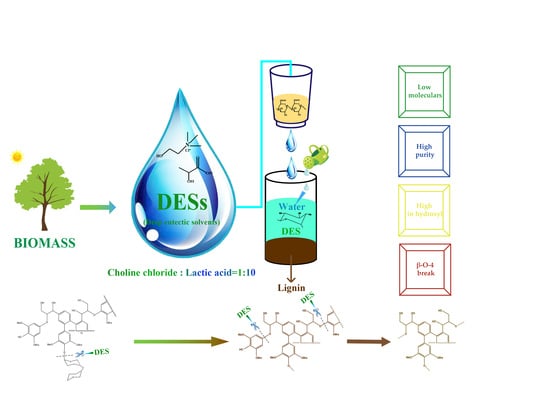
Characterization of Lignin Extracted from Willow by Deep Eutectic Solvent Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation of DES-lignin
2.3. Willow Enzymatic/Mild Acidolysis Lignin (EMAL) Preparation
2.4. Purity Analysis of DES-lignin
2.5. Elemental Analysis of DES-lignin
2.6. Molecular Weight Distribution Analysis of DES-lignin and EMAL
2.7. Particle Size Analysis of DES-lignin
2.8. Atomic Force Microscope (AFM) Analysis of DES-lignin
2.9. 31P NMR Analysis of DES-Lignin
2.10. 2D-HSQC NMR Analysis of DES-Lignin and EMAL
3. Results and Discussion
3.1. Purity Analysis
3.2. Element Analysis
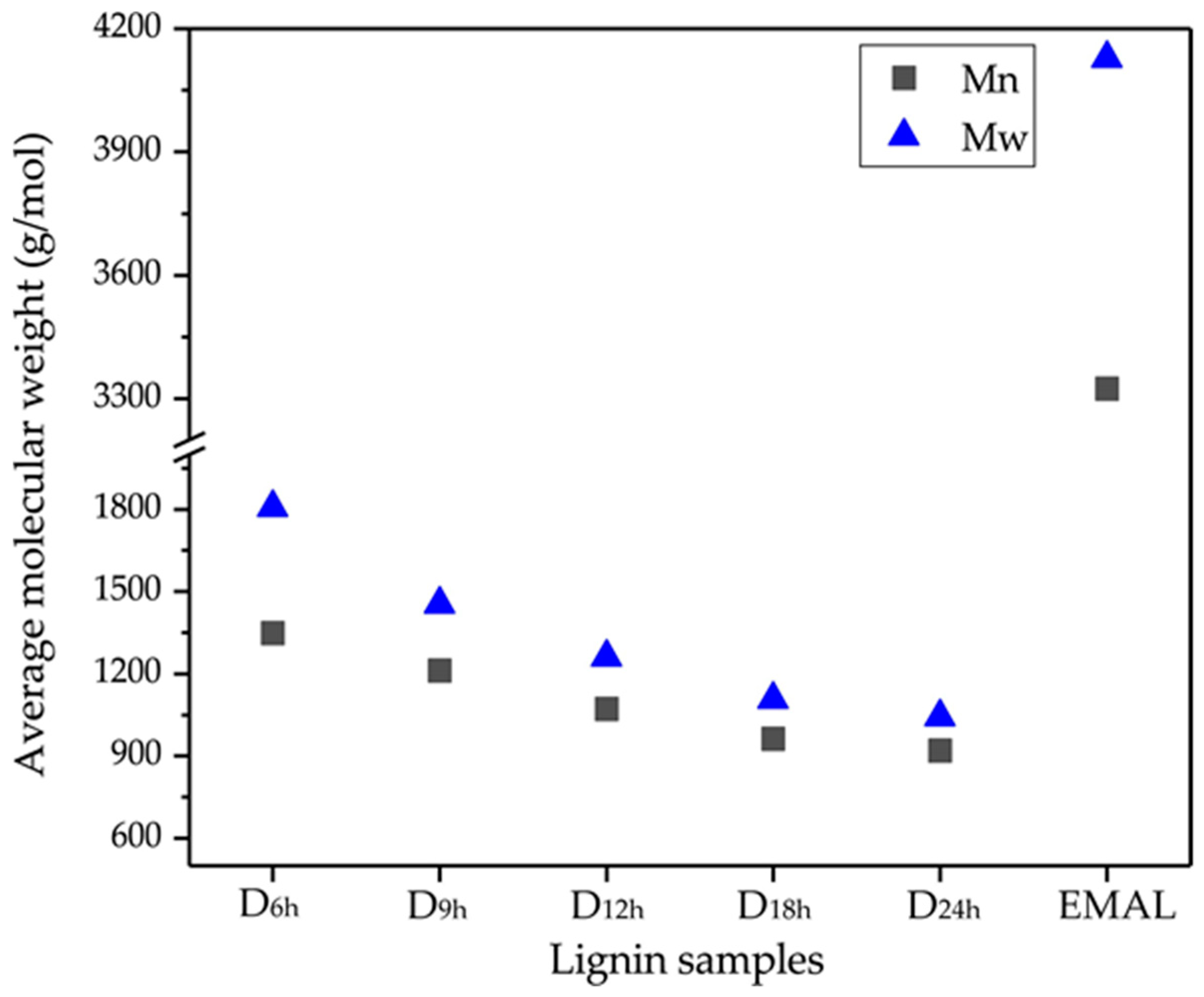
3.3. Molecular Weight Distribution
3.4. Particle Size Analysis
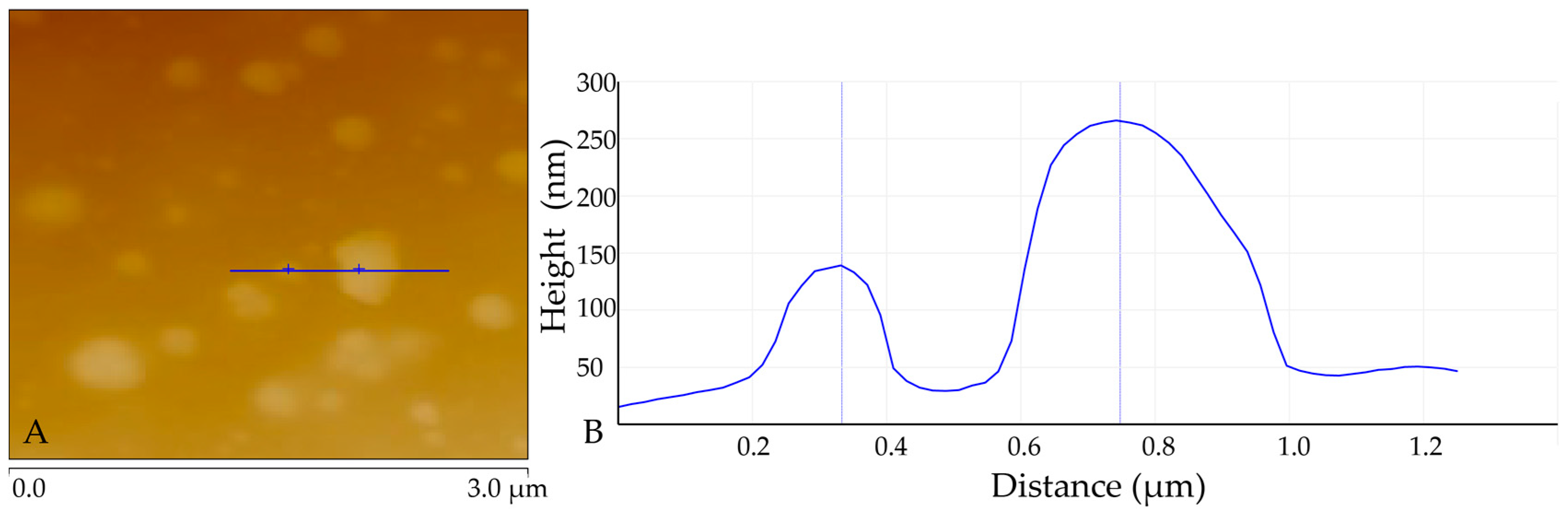
3.5. AFM Analysis of DES-lignin
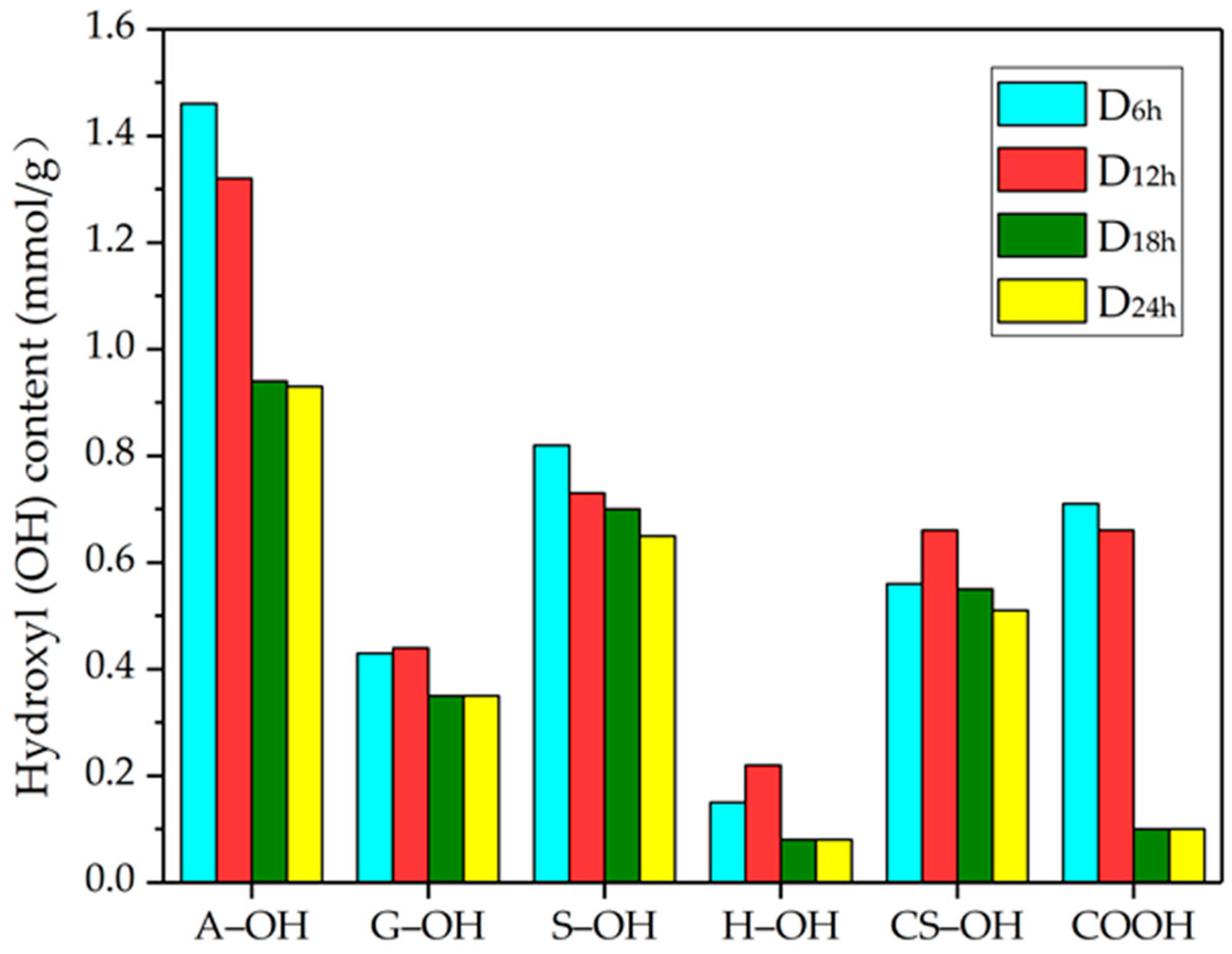
3.6. 31P NMR Analysis of DES-lignin
3.7. 2D-HSQC NMR Spectra of DES-lignin and EMAL
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Junior, H.J.E.; de Melo, R.X.; Sartori, M.M.P.; Guerra, S.P.S.; Ballarin, A.W. Sustainable use of eucalypt biomass grown on short rotation coppice for bioenergy. Biomass Bioenergy 2016, 90, 15–21. [Google Scholar] [CrossRef]
- Lin, X.; Wu, Z.; Zhang, C.; Liu, S.; Nie, S. Enzymatic pulping of lignocellulosic biomass. Ind. Crop. Prod. 2018, 120, 16–24. [Google Scholar] [CrossRef]
- Guo, T.; Liu, Y.; Liu, Y.; Yang, G.; Chen, J.; Lucia, L.A. Chemical elucidation of structurally diverse willow lignins. BioResources 2016, 11, 2043–2054. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Y.; Di, M. Green modification of corn stalk lignin and preparation of environmentally friendly lignin-based wood adhesive. Polymers 2018, 10, 631. [Google Scholar] [CrossRef]
- Zhao, G.; Ni, H.; Ren, S.; Fang, G. Correlation between solubility parameters and properties of alkali lignin/pva composites. Polymers 2018, 10, 290. [Google Scholar] [CrossRef]
- Xiong, F.; Han, Y.; Wang, S.; Li, G.; Qin, T.; Chen, Y.; Chu, F. Preparation and formation mechanism of size-controlled lignin nanospheres by self-assembly. Ind. Crop. Prod. 2017, 100, 146–152. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Li, M.; Wyman, C.E.; Cai, C.M.; Ragauskas, A.J. Fast fractionation of technical lignins by organic cosolvents. ACS Sustain. Chem. Eng. 2018, 6, 6064–6072. [Google Scholar] [CrossRef]
- Jiang, X.; Savithri, D.; Du, X.; Pawar, S.; Jameel, H.; Chang, H.-M.; Zhou, X. Fractionation and characterization of kraft lignin by sequential precipitation with various organic solvents. ACS Sustain. Chem. Eng. 2017, 5, 835–842. [Google Scholar] [CrossRef]
- Stücker, A.; Schütt, F.; Saake, B.; Lehnen, R. Lignins from enzymatic hydrolysis and alkaline extraction of steam refined poplar wood: Utilization in lignin-phenol-formaldehyde resins. Ind. Crop. Prod. 2016, 85, 300–308. [Google Scholar] [CrossRef]
- Prado, R.; Erdocia, X.; Labidi, J. Study of the influence of reutilization ionic liquid on lignin extraction. J. Clean. Prod. 2016, 111, 125–132. [Google Scholar] [CrossRef]
- Lauberts, M.; Sevastyanova, O.; Ponomarenko, J.; Dizhbite, T.; Dobele, G.; Volperts, A.; Lauberte, L.; Telysheva, G. Fractionation of technical lignin with ionic liquids as a method for improving purity and antioxidant activity. Ind. Crop. Prod. 2017, 95, 512–520. [Google Scholar] [CrossRef]
- Liu, E.; Li, M.; Das, L.; Pu, Y.; Frazier, T.; Zhao, B.; Crocker, M.; Ragauskas, A.J.; Shi, J. Understanding lignin fractionation and characterization from engineered switchgrass treated by an aqueous ionic liquid. ACS Sustain. Chem. Eng. 2018, 6, 6612–6623. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, J.; Shen, D.; Xue, J.; Guan, S.; Gu, S.; Luo, K. Catalytic oxidation of lignin in solvent systems for production of renewable chemicals: A review. Polymers 2017, 9, 240. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtureselectronic supplementary information (ESI) available: Spectroscopic data. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jerome, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents-solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Ninomiya, K.; Omote, S.; Ogino, C.; Kuroda, K.; Noguchi, M.; Endo, T.; Kakuchi, R.; Shimizu, N.; Takahashi, K. Saccharification and ethanol fermentation from cholinium ionic liquid-pretreated bagasse with a different number of post-pretreatment washings. Bioresour. Technol. 2015, 189, 203–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Procentese, A.; Johnson, E.; Orr, V.; Garruto Campanile, A.; Wood, J.A.; Marzocchella, A.; Rehmann, L. Deep eutectic solvent pretreatment and subsequent saccharification of corncob. Bioresour. Technol. 2015, 192, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Ding, J.; Han, R.; Dong, J.; Ni, Y. Enhancing cellulose accessibility of corn stover by deep eutectic solvent pretreatment for butanol fermentation. Bioresour. Technol. 2016, 203, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wan, C. Ultrafast fractionation of lignocellulosic biomass by microwave-assisted deep eutectic solvent pretreatment. Bioresour. Technol. 2018, 250, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Lynam, J.G.; Kumar, N.; Wong, M.J. Deep eutectic solvents ability to solubilize lignin, cellulose, and hemicellulose; thermal stability; and density. Bioresour. Technol. 2017, 238, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.; Hou, Y.; Wu, W.; Guo, W.; Peng, W.; Marsh, K.N. Efficient separation of phenols from oils via forming deep eutectic solvents. Green Chem. 2012, 14, 2398. [Google Scholar] [CrossRef]
- Alvarez-Vasco, C.; Ma, R.; Quintero, M.; Guo, M.; Geleynse, S.; Ramasamy, K.K.; Wolcott, M.; Zhang, X. Unique low-molecular-weight lignin with high purity extracted from wood by deep eutectic solvents (DES): A source of lignin for valorization. Green Chem. 2016, 18, 5133–5141. [Google Scholar] [CrossRef]
- Di Marino, D.; Stöckmann, D.; Kriescher, S.; Stiefel, S.; Wessling, M. Electrochemical depolymerisation of lignin in a deep eutectic solvent. Green Chem. 2016, 18, 6021–6028. [Google Scholar] [CrossRef]
- Li, T.; Lyu, G.; Liu, Y.; Lou, R.; Lucia, L.A.; Yang, G.; Chen, J.; Saeed, H.A.M. Deep Eutectic Solvents (DESs) for the isolation of willow lignin (Salix matsudana cv. Zhuliu). Int. J. Mol. Sci. 2017, 18, 2266. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Liu, Y.; Meng, J.; Cheng, W.; Chen, W.; Liu, S.; Liu, Y.; Li, J.; Yu, H. Multiple hydrogen bond coordination in three constituent deep eutectic solvents enhances lignin fractionation from biomass. Green Chem. 2018, 20, 2711–2721. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Technical Report; National Renewable Energy Laboratory: Golden, CO, USA, 2010. [Google Scholar]
- Deshpande, R.; Giummarella, N.; Henriksson, G.; Germgård, U.; Sundvall, L.; Grundberg, H.; Lawoko, M. The reactivity of lignin carbohydrate complex (LCC) during manufacture of dissolving sulfite pulp from softwood. Ind. Crop. Prod. 2018, 115, 315–322. [Google Scholar] [CrossRef]
- You, T.; Zhang, L.; Zhou, S.; Xu, F. Structural elucidation of lignin-carbohydrate complex (LCC) preparations and lignin from Arundo donax Linn. Ind. Crop. Prod. 2015, 71, 65–74. [Google Scholar] [CrossRef]
- Min, D.; Yang, C.; Chiang, V.; Jameel, H.; Chang, H. The influence of lignin-carbohydrate complexes on the cellulase-mediated saccharification II: Transgenic hybrid poplars (Populus nigra L. and Populus maximowiczii A.). Fuel 2014, 116, 56–62. [Google Scholar] [CrossRef]
- Hong, S.; Lian, H.; Sun, X.; Pan, D.; Carranza, A.; Pojman, J.A.; Mota-Morales, J.D. Zinc-based deep eutectic solvent-mediated hydroxylation and demethoxylation of lignin for the production of wood adhesive. RSC Adv. 2016, 6, 89599–89608. [Google Scholar] [CrossRef]
- Chen, L.; Dou, J.; Ma, Q.; Li, N.; Wu, R.; Bian, H.; Yelle, D.J.; Vuorinen, T.; Fu, S.; Pan, X.; et al. Rapid and near-complete dissolution of wood lignin at ≤80 °C by a recyclable acid hydrotrope. Sci. Adv. 2017, 3, e1701735. [Google Scholar] [CrossRef] [PubMed]
- Jääskeläinen, A.; Liitiä, T.; Mikkelson, A.; Tamminen, T. Aqueous organic solvent fractionation as means to improve lignin homogeneity and purity. Ind. Crop. Prod. 2017, 103, 51–58. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, L.; Fang, G.; Yan, N.; Ren, S.; Ma, Y. Hydrogenolysis and activation of soda lignin using [BMIM]Cl as a catalyst and solvent. Polymers 2017, 9, 279. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Liu, L.; Huang, G.; Song, P.; Yu, Y.; Fu, S. Converting industrial alkali lignin to biobased functional additives for improving fire behavior and smoke suppression of polybutylene succinate. ACS Sustain. Chem. Eng. 2016, 4, 4732–4742. [Google Scholar] [CrossRef]
- Wen, J.; Sun, S.; Yuan, T.; Sun, R. Structural elucidation of whole lignin from eucalyptus based on preswelling and enzymatic hydrolysis. Green Chem. 2015, 17, 1589–1596. [Google Scholar] [CrossRef]
- Constant, S.; Wienk, H.L.J.; Frissen, A.E.; Peinder, P.D.; Boelens, R.; van Es, D.S.; Grisel, R.J.H.; Weckhuysen, B.M.; Huijgen, W.J.J.; Gosselink, R.J.A.; et al. New insights into the structure and composition of technical lignins: A comparative characterisation study. Green Chem. 2016, 18, 2651–2665. [Google Scholar] [CrossRef]
- Zhao, J.; Xiu, W.; Hu, J.; Liu, Q.; Shen, D.; Xiao, R. Thermal degradation of softwood lignin and hardwood lignin by TG-FTIR and Py-GC/MS. Polym. Degrad. Stabil. 2014, 108, 133–138. [Google Scholar] [CrossRef]
- Rajić, N.; Logar, N.Z.; Rečnik, A.; El-Roz, M.; Thibault-Starzyk, F.; Sprenger, P.; Hannevold, L.; Andersen, A.; Stöcker, M. Hardwood lignin pyrolysis in the presence of nano-oxide particles embedded onto natural clinoptilolite. Microporous Mesoporous Mater. 2013, 176, 162–167. [Google Scholar] [CrossRef]
- Malaeke, H.; Housaindokht, M.R.; Monhemi, H.; Izadyar, M. Deep eutectic solvent as an efficient molecular liquid for lignin solubilization and wood delignification. J. Mol. Liq. 2018, 263, 193–199. [Google Scholar] [CrossRef]
- Soares, B.; Tavares, D.J.P.; Amaral, J.L.; Silvestre, A.J.D.; Freire, C.S.R.; Coutinho, J.A.P. Enhanced solubility of lignin monomeric model compounds and technical lignins in aqueous solutions of deep eutectic solvents. ACS Sustain. Chem. Eng. 2017, 5, 4056–4065. [Google Scholar] [CrossRef]
- Lourenço, A.; Rencoret, J.; Chemetova, C.; Gominho, J.; Gutiérrez, A.; Pereira, H.; del Río, J.C. Isolation and structural characterization of lignin from cardoon (Cynara cardunculus L.) stalks. Bioenergy Res. 2015, 8, 1946–1955. [Google Scholar] [CrossRef]
- Del Rio, J.C.; Prinsen, P.; Rencoret, J.; Nieto, L.; Jimenez-Barbero, J.; Ralph, J.; Martinez, A.T.; Gutierrez, A. Structural characterization of the lignin in the cortex and pith of elephant grass (Pennisetum purpureum) stems. J. Agric. Food. Chem. 2012, 60, 3619–3634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azarpira, A.; Ralph, J.; Lu, F. Catalytic alkaline oxidation of lignin and its model compounds: A pathway to aromatic biochemicals. Bioenergy Res. 2013, 7, 78–86. [Google Scholar] [CrossRef]
- Kim, K.H.; Dutta, T.; Ralph, J.; Mansfield, S.D.; Simmons, B.A.; Singh, S. Impact of lignin polymer backbone esters on ionic liquid pretreatment of poplar. Biotechnol. Biofuels 2017, 10, 101. [Google Scholar] [CrossRef] [PubMed]

| Component | D6h (%) | D9h (%) | D12h (%) | D18h (%) | D24h (%) |
|---|---|---|---|---|---|
| Acid insoluble lignin (AIL) | 88.54 | 90.86 | 92.57 | 93.45 | 93.69 |
| Acid soluble lignin (ASL) | 1.48 | 1.51 | 1.89 | 1.63 | 1.71 |
| Glucose | 1.12 | 0.77 | 0.21 | 0.11 | - |
| Xylose | 0.96 | 0.56 | 0.15 | - | - |
| Arabinose | 0.12 | - | - | - | - |
| Galactose | 0.05 | - | - | - | - |
| Mannose | - | - | - | - | - |
| Ash | 0.51 | 0.60 | 0.51 | 0.54 | 0.59 |
| Lignin purity * | 90.02 | 92.37 | 94.46 | 95.08 | 95.40 |
| DES-Lignin Samples | Elemental Analysis (wt %) | Element Molar Ratio | ||||
|---|---|---|---|---|---|---|
| C | H | N | O | O/C | H/C | |
| D6h | 59.01 | 4.22 | 0.39 | 35.87 | 0.46 | 0.86 |
| D9h | 59.42 | 4.78 | 0.45 | 34.75 | 0.44 | 0.97 |
| D12h | 60.08 | 5.50 | 0.41 | 33.50 | 0.42 | 1.10 |
| D18h | 59.59 | 6.04 | 0.43 | 33.40 | 0.42 | 1.22 |
| D24h | 58.84 | 6.90 | 0.41 | 33.26 | 0.42 | 1.42 |
| Lignin Samples | / | ||
|---|---|---|---|
| D6h | 1348.1 | 1806.7 | 1.34 |
| D9h | 1212.3 | 1454.2 | 1.20 |
| D12h | 1071.1 | 1261.1 | 1.18 |
| D18h | 962.9 | 1106.3 | 1.15 |
| D24h | 920.2 | 1042.5 | 1.13 |
| EMAL | 3324.9 | 4127.0 | 1.24 |
| DES-Lignin Samples | D6h | D9h | D12h | D18h | D24h |
|---|---|---|---|---|---|
| Particle size (nm) | 704.7 | 612.4 | 494.2 | 400.1 | 402.4 |
| Chemical Shift (ppm) | Assignment | Content (mmol·g−1) | |||
|---|---|---|---|---|---|
| D6h | D12h | D18h | D24h | ||
| 133.7–134.9 | Carboxyl (COOH) | 0.71 | 0.66 | 0.10 | 0.10 |
| 136.9–138.6 | p-Ohenol hydroxyl (H–OH) | 0.15 | 0.22 | 0.08 | 0.08 |
| 138.6–140.3 | Guaiacyl phenol hydroxyl (G–OH) | 0.43 | 0.44 | 0.35 | 0.35 |
| 142.3–143.8 | Syringyl phenol hydroxyl (S–OH) | 0.82 | 0.73 | 0.70 | 0.65 |
| - | G:S:H | 2.9:5.5:1 | 2.0:3.2:1 | 4.4:8.8:1 | 4.4:8.1:1 |
| 140.3–142.3 143.8–144.5 | Condensation phenol hydroxyl (CS–OH) | 0.56 | 0.66 | 0.55 | 0.51 |
| 145.2–150.2 | Aliphatic hydroxyl (A–OH) | 1.46 | 1.32 | 0.94 | 0.93 |
| Total phenol hydroxyl | 1.96 | 2.05 | 1.68 | 1.59 | |
| Total hydroxyl | 3.42 | 3.37 | 2.62 | 2.52 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, G.; Li, T.; Ji, X.; Yang, G.; Liu, Y.; Lucia, L.A.; Chen, J. Characterization of Lignin Extracted from Willow by Deep Eutectic Solvent Treatments. Polymers 2018, 10, 869. https://doi.org/10.3390/polym10080869
Lyu G, Li T, Ji X, Yang G, Liu Y, Lucia LA, Chen J. Characterization of Lignin Extracted from Willow by Deep Eutectic Solvent Treatments. Polymers. 2018; 10(8):869. https://doi.org/10.3390/polym10080869
Chicago/Turabian StyleLyu, Gaojin, Tengfei Li, Xingxiang Ji, Guihua Yang, Yu Liu, Lucian A. Lucia, and Jiachuan Chen. 2018. "Characterization of Lignin Extracted from Willow by Deep Eutectic Solvent Treatments" Polymers 10, no. 8: 869. https://doi.org/10.3390/polym10080869