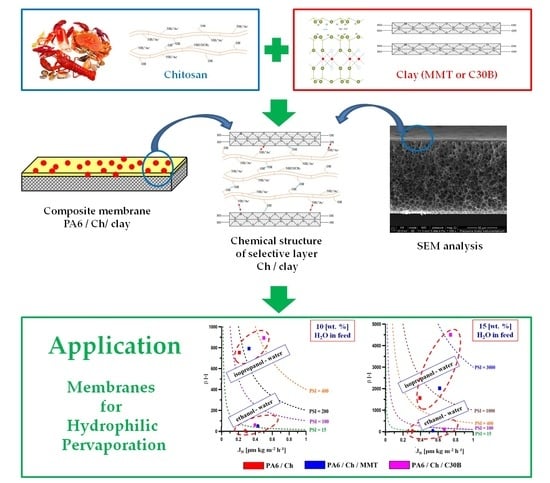
Development and Characterization of Polyamide-Supported Chitosan Nanocomposite Membranes for Hydrophilic Pervaporation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composite Membranes Preparation
2.3. Membrane Characterization
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.2. Thermogravimetric Analysis (TGA)
2.3.3. Membrane Morphology
2.3.4. Mechanical Properties
2.3.5. Contact Angle Measurements
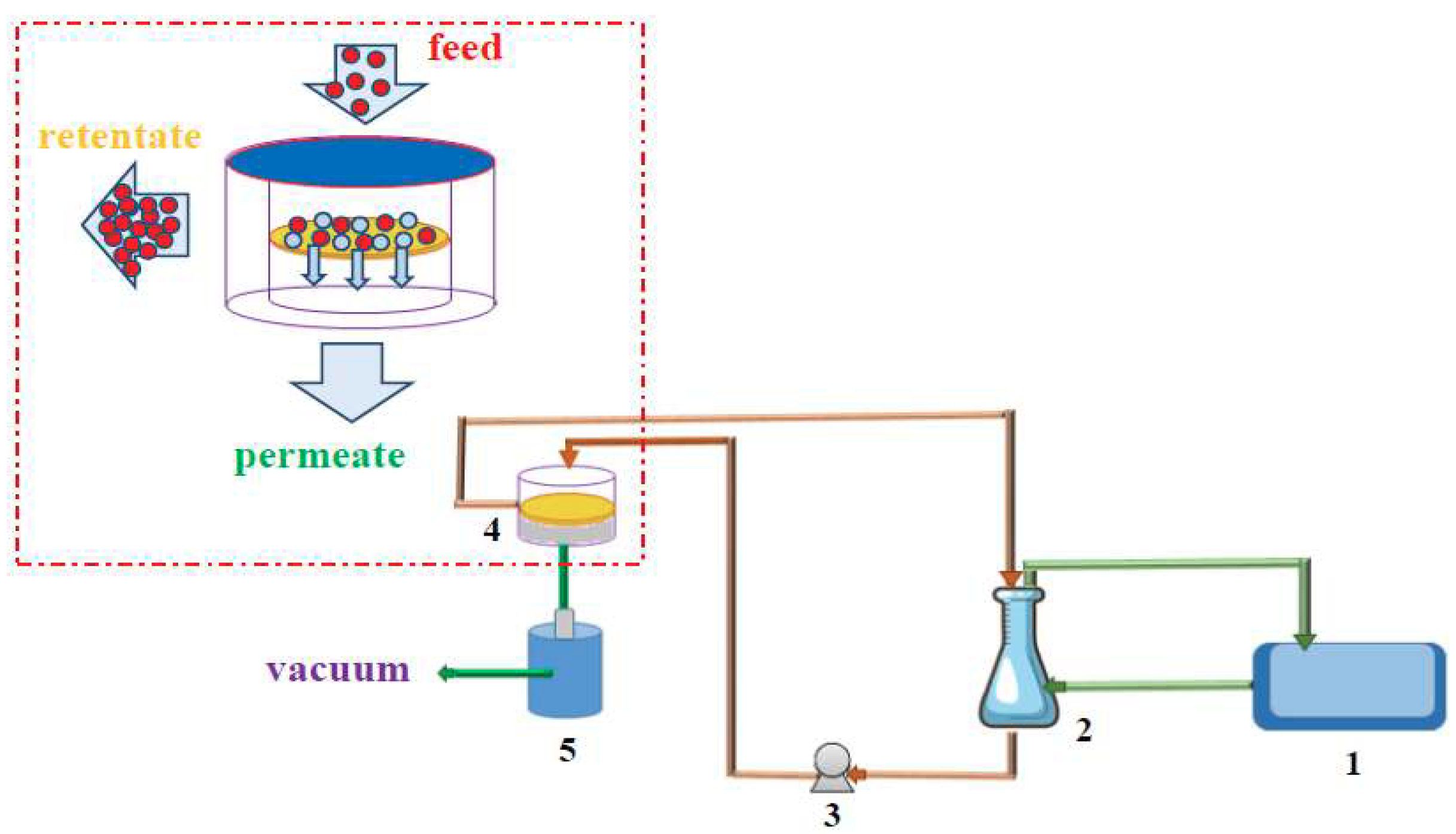
2.3.6. Pervaporation Experiments
3. Results and Discussion
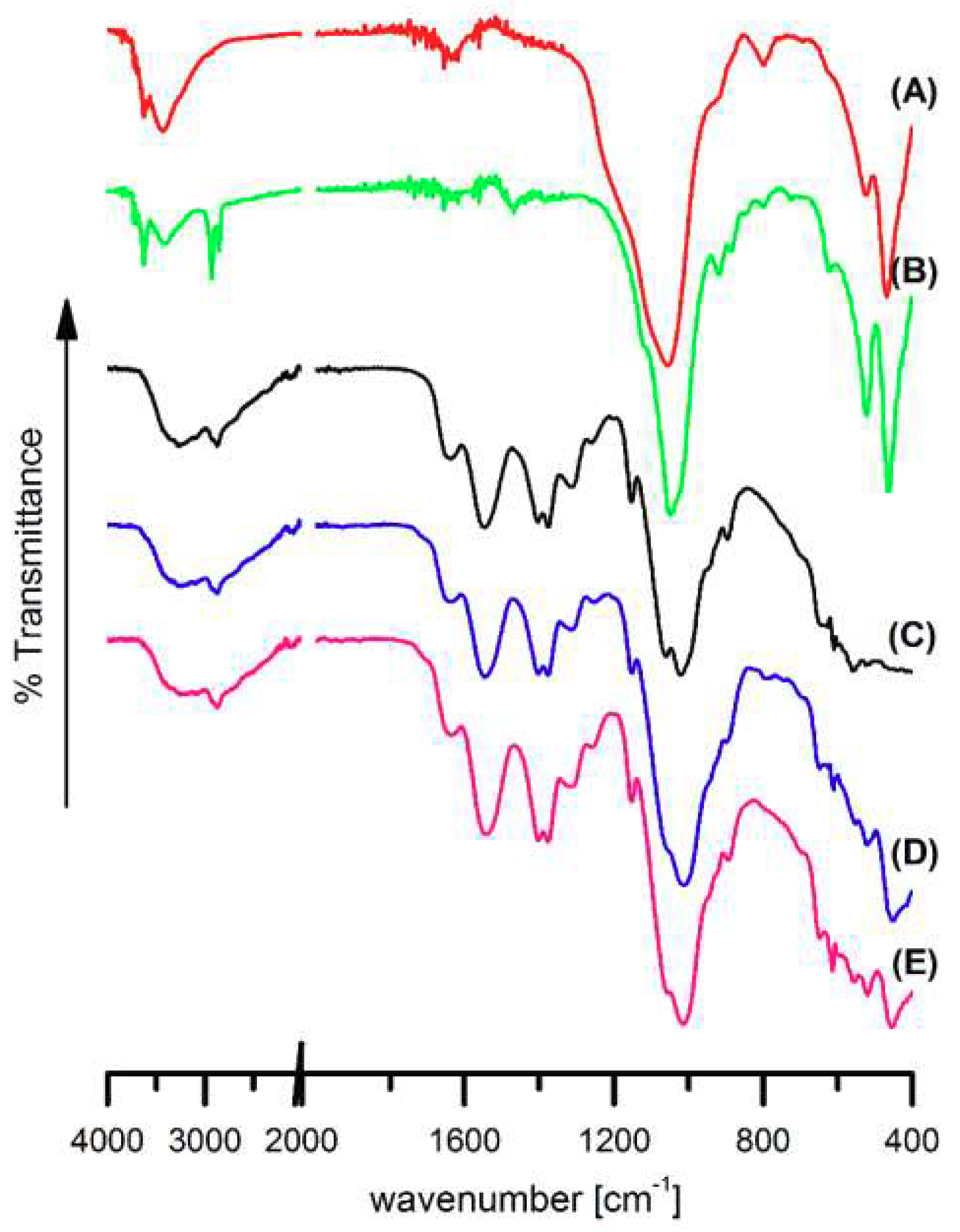
3.1. Fourier Transformed Infrared Spectroscopy
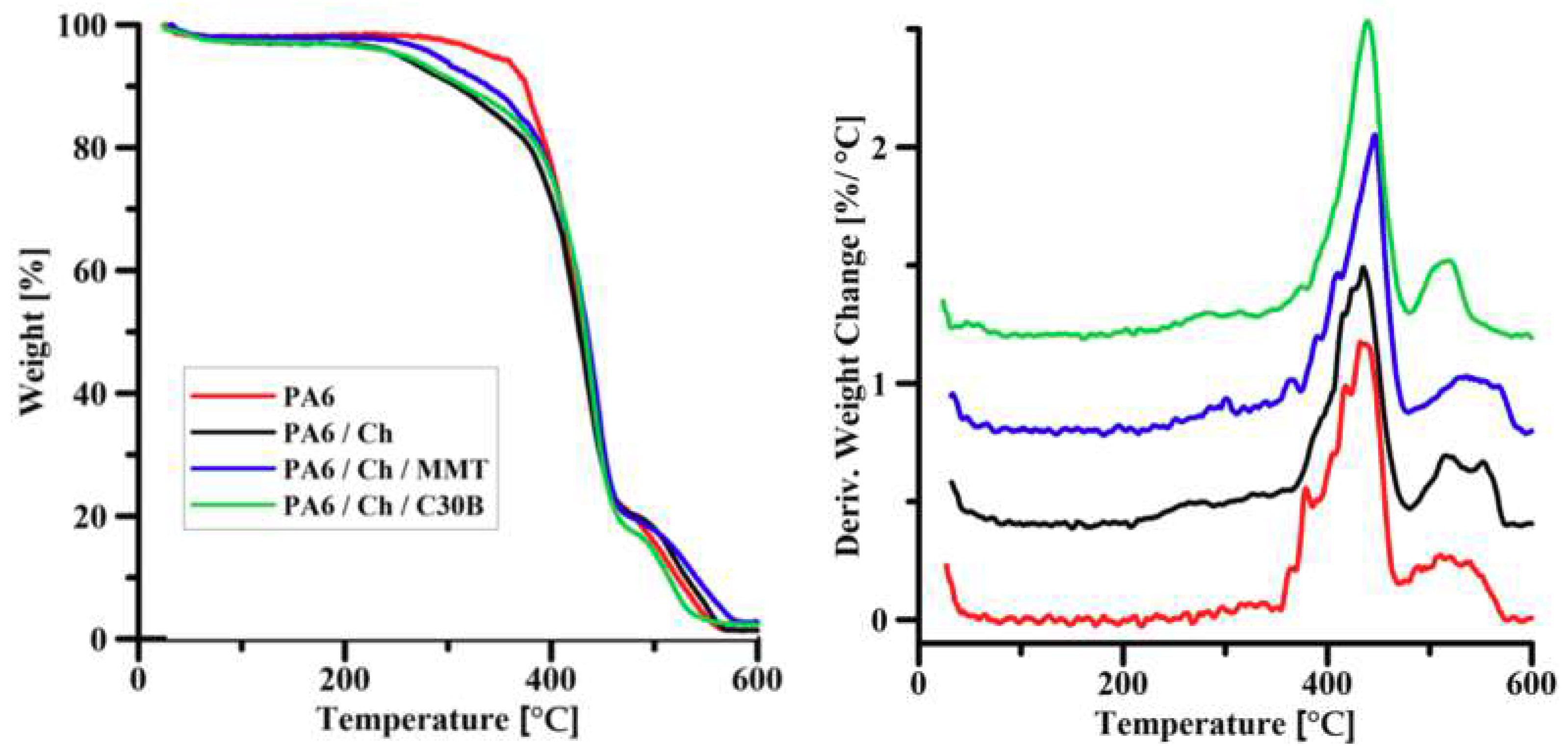
3.2. Thermal Gravimetric Analysis
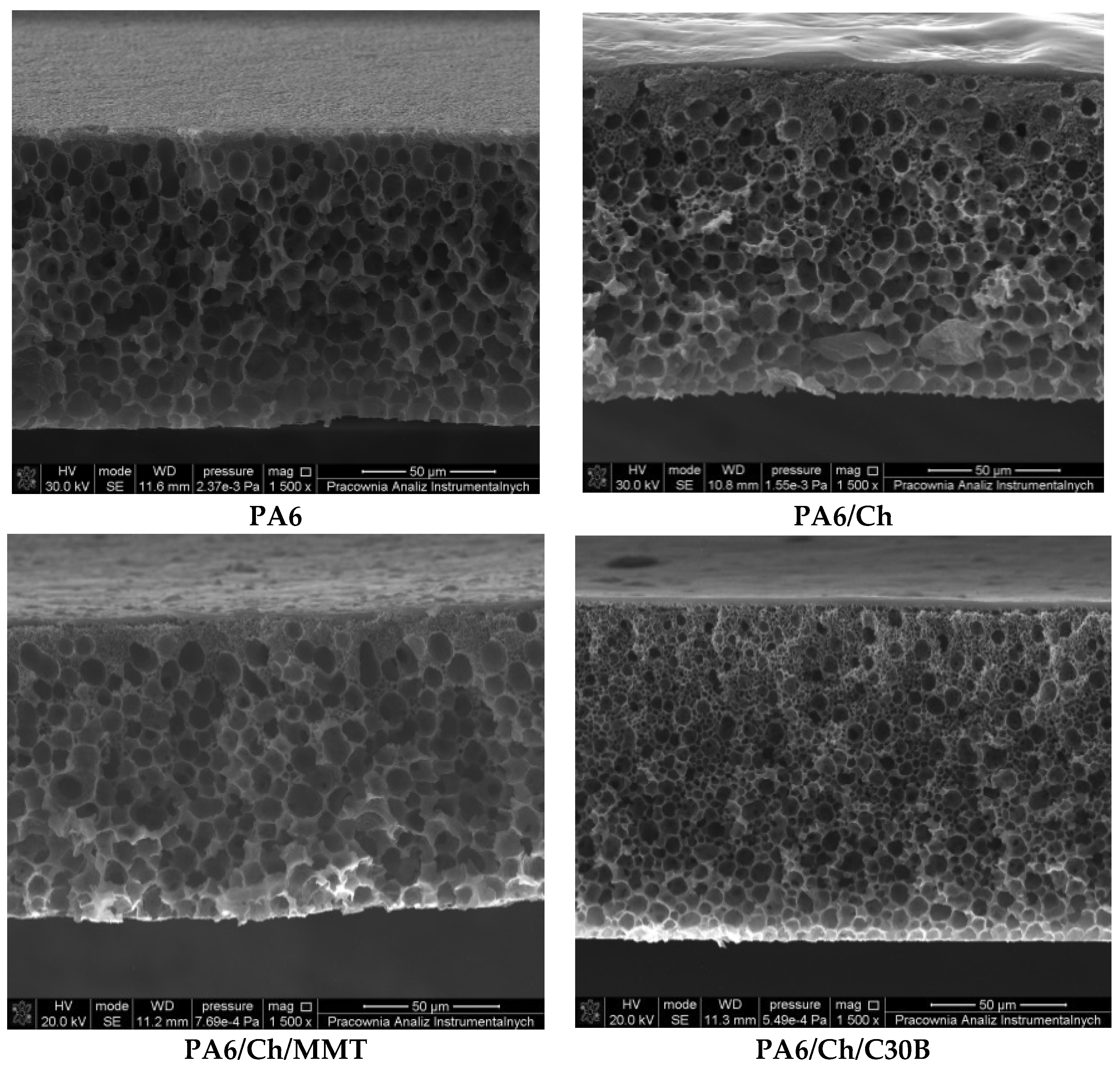
3.3. Scanning Electron Microscopy
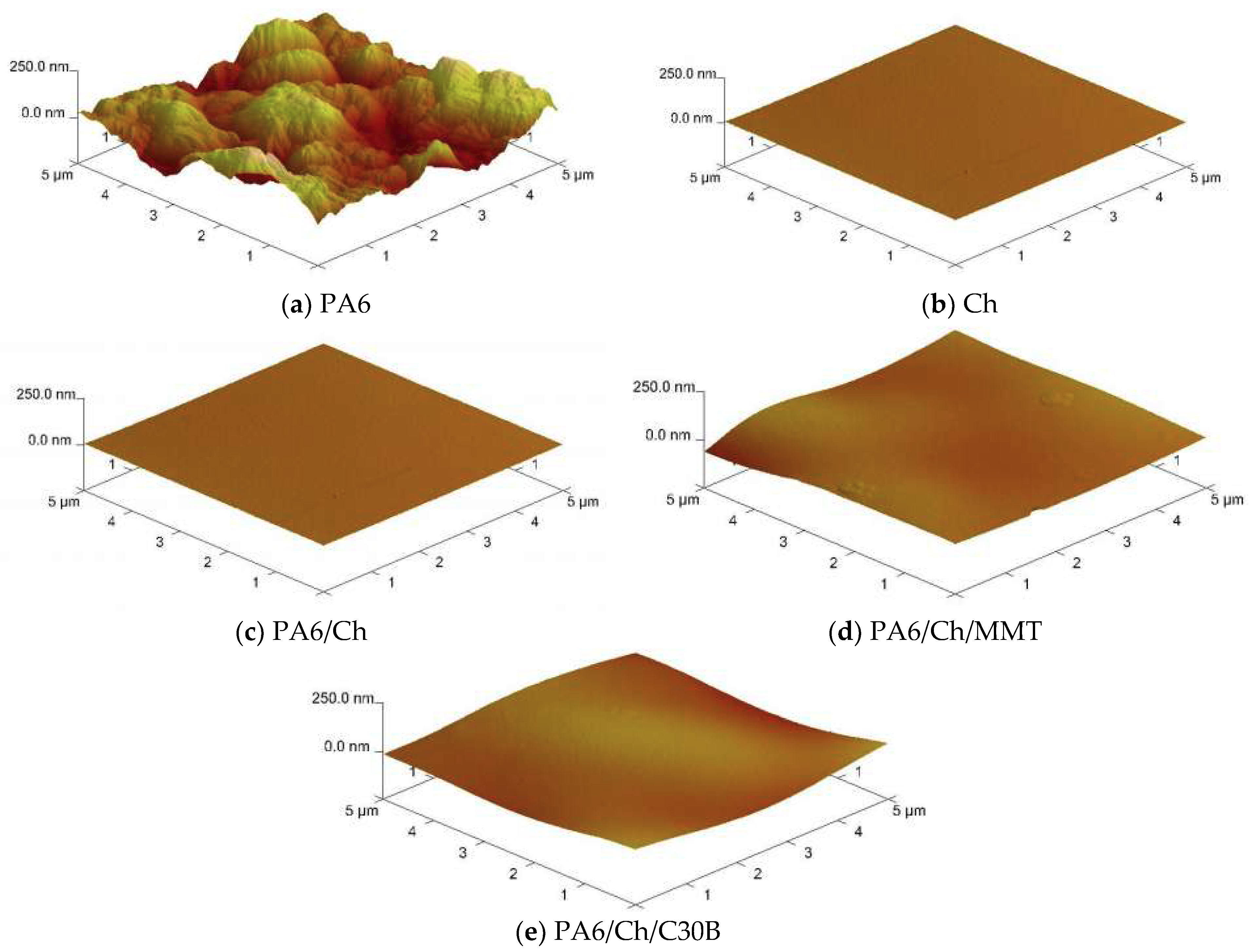
3.4. Atomic Force Microscopy
3.5. Contact Angle Measurements
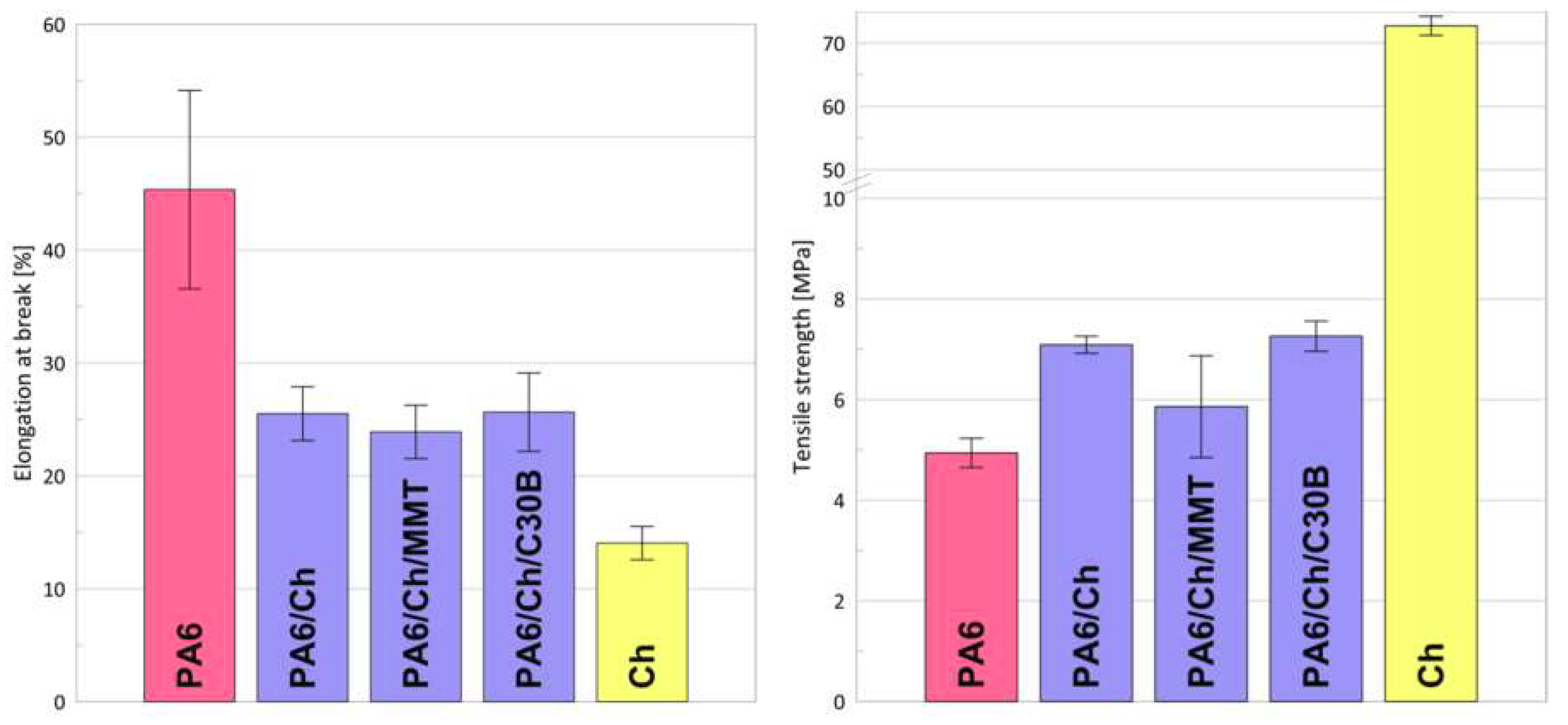
3.6. Mechanical Properties
3.7. Pervaporation Properties of Membranes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; John Wiley and Sons: Oxford, UK, 2012; ISBN 9780470743720. [Google Scholar]
- Ahmad, S.; Ahmed, S.M. Application of Membrane Technology in Food Processing. In Food Processing: Strategies for Quality Assessment; Malik, A., Erginkaya, Z., Ahmad, S., Erten, H., Eds.; Food Engineering Series; Springer: New York, NY, USA, 2014; pp. 379–394. ISBN 978-1-4939-1377-0. [Google Scholar]
- Dhineshkumar, V.; Ramasamy, D. Review on membrane technology applications in food and dairy processing. J. Appl. Biotechnol. Bioeng. 2017, 3, 399–407. [Google Scholar] [CrossRef]
- Song, J.; Huang, T.; Qiu, H.; Niu, X.; Li, X.M.; Xie, Y.; He, T. A critical review on membrane extraction with improved stability: Potential application for recycling metals from city mine. Desalination 2018, 440, 18–38. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Y.; Dong, H.; Meng, M.; Li, Ch.; Yan, Y.; Chen, J. An overview on membrane strategies for rare earths extraction and separation. Sep. Purif. Technol. 2018, 197, 70–85. [Google Scholar] [CrossRef]
- Mänttäri, M.; Kallioinen, M.; Nyström, M. Membrane technologies for water treatment and reuse in the pulp and paper industries. In Advances in Membrane Technologies for Water Treatment; Basile, A., Cassano, A., Rastogi, N.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 581–603. ISBN 978-1-78242-121-4. [Google Scholar]
- Adham, S.; Hussain, A.; Minier-Matar, J.; Janson, A.; Sharma, R. Membrane applications and opportunities for water management in the oil & gas industry. Desalination 2018, 440, 2–17. [Google Scholar] [CrossRef]
- Scholes, C.A.; Stevens, G.W.; Kentish, S.E. Membrane gas separation applications in natural gas processing. Fuel 2012, 96, 15–28. [Google Scholar] [CrossRef]
- Stamatialis, D.F.; Papenburg, B.J.; Giron’es, M.; Saiful, S.; Bettahalli, S.N.M.; Schmitmeier, S.; Wessling, M. Medical applications of membranes: Drug delivery, artificial organs and tissue engineering. J. Membr. Sci. 2008, 308, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Reddy, A.M.; Karthikeyan, R.; Sri. Vejandla, R.; Divya, G.; Babu, S.P. Controlled release matrix drug delivery system—A review. Int. J. Allied Med. Sci. Clin. Res. 2017, 5, 384–398. [Google Scholar]
- Chen, D.; Sirkar, K.K.; Jin, Ch.; Singh, D.; Pfeffer, R. Membrane-based technologies in the pharmaceutical industry and continuous production of polymer-coated crystals/particles. Curr. Pharm. Des. 2017, 23, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.A. Porous-based biomaterials for tissue engineering and drug delivery applications. Biomatter 2012, 2, 237–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, A.; Shyuan, L.K.; Mohamad, A.B.; Kadhum, A.A.H. Review on biopolymer membranes for fuel cell applications. Appl. Mech. Mater. 2013, 291–294, 614–617. [Google Scholar] [CrossRef]
- Ferreira, A.R.V.; Alves, V.D.; Coelhoso, I.M. Polysaccharide-based membranes in food packaging applications. Membranes 2016, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Peter, M.G. Chitin and chitosan from animal sources. In Biopolymers; De Baets’a, S., Vandamme’a, E.J., Stainbuchel, A., Eds.; Polysaccharides II. Polysaccharides from Eukaryotes; Wiley-VCH: Weinheim, Germany, 2002; Volume 6, pp. 481–574. [Google Scholar]
- Uragami, T. Separation membranes from chitin and chitosan derivatives. In Chitin, Chitosan, Oligosaccharides and Their Derivatives; Kim, S.K., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 481–506. [Google Scholar]
- Ma, J.; Sahai, Y. Chitosan biopolymer for fuel cell applications. Carbohydr. Polym. 2013, 92, 955–975. [Google Scholar] [CrossRef] [PubMed]
- Chakrabaty, T.; Kumar, M.; Shahi, V.K. Chitosan based membranes for separation, pervaporation and fuel cell applications: Recent developments. In Biopolymers; Elnashar, M., Ed.; InTech: London, UK, 2010; pp. 201–226. Available online: http://www.intechopen.com/articles/show/title/chitosan-based-membranes (accessed on 28 May 2018).
- Xu, D.; Hein, S.; Wang, K. Chitosan membrane in separation application. Mater. Sci. Technol. 2008, 24, 1076–1087. [Google Scholar] [CrossRef]
- Dudek, G.; Gnus, M.; Turczyn, R.; Strzelewicz, A.; Krasowska, M. Pervaporation with chitosan membranes containing iron oxide nanoparticles. Sep. Purif. Technol. 2014, 133, 8–15. [Google Scholar] [CrossRef]
- Semenova, S.I.; Ohya, H.; Soontraoa, K. Hydrophylic membranes for pervaporation: An analytical review. Desalination 1997, 110, 251–286. [Google Scholar] [CrossRef]
- Veerapur, R.S.; Gudasi, K.B.; Aminabhavi, T.M. Pervaporation dehydration of isopropanol using blend membranes of chitosan and hydroxypropyl cellulose. J. Membr. Sci. 2007, 304, 102–111. [Google Scholar] [CrossRef]
- Shao, P.; Huang, R.Y.M. Polymeric membrane pervaporation. J. Membr. Sci. 2007, 287, 162–179. [Google Scholar] [CrossRef]
- Huang, R.Y.M.; Pal, R.; Moon, G.Y. Crosslinked chitosan composite membrane for the pervaporation dehydration of alcohol mixtures and enhancement of structural stability of chitosan/polysulfone composite membranes. J. Membr. Sci. 1999, 160, 17–30. [Google Scholar] [CrossRef]
- Krishna Rao, K.S.V.; Lokesh, B.G.; Srinivasa Rao, P.; Chowdoji Rao, K. Synthesis and characterization of biopolymeric blend membranes based on sodium alginate for the pervaporation dehydration of isopropanol/water mixtures. Sep. Sci. Technol. 2008, 43, 1065–1082. [Google Scholar] [CrossRef]
- Choudhari, S.K.; Kittur, A.A.; Kulkarni, S.S.; Mahadevappa, Y.; Kariduraganavar, M.Y. Development of novel blocked diisocyanate crosslinked chitosan membranes for pervaporation separation of water–isopropanol mixtures. J. Membr. Sci. 2007, 302, 197–206. [Google Scholar] [CrossRef]
- Ghazali, M.; Nawawi, M.; Huang, R.Y.M. Pervaporation dehydration of isopropanol with chitosan membranes. J. Membr. Sci. 1997, 124, 53–62. [Google Scholar] [CrossRef]
- Ong, Y.K.; Shi, G.M.; Le, N.L.; Tang, Y.P.; Zuo, J.; Nunes, S.P.; Chung, T.S. Recent membrane development for pervaporation processes. Prog. Polym. Sci. 2016, 57, 1–31. [Google Scholar] [CrossRef]
- Ray, S.S.; Bousmina, M. Biodegradable polymers and their layered silicate nanocomposites: In greening the 21st century materials world. Prog. Mater. Sci. 2005, 50, 962–1079. [Google Scholar] [CrossRef]
- George, S.C.; Thomas, S. Transport phenomena through polymeric system. Prog. Polym. Sci. 2001, 26, 985–1017. [Google Scholar] [CrossRef]
- Qiu, S.; Wu, L.; Shi, G.; Zhang, L.; Chen, H.; Gao, C. Preparation and pervaporation property of chitosan membrane with functionalized multiwalled carbon nanotubes. Ind. Eng. Chem. Res. 2010, 49, 11667–11675. [Google Scholar] [CrossRef]
- Gao, Z.; Yue, Y.; Li, W. Application of zeolite-filled pervaporation membranes. Zeolite 1996, 16, 70–74. [Google Scholar] [CrossRef]
- Choudhari, S.K.; Kariduraganava, M.Y. Development of novel composite membranes using quaternized chitosan and Na+-MMT clay for the pervaporation dehydration of isopropanol. J. Colloid Interface Sci. 2009, 338, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.P.; Shen, Z.Q.; Zhang, F.Y.; Zhang, Y.F. A novel composite chitosan membrane for separation of alcohol-water mixtures. J. Membr. Sci. 1996, 119, 191–198. [Google Scholar] [CrossRef]
- EL-Gendi, A.; Deratani, A.; Ahmed, S.A.; Ali, S.S. Development of polyamide-6/chitosan membranes for desalination. Egypt. J. Pet. 2014, 23, 169–173. [Google Scholar] [CrossRef]
- Akbari, A.; Derikvandi, Z.; Rostami, S.M.M. Influence of chitosan coating on the separation performance, morphology and anti-fouling properties of the polyamide nanofiltration membranes. J. Ind. Eng. Chem. 2015, 28, 268–276. [Google Scholar] [CrossRef]
- Xu, J.; Feng, X.; Gao, C. Surface modification of thin-film-composite polyamide membranes for improved reverse osmosis performance. J. Membr. Sci. 2011, 370, 116–123. [Google Scholar] [CrossRef]
- Albo, J.; Hagiwara, H.; Yanagishita, H.; Ito, K.; Tsuru, T. Structural characterization of thin-film polyamide reverse osmosis membranes. Ind. Eng. Chem. Res. 2014, 53, 1442–1451. [Google Scholar] [CrossRef]
- Albo, J.; Wang, J.; Tsuru, T. Gas transport properties of interfacially polymerized polyamide composite membranes under different pre-treatments and temperatures. J. Membr. Sci. 2014, 449, 109–118. [Google Scholar] [CrossRef]
- Albo, J.; Wang, J.; Tsuru, T. Application of interfacially polymerized polyamide composite membranes to isopropanol dehydration: Effect of membrane pre-treatment and temperature. J. Membr. Sci. 2014, 453, 384–393. [Google Scholar] [CrossRef]
- Meier-Haack, J.; Lenk, W.; Berwald, S.; Rieser, T.; Lunkwitz, K. Influence of thermal treatment on the pervaporation separation properties of polyamide-6 membranes. Sep. Purif. Technol. 2000, 19, 199–207. [Google Scholar] [CrossRef]
- Kujawski, W.; Waczyński, M.; Lasota, M. Pervaporation properties of dense polyamide-6 membranes in separation of water–ethanol mixtures. Sep. Sci. Technol. 1996, 31, 953–963. [Google Scholar] [CrossRef]
- Olewnik, E.; Richert, J. Effect of the compatibilizing agent on the structure, mechanical and thermal properties of polylactide filled with modified and unmodified montmorillonite. Polym. Compos. 2014, 35, 1330–1337. [Google Scholar] [CrossRef]
- Kopeć, R.; Meller, M.; Kujawski, W.; Kujawa, J. Polyamide-6 based pervaporation membranes for organic–organic separation. Sep. Purif. Technol. 2013, 110, 63–73. [Google Scholar] [CrossRef]
- Ceynowa, J.; Adamczak, P. Enzyme membrane based upon polyamide-6 for oil hydrolysis. J. Appl. Polym. Sci. 1992, 46, 749–756. [Google Scholar] [CrossRef]
- Zielińska, K.; Kujawski, W.; Chostenko, A.G. Chitosan hydrogel membranes for pervaporative dehydration of alcohols. Sep. Purif. Technol. 2011, 83, 114–120. [Google Scholar] [CrossRef]
- Żenkiewicz, M. Comparative study on the surface free energy of a solid calculated by different methods. Polym. Test. 2007, 26, 14–19. [Google Scholar] [CrossRef]
- Kujawska, A.; Knozowska, K.; Kujawa, J.; Kujawski, W. Influence of downstream pressure on pervaporation properties of PDMS and POMS based membranes. Sep. Purif. Technol. 2016, 159, 68–80. [Google Scholar] [CrossRef]
- Ahmad, F.; Lau, K.K.; Shariff, A.M.; Yeong, Y.F. Temperature and pressure dependence of membrane permeance and its effect on process economics of hollow fiber gas separation system. J. Membr. Sci. 2013, 430, 44–55. [Google Scholar] [CrossRef]
- Baker, R.W.; Wijmans, J.G.; Huang, Y. Permeability, permeance and selectivity: A preferred way of reporting pervaporation performance data. J. Membr. Sci. 2010, 348, 346–352. [Google Scholar] [CrossRef]
- Sampranpiboon, P.; Jiraratananon, R.; Uttapap, D.; Feng, X.; Huang, R.Y.M. Pervaporation separation of ethyl butyrate and isopropanol with polyether block amide (PEBA) membranes. J. Membr. Sci. 2000, 173, 53–59. [Google Scholar] [CrossRef]
- Katti, K.S.; Katti, D.R.; Dash, R. Synthesis and characterization of a novel chitosan/montmorillonite/hydroxyapatite nanocomposite for bone tissue engineering. Biomed. Mater. 2008, 3, 034122. [Google Scholar] [CrossRef] [PubMed]
- Silvino, A.C.; de Souza, K.S.; Dahmouche, K.; Dias, M.L. Polylactide/clay nanocomposites: A fresh look into the in situ polymerization process. J. Appl. Polym. Sci. 2012, 124, 1217–1224. [Google Scholar] [CrossRef]
- Tyagi, B.; Chudasama, C.D.; Jasra, R.C.V. Determination of structural modification in acid activated montmorillonite clay by FT-IR spectroscopy. Specrochim. Acta Part A 2006, 64, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska-Czubenko, J.; Gierszewska Drużyńska, M. Effect of ionic crosslinking on the water state in hydrogel chitosan membranes. Carbohydr. Polym. 2009, 77, 590–598. [Google Scholar] [CrossRef]
- Wang, S.F.; Shen, L.; Tong, Y.J.; Chen, L.; Phang, I.Y.; Lim, P.Q.; Liu, T.X. Biopolymer chitosan/montmorillonite nanocomposites: Preparation and characterization. Polym. Degrad. Stab. 2005, 90, 123–131. [Google Scholar] [CrossRef]
- Thakur, G.; Singh, A.; Singh, I. Chitosan-montmorillonite polymer composites: Formulation and evaluation of sustained release tablets of aceclofenac. Sci. Pharm. 2016, 84, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tong, L.; Fang, Z.; Gu, A.; Xu, Z. Thermal degradation behavior of multi- walled carbon nanotubes/polyamide 6 composites. Polym. Degrad. Stab. 2006, 91, 2046–2052. [Google Scholar] [CrossRef]
- Tortora, M.; Vittoria, V.; Galli, G.; Ritrovati, S.; Chiellini, E. Transport properties of modified montmorillonite-poly(ε-caprolactone) nanocomposites. Macromol. Mater. Eng. 2002, 287, 243–249. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, X.; Hanna, M.A. Chitosan/clay a nonocomposite film preparation and characterization. J. Appl. Polym. Sci. 2006, 99, 1684–1691. [Google Scholar] [CrossRef]
- Kim, J.T.; Lee, D.Y.; Oh, T.S.; Lee, D.H. Characteristics of nitrile–butadiene rubber layered silicate nanocomposites with silane coupling agent. J. Appl. Polym. Sci. 2003, 89, 2633–2640. [Google Scholar] [CrossRef]
- Choi, W.M.; Kim, T.W.; Park, O.O.; Chang, Y.K.; Lee, J.W. Preparation and characterization of poly(hydroxybutyrate-co-hydroxyvalerate)-organoclay nanocomposites. J. Appl. Polym. Sci. 2003, 90, 525–529. [Google Scholar] [CrossRef]
- Lewandowska, K.; Sionkowska, A.; Kaczmarek, B.; Furtos, G. Characterization of chitosan composites with various clays. Int. J. Biol. Macromol. 2014, 65, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.S.; Lee, S.H.; Choi, K.H.; Park, I. Preparation and characterization of chitosan–clay nanocomposites with antimicrobial activity. J. Phys. Chem. Solids 2010, 71, 464–467. [Google Scholar] [CrossRef]
- Pankaj, G.; Ashim, J.T.; Rashmi, R.D.; Bodhaditya, D.; Tarun, K.M. A comparative study on sorption of arsenate ions from water by crosslinked chitosan and crosslinked chitosan/MMT nanocomposite. J. Environ. Chem. Eng. 2016, 4, 4248–4257. [Google Scholar] [CrossRef]
- Kujawski, W.; Adamczak, P.; Narebska, A. A fully automated system for the determination of pore size distribution in microfiltration and ultrafiltration membranes. Sep. Sci. Technol. 1989, 24, 495–506. [Google Scholar] [CrossRef]
- Hernández, A.; Calvo, J.I.; Prádanos, P.; Tejerina, F. Pore size distributions in microporous membranes. A critical analysis of the bubble point extended method. J. Membr. Sci. 1996, 112, 1–12. [Google Scholar] [CrossRef]
- Szczerbińska, J.; Kujawski, W.; Arszyńska, J.; Kujawa, J. Assessment of air-gap membrane distillation with hydrophobic porous membranes utilized for damaged paintings humidification. J. Membr. Sci. 2017, 538, 1–8. [Google Scholar] [CrossRef]
- Lewandowska, K.; Sionkowska, A.; Kaczmarek, B.; Furtos, G. Mechanical and morphological studies of chitosan/clay composites, molecular crystals and liquid crystals. Mol. Cryst. Liq. Cryst. 2014, 590, 193–198. [Google Scholar] [CrossRef]
- Drelich, J.; Miller, J.D.; Good, R.J. The effect of drop (bubble) size on advancing and receding contact angles for heterogeneous and rough solid surfaces as observed with sessile-drop and captive-bubble techniques. J. Colloid Interface Sci. 1996, 179, 37–50. [Google Scholar] [CrossRef]
- Amaral, I.F.; Granja, P.L.; Melo, L.V.; Saramago, B.; Barbosa, M.A. Functionalization of chitosan membranes through phosphorylation: Atomic force microscopy, wettability, and cytotoxicity studies. J. Appl. Polym. Sci. 2006, 102, 276–284. [Google Scholar] [CrossRef]
- Solid Surface Energy Data (SFE) for Common Polymers. Available online: http://www.surface-tension.de/solid-surface-energy.htm (accessed on 6 July 2018).
- Mittal, K.L. (Ed.) Advances in Contact Angle, Wettability and Adhesion; Scrivener Publishing: Beverly, MA, USA, 2015; Volume 2, ISBN 978-1-119-11698-1. [Google Scholar]
- Taguet, A.; Cassagnau, P.; Lopez-Cuesta, J.-M. Structuration, selective dispersion and compatibilizing effectof (nano)fillers in polymer blends. Prog. Polym. Sci. 2014, 39, 1526–1563. [Google Scholar] [CrossRef]
- Sionkowska, A.; Płanecka, A.; Kozłowska, J.; Skopińska-Wiśniewska, J.; Łoś, P. Weathering of chitosan films in the presence of low- and high-molecular weight additives. Carbohydr. Polym. 2011, 84, 900–906. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A. Phase inversion technique-based polyamide films and their applications: A comprehensive review. Polym. Plast. Technol. Eng. 2017, 56, 1421–1437. [Google Scholar] [CrossRef]
- Tang, Ch.; Xiang, L.; Su, J.; Wang, K.; Yang, C.; Zhang, Q.; Qiang, F. Largely improved tensile properties of chitosan film via unique synergistic reinforcing effect of carbon nanotube and clay. J. Phys. Chem. B 2008, 112, 3876–3881. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.W.; Park, H.M.; Ha, C.S. Bio-nanocomposites: For food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Araújo, E.M.; Barbosa, R.; Morais, C.R.S.; Soledade, L.E.B.; Souza, A.G.; Vieira, M.Q. Effects of organoclays on the thermal processing of pe/clay nanocomposites. J. Therm. Anal. Calorim. 2007, 90, 841–848. [Google Scholar] [CrossRef]
- Chiu, F.C.; Lai, S.M.; Hsieh, I.C.; Don, T.M.; Huang, C.Y. Preparation and properties of chitosan/clay (nano)composites: A silanol quaternary ammonium intercalated clay. J. Polym. Res. 2012, 19, 9781. [Google Scholar] [CrossRef]
- Prateepchanachai, S.; Thakhiew, W.; Devahastin, S.; Soponronnarit, S. Mechanical properties improvement of chitosan films via the use of plasticizer, charge modifying agent and film solution homogenization. Carbohydr. Polym. 2017, 174, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Niamsa, N.; Baima, Y. Preparation and characterization of highly flexible chitosan films for use as food packaging. Am. J. Food Technol. 2009, 4, 162–169. [Google Scholar] [CrossRef]
- Kasirga, Y.; Oral, A.; Caner, C. Preparation and characterization of chitosan/montmorillonite-K10 nanocomposites films for food packaging applications. Polym. Compos. 2012, 33, 1330–1337. [Google Scholar] [CrossRef]
- Ge, J.; Cui, Y.; Yan, Y.; Jiang, W. The effect of structure on pervaporation of chitosan membrane. J. Membr. Sci. 2000, 165, 75–81. [Google Scholar] [CrossRef]
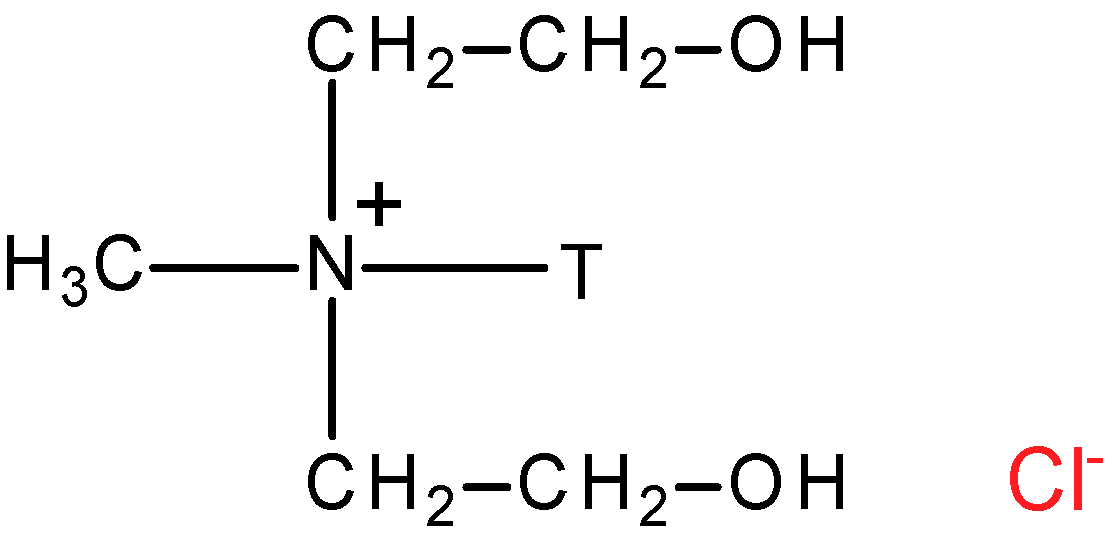
| Compound | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O |
| Content (wt %) | 73.0 | 14.0 | 2.7 | 0.2 | 1.1 | 0.6 | 1.9 |
| Sample | Temperature/°C at mass loss | Residual mass at 600 °C | ||
|---|---|---|---|---|
| T5% | T10% | T50% | (%) | |
| MMT | 86.1 | 592.3 | - | 90.4 |
| C30B | 244.6 | 288.1 | - | 77.5 |
| Ch | 66.9 | 124.4 | 332.0 | 0.9 |
| PA6 | 341.1 | 375.7 | 431.1 | 1.3 |
| PA6/Ch | 257.3 | 307.6 | 427.3 | 1.4 |
| PA6/Ch/MMT | 288.3 | 338.0 | 434.9 | 2.7 |
| PA6/Ch/C30B | 297.9 | 314.2 | 432.8 | 2.3 |
| Membrane | Ra (nm) | Rq (nm) |
|---|---|---|
| PA6 | 63.1 ± 0.5 | 50.3 ± 1.1 |
| Ch | 1.9 ± 0.3 | 1.6 ± 0.2 |
| PA6/Ch | 7.2 ± 0.3 | 5.3 ± 0.5 |
| PA6/Ch/MMT | 13.1 ± 0.1 | 10.8 ± 0.5 |
| PA6/Ch/C30B | 16.2 ± 0.7 | 12.6 ± 0.8 |
| Sample | Contact angle (°) | Surface free energy (mN·m−1) | ||||
|---|---|---|---|---|---|---|
| H2O | DIM | G | ||||
| PA6 | 51.5 ± 2.1 | 20.6 ± 0.8 | 57.5 ± 1.4 | 47.61 | 45.31 | 2.30 |
| Ch | 77.3 ± 3.6 | 47.6 ± 1.9 | 71.0 ± 2.1 | 35.6 | 33.97 | 1.63 |
| PA6/Ch | 74.9 ± 3.3 | 47.2 ± 1.6 | 64.7 ± 1.8 | 36.53 | 32.14 | 4.39 |
| PA6/Ch/MMT | 74.9 ± 2.3 | 47.6 ± 3.2 | 65.8 ± 4.1 | 36.13 | 32.21 | 3.92 |
| PA6/Ch/C30B | 68.9 ± 1.2 | 46.1 ± 1.5 | 60.7 ± 4.6 | 38.04 | 31.65 | 6.40 |
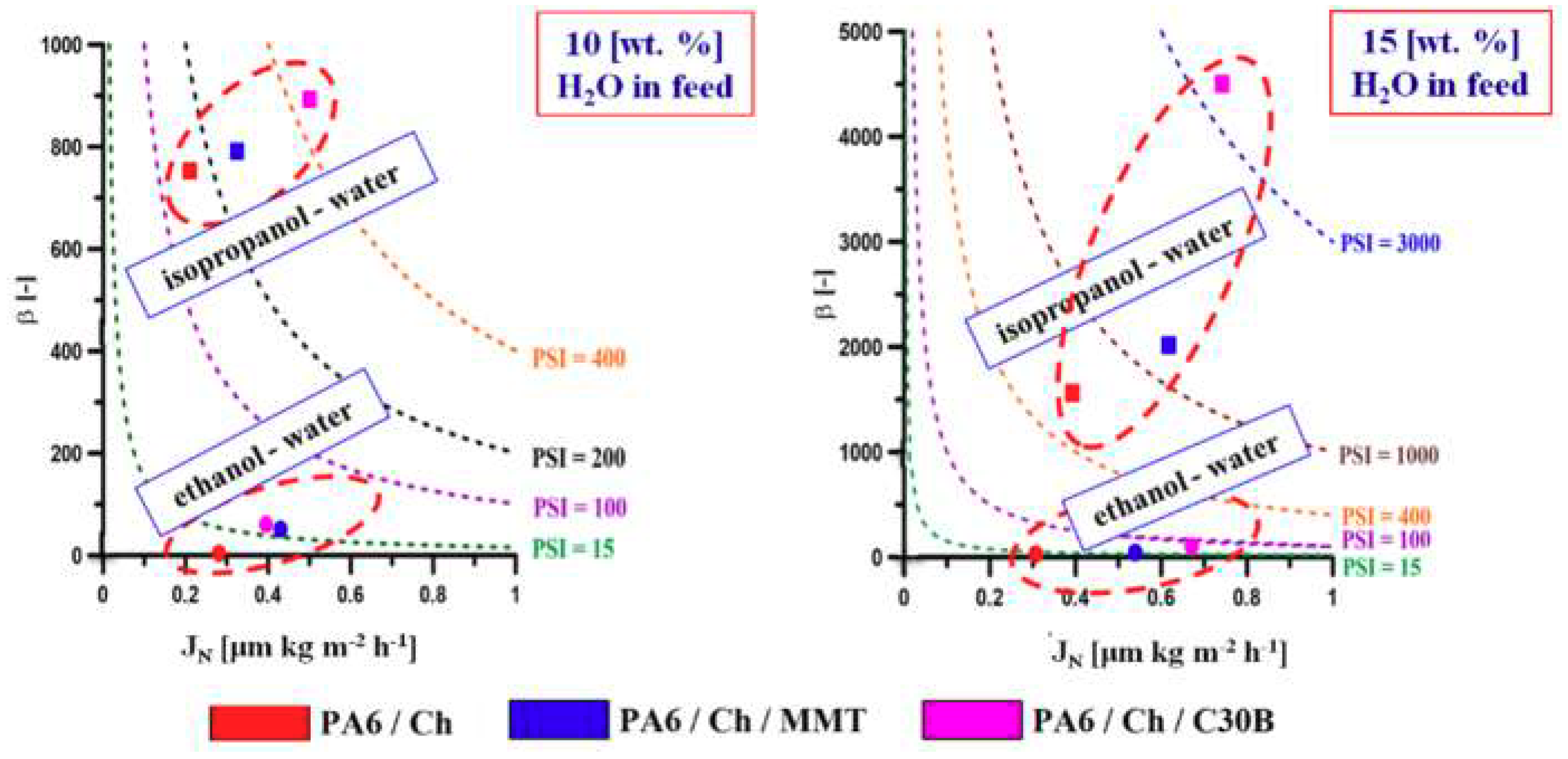
| Membrane | Thickness (μm) | EtOH/H2O (90/10 wt %) | EtOH/H2O (85/15 wt %) | iPrOH/H2O (90/10 wt %) | iPrOH/H2O (85/15 wt %) | ||||
|---|---|---|---|---|---|---|---|---|---|
| β (-) | JN (μm·kg·m−2·h−1) | β (-) | JN (μm·kg·m−2·h−1) | β (-) | JN (μm·kg·m−2·h−1) | β (-) | JN (μm·kg·m−2·h−1) | ||
| Ch | 36.5 ± 0.1 | 18 ± 2 | 0.0016 ± 0.0001 | 15 ± 2 | 0.0035 ± 0.0002 | 431.0 ± 0.5 | 0.0073 ± 0.0003 | 980.0 ± 0.6 | 0.0057 ± 0.0011 |
| PA6/Ch | 124.3 ± 0.1 | 39 ± 1 | 0.281 ± 0.005 | 31 ± 3 | 0.307 ± 0.001 | 756.0 ± 1.0 | 0.210 ± 0.002 | 1562.0 ± 0.5 | 0.392 ± 0.006 |
| PA6/Ch/MMT | 110.8 ± 0.3 | 51 ± 1 | 0.430 ± 0.003 | 45 ± 1 | 0.539 ± 0.005 | 791.0 ± 0.9 | 0.325 ± 0.002 | 2017.0 ± 0.7 | 0.617 ± 0.008 |
| PA6/Ch/C30B | 130.1 ± 0.1 | 60 ± 2 | 0.395 ± 0.003 | 105 ± 1 | 0.671 ± 0.004 | 893.0 ± 0.8 | 0.501 ± 0.003 | 4500.0 ± 1.2 | 0.742 ± 0.002 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrzanowska, E.; Gierszewska, M.; Kujawa, J.; Raszkowska-Kaczor, A.; Kujawski, W. Development and Characterization of Polyamide-Supported Chitosan Nanocomposite Membranes for Hydrophilic Pervaporation. Polymers 2018, 10, 868. https://doi.org/10.3390/polym10080868
Chrzanowska E, Gierszewska M, Kujawa J, Raszkowska-Kaczor A, Kujawski W. Development and Characterization of Polyamide-Supported Chitosan Nanocomposite Membranes for Hydrophilic Pervaporation. Polymers. 2018; 10(8):868. https://doi.org/10.3390/polym10080868
Chicago/Turabian StyleChrzanowska, Ewelina, Magdalena Gierszewska, Joanna Kujawa, Aneta Raszkowska-Kaczor, and Wojciech Kujawski. 2018. "Development and Characterization of Polyamide-Supported Chitosan Nanocomposite Membranes for Hydrophilic Pervaporation" Polymers 10, no. 8: 868. https://doi.org/10.3390/polym10080868