Enhanced Electrorheological Response of Cellulose: A Double Effect of Modification by Urea-Terminated Silane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Silane-Modified Cellulose (Cel-Si-Urea)
2.3. Characterization
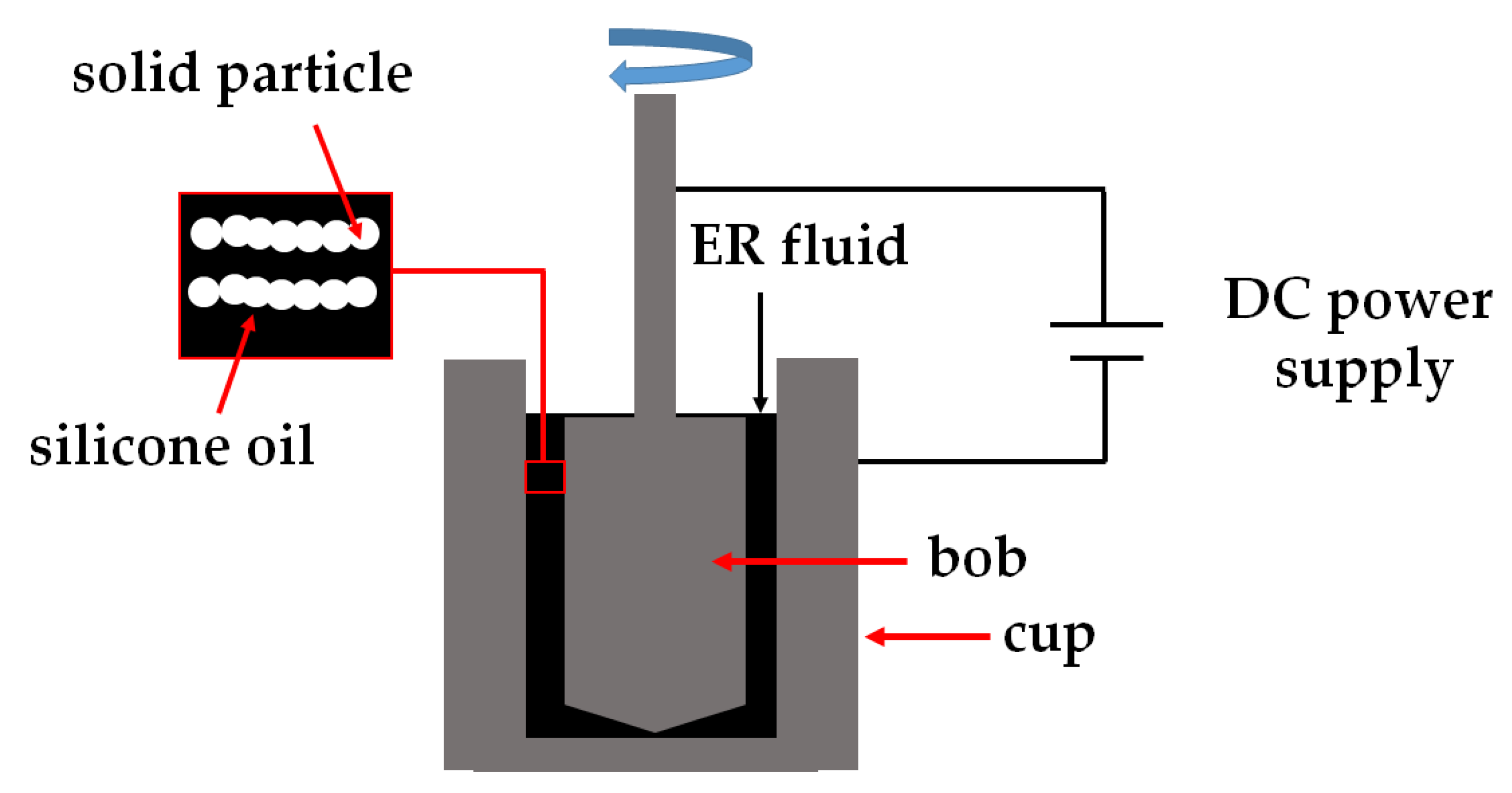
2.4. Preparation of ER Fluids and Rheological Measurements
3. Results and Discussion
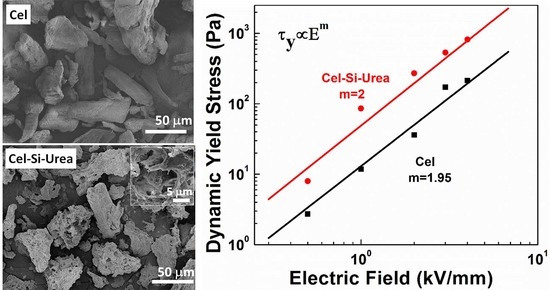
3.1. Morphologies and Structures
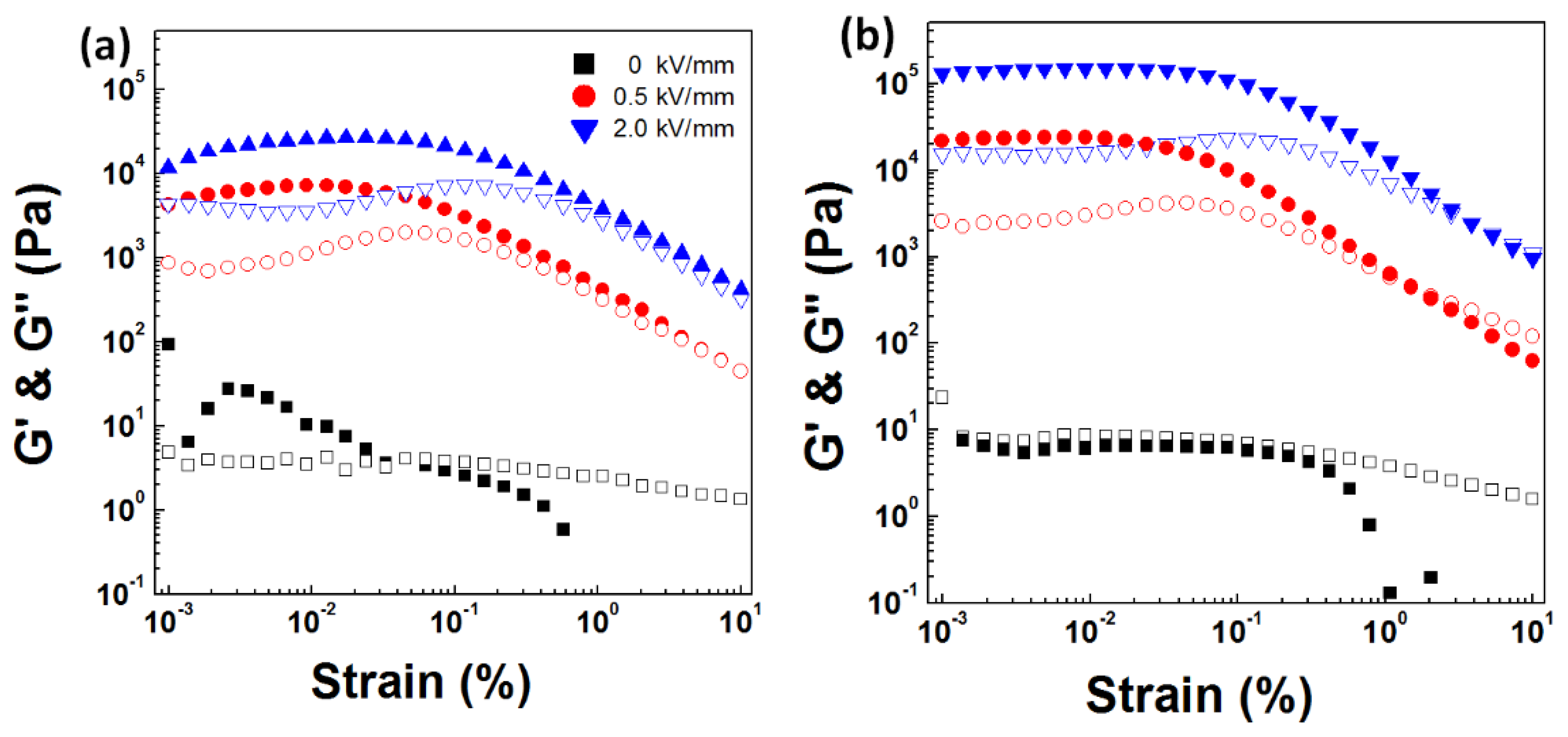
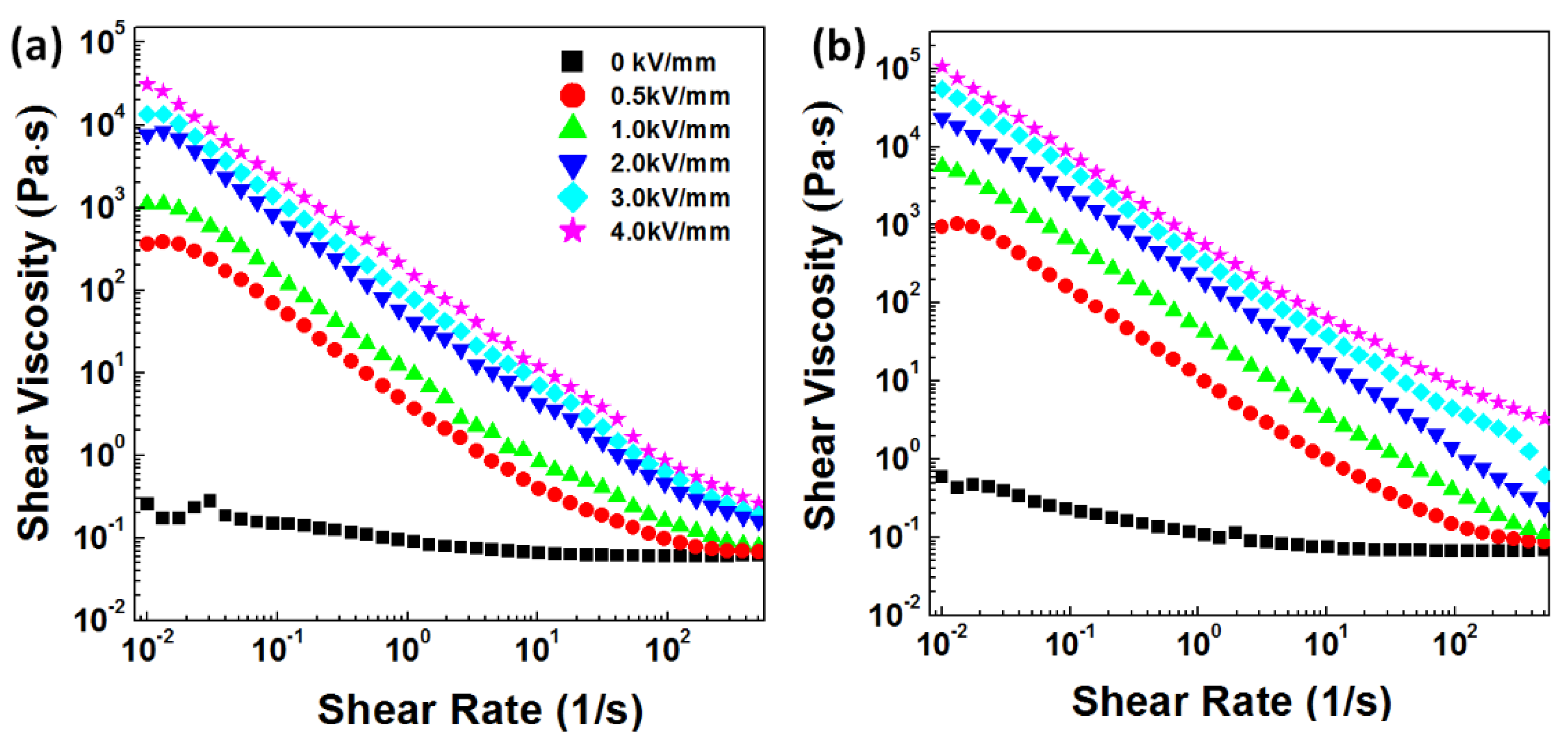
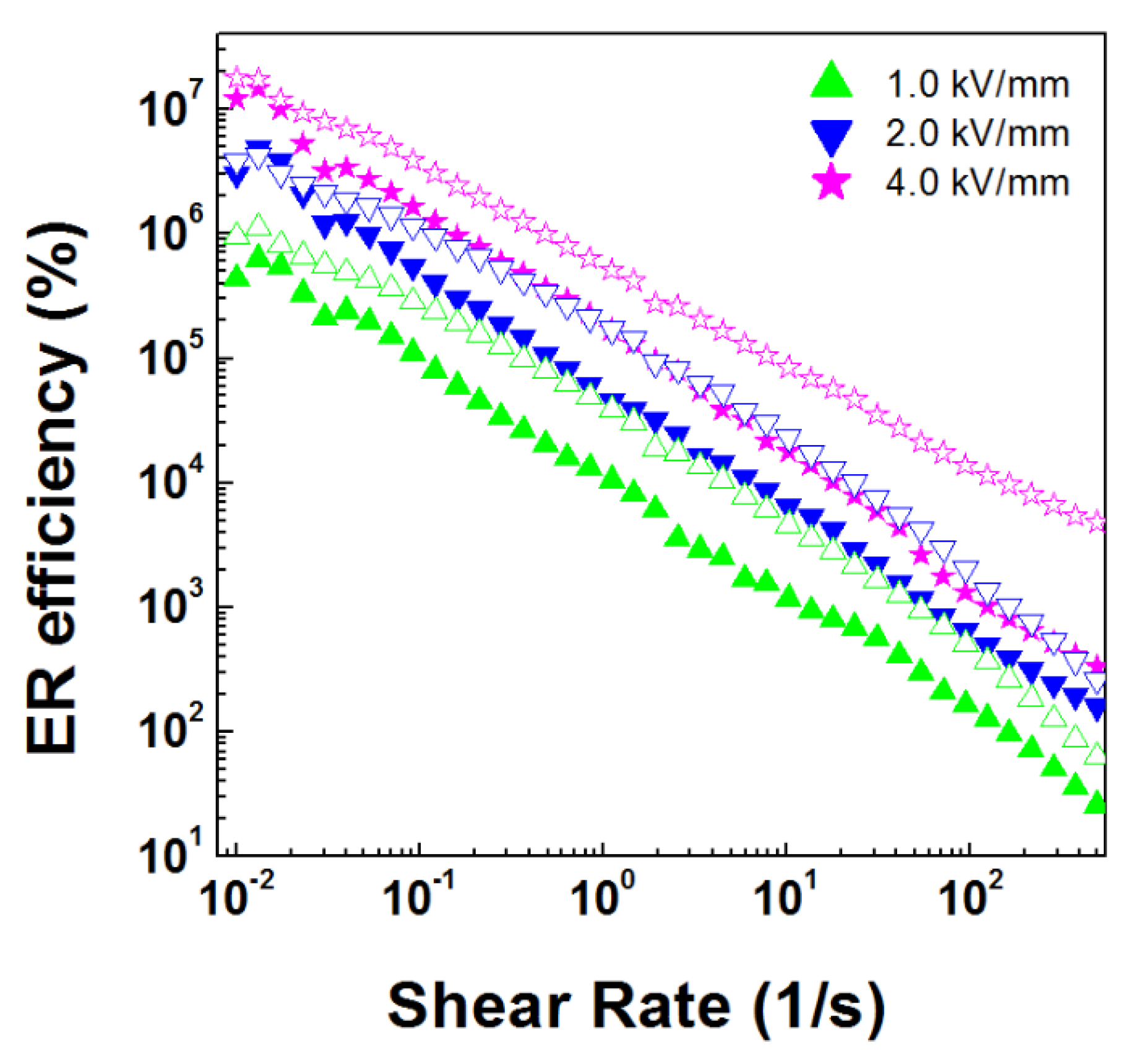
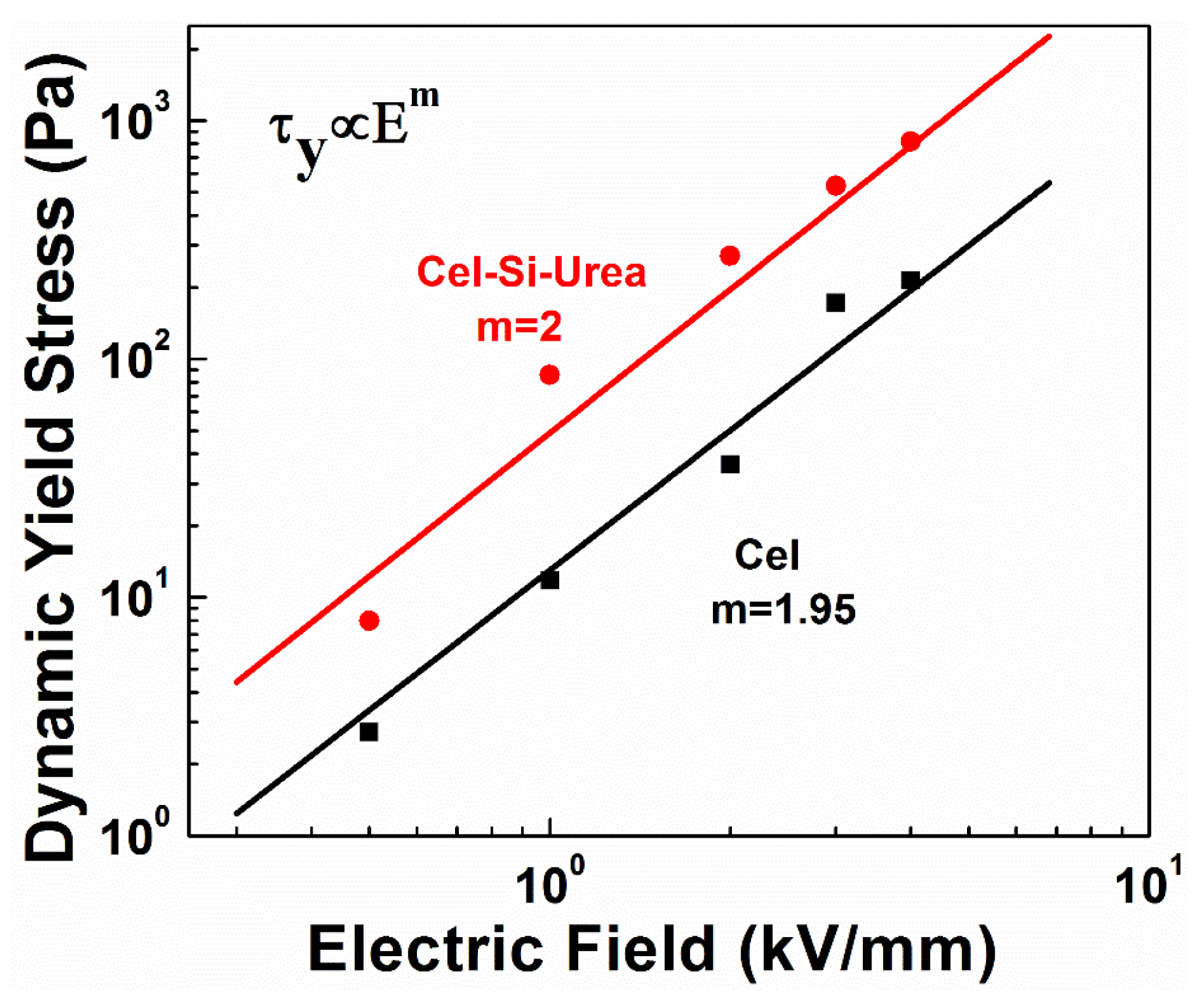
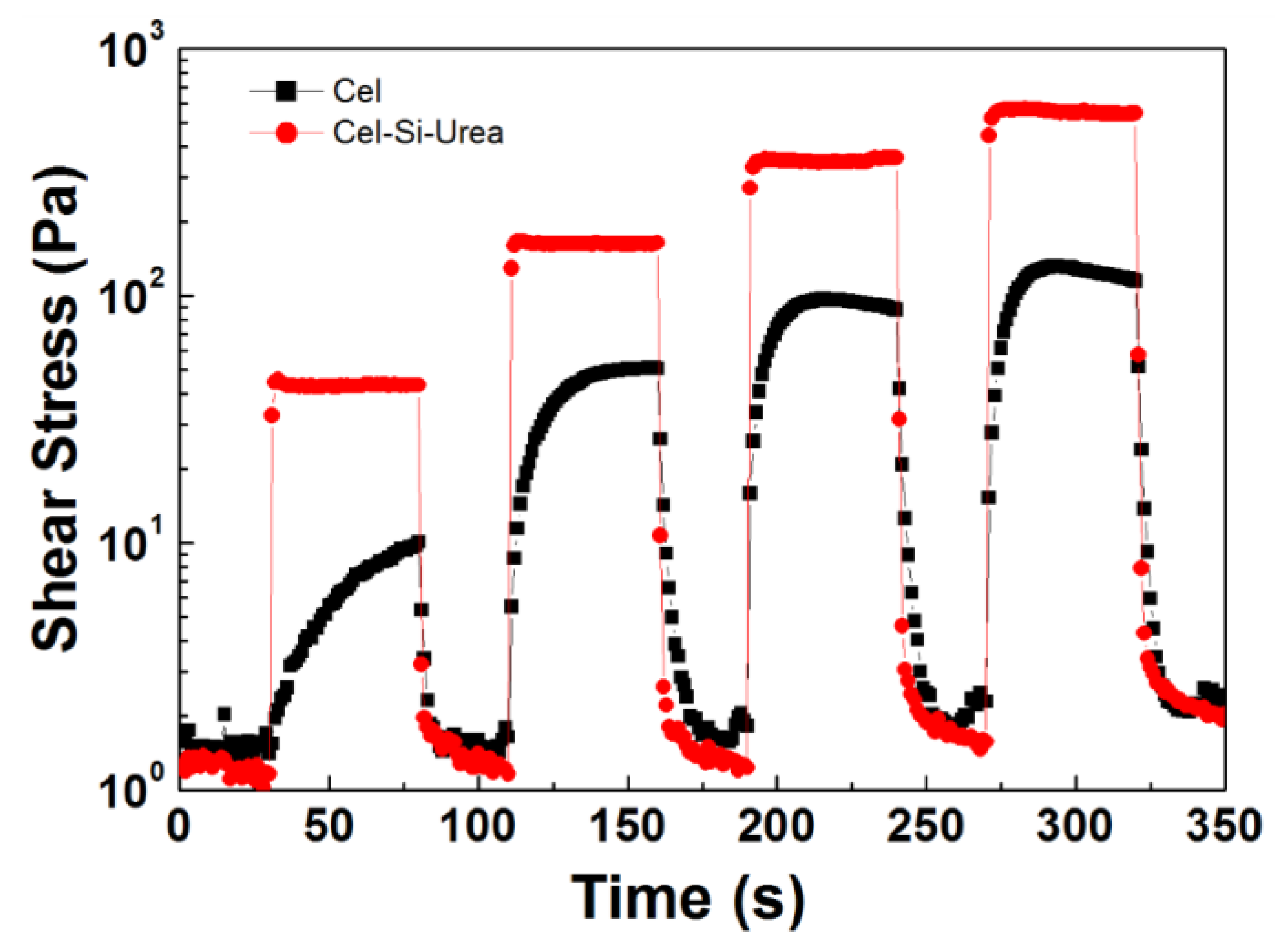
3.2. ER Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.L. Biopolymers from Renewable Resources; Springer: Berlin/Heidelberg, German, 1998; pp. 1–29. [Google Scholar]
- Credou, J.; Berthelot, T. Cellulose: From Biocompatible to Bioactive Material. J. Mater. Chem. B 2014, 2, 4767–4788. [Google Scholar] [CrossRef]
- Gelin, K.; Bodin, A.; Gatenholm, P.; Mihranyan, A.; Edwards, K.; Strømme, M. Characterization of Water in Bacterial Cellulose Using Dielectric Spectroscopy and Electron Microscopy. Polymer 2007, 48, 7623–7631. [Google Scholar] [CrossRef]
- Zugenmaier, P. Conformation and Packing of Various Crystalline Cellulose Fibers. Prog. Polym. Sci. 2001, 26, 1341–1417. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal Structure and Hydrogen-Bonding System in Cellulose Ibeta from Synchrotron X-ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2003, 125, 14300–14306. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y. Key Advances in the Chemical Modification of Nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.; Eichhorn, S.J. Crystalline and Amorphous Deformation of Process-Controlled Cellulose-II Fibres. Polymer 2005, 46, 6380–6390. [Google Scholar] [CrossRef]
- Sullivan, A.C.O. Cellulose: The Structure Slowly Unravels. Cellulose 1997, 4, 173–197. [Google Scholar] [CrossRef]
- Paavilainen, S.; Róg, T.; Vattulainen, I. Analysis of Twisting of Cellulose Nanofibrils in Atomistic Molecular Dynamics Simulations. J. Phys. Chem. B 2011, 115, 3747–3755. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, R.; Shen, D.; Wu, Z.; Huang, Y. Arrangement of Cellulose Microfibrils in the Wheat Straw Cell Wall. Carbohydr. Polym. 2008, 72, 76–85. [Google Scholar] [CrossRef]
- Roy, D.; Semsarilar, M.; Guthrie, J.T.; Perrier, S. Cellulose Modification by Polymer Grafting: A Review. Chem. Soc. Rev. 2009, 38, 2046–2064. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.M. Cellulose Structure and Biosynthesis: What Is in Store for the 21st Century? J. Polym. Sci. Pol. Chem. 2010, 42, 487–495. [Google Scholar] [CrossRef]
- Long, L.-Y.; Weng, Y.-X.; Wang, Y.-Z. Cellulose Aerogels: Synthesis, Applications, and Prospects. Polymers 2018, 10, 20734360. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Sugiyama, J.; Chanzy, H.; Langan, P. Crystal Structure and Hydrogen Bonding System in Cellulose I (alpha) from Synchrotron X-ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2003, 125, 9074–9082. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose Nanomaterials Review: Structure, Properties and Nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Gu, J.; Zhang, W.; Tu, D.; Hu, C. Cellulose I and II Nanocrystals Produced by Sulfuric Acid Hydrolysis of Tetra Pak Cellulose I. Carbohydr. Polym. 2018, 192, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Tsimogiannis, D.; Stavrakaki, M.; Oreopoulou, V. Isolation and Characterization of Cellulose Nanofibrils from Wheat Straw Using Steam Explosion Coupled with High Shear Homogenization. Carbohydr. Res. 2011, 346, 76–85. [Google Scholar]
- Wang, H.; Chen, W.; Zhang, X.; Wei, Y.; Zhang, A.; Liu, S.; Wang, X.; Liu, C. Structural Changes of Bagasse during the Homogeneous Esterification with Maleic Anhydride in Ionic Liquid 1-Allyl-3-methylimidazolium Chloride. Polymers 2018, 10, 433. [Google Scholar] [CrossRef]
- Avolio, R.; Bonadies, I.; Capitani, D.; Errico, M.E.; Gentile, G.; Avella, M. A Multitechnique Approach to Assess the Effect of Ball Milling on Cellulose. Carbohydr. Polym. 2012, 87, 265–273. [Google Scholar] [CrossRef]
- Zhang, L.; Tsuzuki, T.; Wang, X. Preparation of Cellulose Nanofiber from Softwood Pulp by Ball Milling. Cellulose 2015, 22, 1729–1741. [Google Scholar] [CrossRef]
- Kontturi, K.S.; Biegaj, K.W.; Mautner, A.; Woodward, R.T.; Wilson, B.P.; Johansson, L.S.; Lee, K.Y.; Jyy, H.; Bismarck, A.; Kontturi, E. Non-Covalent Surface Modification of Cellulose Nanopapers by Adsorption of Polymers from Aprotic Solvents. Langmuir 2017, 33, 5707–5712. [Google Scholar] [CrossRef] [PubMed]
- Shaabani, A.; Rahmati, A.; Badri, Z. Sulfonated Cellulose and Starch: New Biodegradable and Renewable Solid Acid Catalysts for Efficient Synthesis of Quinolines. Catal. Commun. 2008, 9, 13–16. [Google Scholar] [CrossRef]
- Wang, B.; Yang, D.; Zhang, H.-R.; Huang, C.; Xiong, L.; Luo, J.; Chen, X.-D. Preparation of Esterified Bacterial Cellulose for Improved Mechanical Properties and the Microstructure of Isotactic Polypropylene/Bacterial Cellulose Composites. Polymers 2016, 8, 129. [Google Scholar] [CrossRef]
- Manaf, M.E.A.; Tsuji, M.; Nobukawa, S.; Yamaguchi, M. Effect of Moisture on the Orientation Birefringence of Cellulose Esters. Polymers 2011, 3, 955–966. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.; Gao, C.Y.; Nam, J.D.; Choi, H.J. Cellulose-Based Smart Fluids Under Applied Electric Fields. Materials 2017, 10, 1060. [Google Scholar] [CrossRef] [PubMed]
- Bae, D.H.; Choi, H.J.; Choi, K.; Nam, J.D.; Islam, M.S.; Kao, N. Fabrication of Phosphate Microcrystalline Rice Husk Based Cellulose Particles and Their Electrorheological Response. Carbohydr. Polym. 2017, 165, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Patural, L.; Marchal, P.; Govin, A.; Grosseau, P.; Ruot, B.; Devès, O. Cellulose Ethers Influence on Water Retention and Consistency in Cement-Based Mortars. Cem. Concr. Res. 2011, 41, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Ly, B.; Belgacem, M.N.; Bras, J.; Salon, M.C.B. Grafting of Cellulose by Fluorine-Bearing Silane Coupling Agents. Mater. Sci. Eng. C 2010, 30, 343–347. [Google Scholar] [CrossRef]
- Abdelmouleh, M.; Boufi, S.; Salah, A.B.; Belgacem, M.N.; Gandini, A. Interaction of Silane Coupling Agents with Cellulose. Langmuir 2002, 18, 3203–3208. [Google Scholar] [CrossRef]
- Zhang, W.L.; Choi, H.J. Stimuli-Responsive Polymers and Colloids under Electric and Magnetic Fields. Polymers 2014, 6, 2803–2818. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.D.; Choi, H.J. Electrorheological Fluids: Smart Soft Matter and Characteristics. Soft Matter 2012, 8, 11961–11978. [Google Scholar] [CrossRef]
- Zheng, C.; Dong, Y.; Liu, Y.; Zhao, X.; Yin, J. Enhanced Stimuli-Responsive Electrorheological Property of Poly(ionic liquid)s-Capsulated Polyaniline Particles. Polymers 2017, 9, 385. [Google Scholar] [CrossRef]
- Sheng, P.; Wen, W. Electrorheological Fluids: Mechanisms, Dynamics, and Microfluidics Applications. Annu. Rev. Fluid Mech. 2012, 44, 143–174. [Google Scholar] [CrossRef]
- Shen, R.; Wang, X.; Wen, W.; Lu, K. TiO2 Based Electrorheological Fluid with High Yield Stress Int. J. Mod. Phys. B 2008, 19, 1104–1109. [Google Scholar] [CrossRef]
- Wen, W.; Huang, X.; Yang, S.; Lu, K.; Sheng, P. The Giant Electrorheological Effect in Suspensions of Nanoparticles. Nat. Mater. 2003, 2, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.B.; Zhao, X.P. Giant Electrorheological Activity of High Surface Area Mesoporous Cerium-Doped TiO2 Templated by Block Copolymer. Chem. Phys. Lett. 2004, 398, 393–399. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Lee, S.; Cheong, O.J.; Jane, J. Enhanced Electroresponse of Alkaline Earth Metal-Doped Silica/Titania Spheres by Synergetic Effect of Dispersion Stability and Dielectric Property. ACS Appl. Mater. Interfaces 2015, 7, 18977–18984. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Gonçalves, P.; Cidade, M.T. Electrorheological behavior of suspensions of camphorsulfonic acid (CSA) doped polyaniline nanofibers in silicone oil. Phys. Scr. 2017, 92, 075801. [Google Scholar] [CrossRef]
- Goswami, S.; Brehm, T.; Filonovich, S.; Cidade, M.T. Electrorheological properties of polyaniline-vanadium oxide nanostructures suspended in silicone oil. Smart Mater. Struct. 2014, 23, 105012. [Google Scholar] [CrossRef]
- Wei, C.; Zhu, Y.; Yang, X.; Li, C. One-pot synthesis of polyaniline-doped in mesoporous TiO2 and its electrorheological behavior. Mater. Sci. Eng. B 2007, 137, 213–216. [Google Scholar] [CrossRef]
- Chotpattananont, D.; Sirivat, A.; Jamieson, A.M. Electrorheological properties of perchloric acid-doped polythiophene suspensions. Colloid Polym. Sci. 2004, 282, 357–365. [Google Scholar] [CrossRef]
- Cabuk, M.; Yavuz, M.; Unal, H.I.; Erol, O. Synthesis, Characterization and Electrorheological Properties of Biodegradable Chitosan/Bentonite Composites. Clay Miner. 2013, 48, 129–141. [Google Scholar] [CrossRef]
- Sim, B.; Bae, D.H.; Choi, H.J.; Choi, K.; Islam, M.S.; Kao, N. Fabrication and Stimuli Response of Rice Husk-Based Microcrystalline Cellulose Particle Suspension under Electric Fields. Cellulose 2016, 23, 185–197. [Google Scholar] [CrossRef]
- Ko, Y.G.; Lee, H.J.; Shin, S.S.; Choi, U.S. Dipolar-Molecule Complexed Chitosan Carboxylate, Phosphate, and Sulphate Dispersed Electrorheological Suspensions. Soft Matter 2012, 8, 6273–6279. [Google Scholar] [CrossRef]
- Xie, Y.; Hill, C.A.S.; Xiao, Z.; Militz, H.; Mai, C. Silane Coupling Agents Used for Natural Fiber/Polymer Composites: A review. Compos. Part A-Appl. Sci. Manu. 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Jiao, C.; Xiong, J. Accessibility and Morphology of Cellulose Fibres Treated with Sodium Hydroxide. Bioresources 2014, 9, 6504–6513. [Google Scholar] [CrossRef]
- Thakur, M.K.; Gupta, R.K.; Thakur, V.K. Surface modification of cellulose using silane coupling agent. Carbohydr. Polym. 2014, 111, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, S.J.; Young, R.J.; Davies, R.J.; Riekel, C. Characterisation of the Microstructure and Deformation of High Modulus Cellulose Fibres. Polymer 2003, 44, 5901–5908. [Google Scholar] [CrossRef]
- Ilharco, L.M.; Garcia, A.R.; Lopes, D.S. Infrared Approach to the Study of Adsorption on Cellulose: Influence of Cellulose Crystallinity on the Adsorption of Benzophenone. Langmuir 1997, 13, 4126–4132. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction Preparation and Characterization of Cellulose Fibres and Nanocrystals from Rice Husk. Ind. Crop. Prod. 2012, 1, 93–99. [Google Scholar] [CrossRef]
- He, K.; Wen, Q.; Wang, C.; Wang, B.; Yu, S.; Hao, C.; Chen, K. Porous TiO2 Nanoparticles Derived from Titanium Metal-Organic Framework and Its Improved Electrorheological Performance. Ind. Eng. Chem. Res. 2018, 57, 6888–6896. [Google Scholar] [CrossRef]
- Noh, J.; Yoon, C.M.; Jang, J. Enhanced Electrorheological Activity of Polyaniline Coated Mesoporous Silica with High Aspect ratio. J. Colloid Interface. Sci. 2016, 470, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Sever, E.; Unal, H.I. Electrorheological, Viscoelastic and Creeprecovery Behaviors of Covalently Bonded Nanocube-TiO2/Poly (3-octylthiophene) Colloidal Dispersions. Polym. Compos. 2016, 39, 351–359. [Google Scholar] [CrossRef]
- Zhang, W.L.; Jiang, D.; Wang, X.; Hao, B.N.; Liu, Y.D.; Liu, J. Growth of Polyaniline Nanoneedles on MoS2 Nanosheets, Tunable Electroresponse, and Electromagnetic Wave Attenuation Analysis. J. Phys. Chem. C 2017, 121, 4989–4998. [Google Scholar] [CrossRef]
- Cho, M.S.; Choi, H.J.; Jhon, M.S. Shear Stress Analysis of A Semiconducting Polymer Based Electrorheological Fluid System. Polymer 2005, 46, 11484–11488. [Google Scholar] [CrossRef]
- Choi, H.J.; Jhon, M.S. Electrorheology of Polymers and Nanocomposites. Soft Matter 2009, 5, 1562–1567. [Google Scholar] [CrossRef]
- Choi, H.J.; Cho, M.S.; Kim, J.W. A Yield Stress Scaling Function for Electrorheological Fluids. Appl. Phys. Lett. 2001, 78, 3806–3808. [Google Scholar] [CrossRef]
- Vemuri, S.H.; Jhon, M.S.; Zhang, K. New Analysis of Yield Stress on Giant Electrorheological Fluids. Colloid Polym. Sci. 2012, 290, 189–192. [Google Scholar] [CrossRef]
- Shen, R.; Liu, R.; Wang, D.; Chen, K.; Sun, G.; Lu, K. Frequency Response of Giant Electrorheological Fluids in AC Electric Field. RSC Adv. 2014, 4, 61968–61974. [Google Scholar] [CrossRef]
- Tu, W.; Li, X.; Chen, Z.; Liu, Y.D.; Labardi, M.; Capaccioli, S.; Paluch, M.; Wang, L.M. Glass Formability in Medium-Sized Molecular Systems/Pharmaceuticals. I. Thermodynamics vs. Kinetics. J. Chem. Phys. 2016, 144, 174502. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, X.; Guo, Y.; Wu, T.; Liu, Y.D.; Ngai, K.L.; Wang, L.M. A New Secondary Relaxation in the Rigid and Planar 1-Methylindole: Evidence from Binary Mixture Studies. J. Chem. Phys. 2016, 145, 214501. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, Y.; Zheng, C.; Lei, Q.; Dong, Y.; Zhao, X.P.; Yin, J.B. Pickering Emulsion Polymerization of Poly(ionic liquid)s Encapsulated Nano-SiO2 Composite Particles with Enhanced Electro-Responsive Characteristic. Polymer 2018, 146, 109–119. [Google Scholar] [CrossRef]







| Sample | E (kV/mm) | 0.5 | 1.0 | 2.0 | 3.0 | 4.0 |
|---|---|---|---|---|---|---|
| Cel-Si-Urea | τy | 7.99 | 85.7 | 271 | 532 | 817 |
| α | 1.14 | 0.17 | 0.31 | 0.32 | 0.79 | |
| β | 0.98 | 0.30 | 0.50 | 0.24 | 0.90 | |
| t2 | 1.49 | 0.05 | 0.01 | 0.01 | 8.37 | |
| t3 | 0.01 | 0.47 | 0.06 | 0.04 | 0.002 | |
| η∞ | 0.07 | 0.08 | 0.09 | 0.13 | 1.49 | |
| Pristine cellulose | τy | 2.72 | 11.7 | 36.2 | 173 | 214 |
| α | 1.81 | 1.25 | 1.78 | 0.28 | 0.36 | |
| β | 1.00 | 0.90 | 1.09 | 0.18 | 1.44 | |
| t2 | 2.93 | 2.86 | 10.4 | 0.30 | 0.07 | |
| t3 | 0.02 | 0.004 | 0.002 | 0.30 | 0.04 | |
| η∞ | 0.06 | 0.52 | 0.08 | 0.09 | 0.17 |
| Cel-Si-Urea | Δε’ | ε’0 | ε’∞ | α | γ | τ | σ | A | n |
| 1.38 | 3.63 | 2.25 | 0.65 | 0.82 | 0.0098 | 0.048 | 0.19 | −0.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Chen, P.; Jin, X.; Wang, L.-M.; Liu, Y.D.; Choi, H.J. Enhanced Electrorheological Response of Cellulose: A Double Effect of Modification by Urea-Terminated Silane. Polymers 2018, 10, 867. https://doi.org/10.3390/polym10080867
Liu Z, Chen P, Jin X, Wang L-M, Liu YD, Choi HJ. Enhanced Electrorheological Response of Cellulose: A Double Effect of Modification by Urea-Terminated Silane. Polymers. 2018; 10(8):867. https://doi.org/10.3390/polym10080867
Chicago/Turabian StyleLiu, Zhao, Panpan Chen, Xiao Jin, Li-Min Wang, Ying Dan Liu, and Hyoung Jin Choi. 2018. "Enhanced Electrorheological Response of Cellulose: A Double Effect of Modification by Urea-Terminated Silane" Polymers 10, no. 8: 867. https://doi.org/10.3390/polym10080867