteochondral tissue engineering. Sci. Rep. 2014, 4, 4457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, T.I.; Sakuragi, M.; Takahashi, S.; Obuse, S.; Kang, J.; Fujishiro, M.; Matsushita, H.; Gong, J.; Shimizu, S.; Tajima, Y.; et al. Visible light-induced crosslinkable gelatin. Acta Biomater. 2010, 6, 4005–4010. [Google Scholar] [CrossRef] [PubMed]
- Han, M.E.; Kang, B.J.; Kim, S.H.; Kim, H.D.; Hwang, N.S. Gelatin-based extracellular matrix cryogels for cartilage tissue engineering. J. Ind. Eng. Chem. 2017, 45, 421–429. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lin, R.Z.; Qi, H.; Yang, Y.; Bae, H.; Melero-Martin, J.M.; Khademhosseini, A. Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv. Funct. Mater. 2012, 22, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Liu, X.; Wei, D.; Sun, J.; Xiao, W.; Zhao, H.; Guo, L.; Wei, Q.; Fan, H.; Zhang, X. Photo-cross-linkable methacrylated gelatin and hydroxyapatite hybrid hydrogel for modularly engineering biomimetic osteon. ACS Appl. Mater. Interfaces 2015, 7, 10386–10394. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerhardt, L.C.; Boccaccini, A.R. Bioactive glass and glass-ceramic scaffolds for bone tissue engineering. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef] [PubMed]
- Ros-Tarraga, P.; Rabadan-Ros, R.; Murciano, A.; Meseguer-Olmo, L.; De Aza, P.N. Assessment of effects of si-ca-p biphasic ceramic on the osteogenic differentiation of a population of multipotent adult human stem cells. Materials 2016, 9, 969. [Google Scholar] [CrossRef] [PubMed]
- Barradas, A.M.; Fernandes, H.A.; Groen, N.; Chai, Y.C.; Schrooten, J.; van de Peppel, J.; van Leeuwen, J.P.; van Blitterswijk, C.A.; de Boer, J. A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials 2012, 33, 3205–3215. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, J.; Chonchol, M. The role of phosphorus in the development and progression of vascular calcification. Am. J. Kidney Dis. 2011, 58, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.H.; Yao, C.H.; Huang, C.W.; Liu, H.C.; Sun, J.S.; Wang, C.Y. The bonding behavior of dp-bioglass and bone tissue. Mater. Chem. Phys. 1996, 46, 36–42. [Google Scholar] [CrossRef]
- Gomez-Vega, J.M.; Saiz, E.; Tomsia, A.P.; Marshall, G.W.; Marshall, S.J. Bioactive glass coatings with hydroxyapatite and bioglass particles on ti-based implants. 1. Processing. Biomaterials 2000, 21, 105–111. [Google Scholar] [CrossRef]
- Qu, T.; Liu, X. Nano-structured gelatin/bioactive glass hybrid scaffolds for the enhancement of odontogenic differentiation of human dental pulp stem cells. J. Mater. Chem. B 2013, 1, 4764–4772. [Google Scholar] [CrossRef] [PubMed]
- Allan, I.U.; Tolhurst, B.A.; Shevchenko, R.V.; Dainiak, M.B.; Illsley, M.; Ivanov, A.; Jungvid, H.; Galaev, I.Y.; James, S.L.; Mikhalovsky, S.V.; et al. An in vitro evaluation of fibrinogen and gelatin containing cryogels as dermal regeneration scaffolds. Biomater. Sci. 2016, 4, 1007–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Hutson, C.B.; Nichol, J.W.; Aubin, H.; Bae, H.; Yamanlar, S.; Al-Haque, S.; Koshy, S.T.; Khademhosseini, A. Synthesis and characterization of tunable poly(ethylene glycol): Gelatin methacrylate composite hydrogels. Tissue Eng. Part A 2011, 17, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Reis, R.L.; Mano, J.F. Preparation and in vitro characterization of novel bioactive glass ceramic nanoparticles. J. Biomed. Mater. Res. A 2009, 88, 304–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constantz, B.R.; Ison, I.C.; Fulmer, M.T.; Poser, R.D.; Smith, S.T.; Vanwagoner, M.; Ross, J.; Goldstein, S.A.; Jupiter, J.B.; Rosenthal, D.I. Skeletal repair by in-situ formation of the mineral phase of bone. Science 1995, 267, 1796–1799. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Ducheyne, P.; Radin, S. Determination of the ca/p ratio in calcium-deficient hydroxyapatite using x-ray-diffraction analysis. J. Mater. Sci. Mater. Med. 1993, 4, 165–168. [Google Scholar] [CrossRef]
- Benton, J.A.; DeForest, C.A.; Vivekanandan, V.; Anseth, K.S. Photocrosslinking of gelatin macromers to synthesize porous hydrogels that promote valvular interstitial cell function. Tissue Eng. Part A 2009, 15, 3221–3230. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T.; Kamiura, Y.; Yamamoto, S.; Kohgo, T.; Amemiya, A.; Ukai, H.; Murakami, K.; Asaoka, K. Early bone formation around calcium-ion-implanted titanium inserted into rat tibia. J. Biomed. Mater. Res. 1997, 36, 131–136. [Google Scholar] [CrossRef]
- Maeda, H.; Kasuga, T.; Hench, L.L. Preparation of poly (L-lactic acid)-polysiloxane-calcium carbonate hybrid membranes for guided bone regeneration. Biomaterials 2006, 27, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.F.; Saleem, M.; Afzal, A.; Ali, A.; Khan, A.; Khan, A.R. Bioactive behavior of silicon substituted calcium phosphate based bioceramics for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 35, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Shie, M.Y.; Ding, S.J.; Chang, H.C. The role of silicon in osteoblast-like cell proliferation and apoptosis. Acta Biomater. 2011, 7, 2604–2614. [Google Scholar] [CrossRef] [PubMed]
- Boonrungsiman, S.; Gentleman, E.; Carzaniga, R.; Evans, N.D.; McComb, D.W.; Porter, A.E.; Stevens, M.M. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc. Natl. Acad. Sci. USA 2012, 109, 14170–14175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shekaran, A.; Garcia, A.J. Extracellular matrix-mimetic adhesive biomaterials for bone repair. J. Biomed. Mater. Res. A 2011, 96, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.; Mezour, M.A.; Badran, Z.; Tamimi, F. Extracellular matrices for bone regeneration: A literature review. Tissue Eng. Part A 2017, 23, 1436–1451. [Google Scholar] [CrossRef] [PubMed]
- Hixon, K.R.; Eberlin, C.T.; Lu, T.; Neal, S.M.; Case, N.D.; McBride-Gagyi, S.H.; Sell, S.A. The calcification potential of cryogel scaffolds incorporated with various forms of hydroxyapatite for bone regeneration. Biomed. Mater. 2017, 12, 025005. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Jang, H.L.; Ahn, H.Y.; Lee, H.K.; Park, J.; Lee, E.S.; Lee, E.A.; Jeong, Y.H.; Kim, D.G.; Nam, K.T.; et al. Biomimetic whitlockite inorganic nanoparticles-mediated in situ remodeling and rapid bone regeneration. Biomaterials 2017, 112, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Pluharova, E.; Baer, M.D.; Mundy, C.J.; Schmidt, B.; Jungwirth, P. Aqueous cation-amide binding: Free energies and ir spectral signatures by ab initio molecular dynamics. J. Phys Chem. Lett. 2014, 5, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chang, J.; Zhai, W.; Ni, S. A novel bioactive porous bredigite (ca7mgsi4o16) scaffold with biomimetic apatite layer for bone tissue engineering. J. Mater. Sci. Mater. Med. 2007, 18, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Ghomi, H.; Fathi, M.H.; Edris, H. Effect of the composition of hydroxyapatite/bioactive glass nanocomposite foams on their bioactivity and mechanical properties. Mater. Res Bull. 2012, 47, 3523–3532. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics—From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Filgueiras, M.R.; La Torre, G.; Hench, L.L. Solution effects on the surface reactions of a bioactive glass. J. Biomed. Mater. Res. 1993, 27, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Sepulveda, P.; Hench, L.L. Dose-dependent behavior of bioactive glass dissolution. J. Biomed. Mater. Res. 2001, 58, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Pickrell, G.; Kimsawatde, G.; Homa, D.; Allbee, H.A.; Sriranganathan, N. Synthesis, cytotoxicity, and hydroxyapatite formation in 27-tris-sbf for sol-gel based cao-p2o5-sio2-b2o3-zno bioactive glasses. Sci. Rep. 2014, 4, 4392. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Matsumoto, T.; Sasaki, J.; Egusa, H.; Lee, K.Y.; Nakano, T.; Sohmura, T.; Nakahira, A. Effect of calcium ion concentrations on osteogenic differentiation and hematopoietic stem cell niche-related protein expression in osteoblasts. Tissue Eng. Part A 2010, 16, 2467–2473. [Google Scholar] [CrossRef] [PubMed]
- Maeno, S.; Niki, Y.; Matsumoto, H.; Morioka, H.; Yatabe, T.; Funayama, A.; Toyama, Y.; Taguchi, T.; Tanaka, J. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3d culture. Biomaterials 2005, 26, 4847–4855. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, S.; Lee, S.S.; Sivashanmugam, A.; Kwon, J.; Kim, S.H.L.; Noh, M.Y.; Kwon, S.K.; Jayakumar, R.; Hwang, N.S. Bioglass-Incorporated Methacrylated Gelatin Cryogel for Regeneration of Bone Defects. Polymers 2018, 10, 914. https://doi.org/10.3390/polym10080914
Kwon S, Lee SS, Sivashanmugam A, Kwon J, Kim SHL, Noh MY, Kwon SK, Jayakumar R, Hwang NS. Bioglass-Incorporated Methacrylated Gelatin Cryogel for Regeneration of Bone Defects. Polymers. 2018; 10(8):914. https://doi.org/10.3390/polym10080914
Chicago/Turabian StyleKwon, Song, Seunghun S. Lee, A. Sivashanmugam, Janet Kwon, Seung Hyun L. Kim, Mi Yeon Noh, Seong Keun Kwon, R. Jayakumar, and Nathaniel S. Hwang. 2018. "Bioglass-Incorporated Methacrylated Gelatin Cryogel for Regeneration of Bone Defects" Polymers 10, no. 8: 914. https://doi.org/10.3390/polym10080914