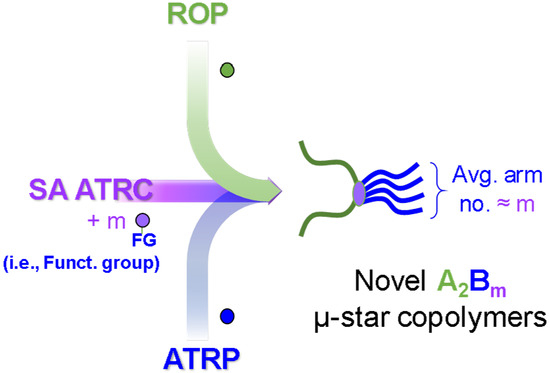
Synthesis of Poly(ε-caprolactone)-Based Miktoarm Star Copolymers through ROP, SA ATRC, and ATRP
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. ROP with ε-Caprolactone and Chain End Modification (Shown in Scheme 1a,b, respectively)
2.3. SA ATRC of PCL–Br with VBC (Shown in Scheme 1c)
2.4. Chain Extension of PCL-VBCm-PCL with St or tBA through ATRP (Shown in Scheme 1d)
2.5. Deprotection of PCL-μ-PtBA
2.6. Characterization
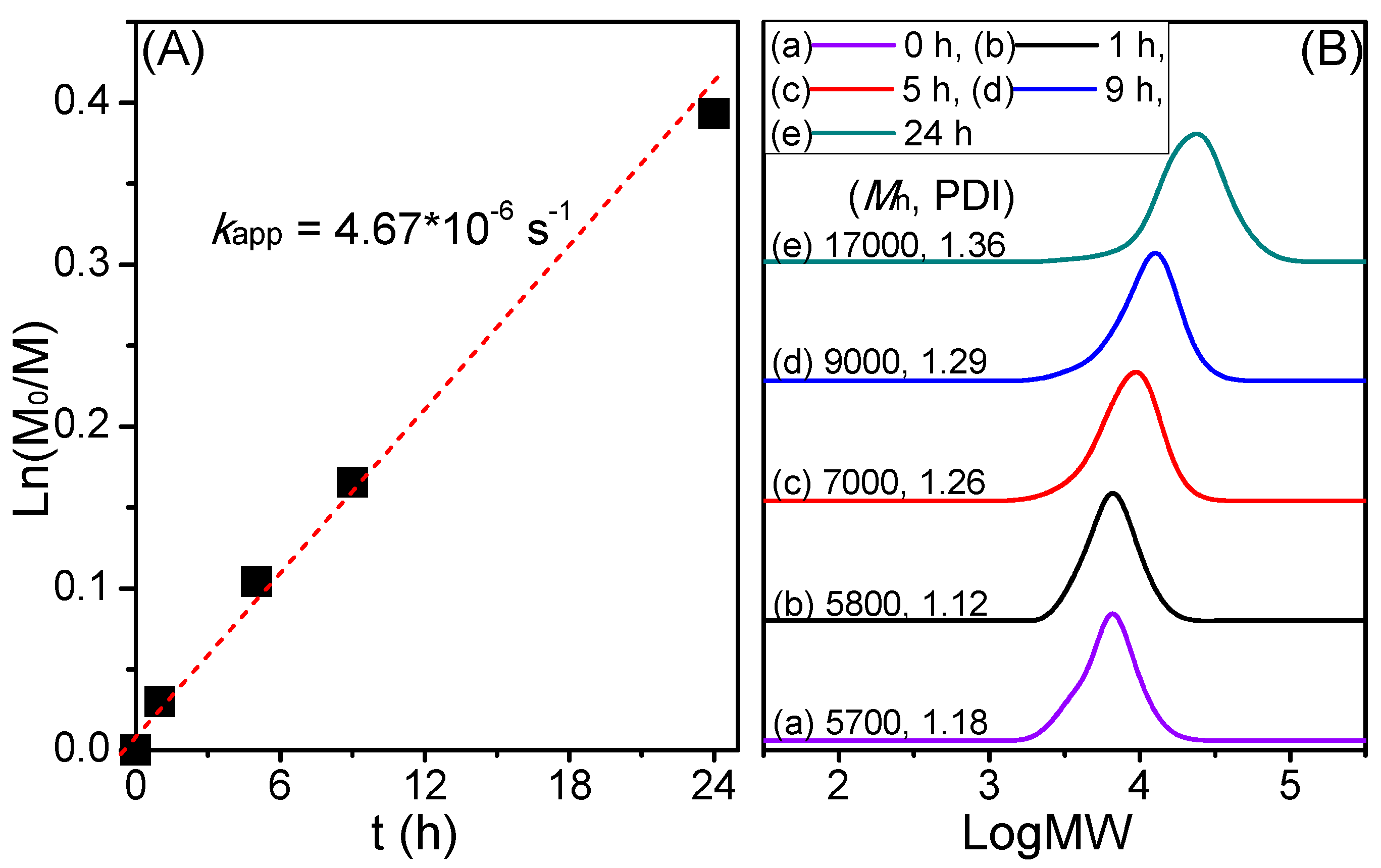
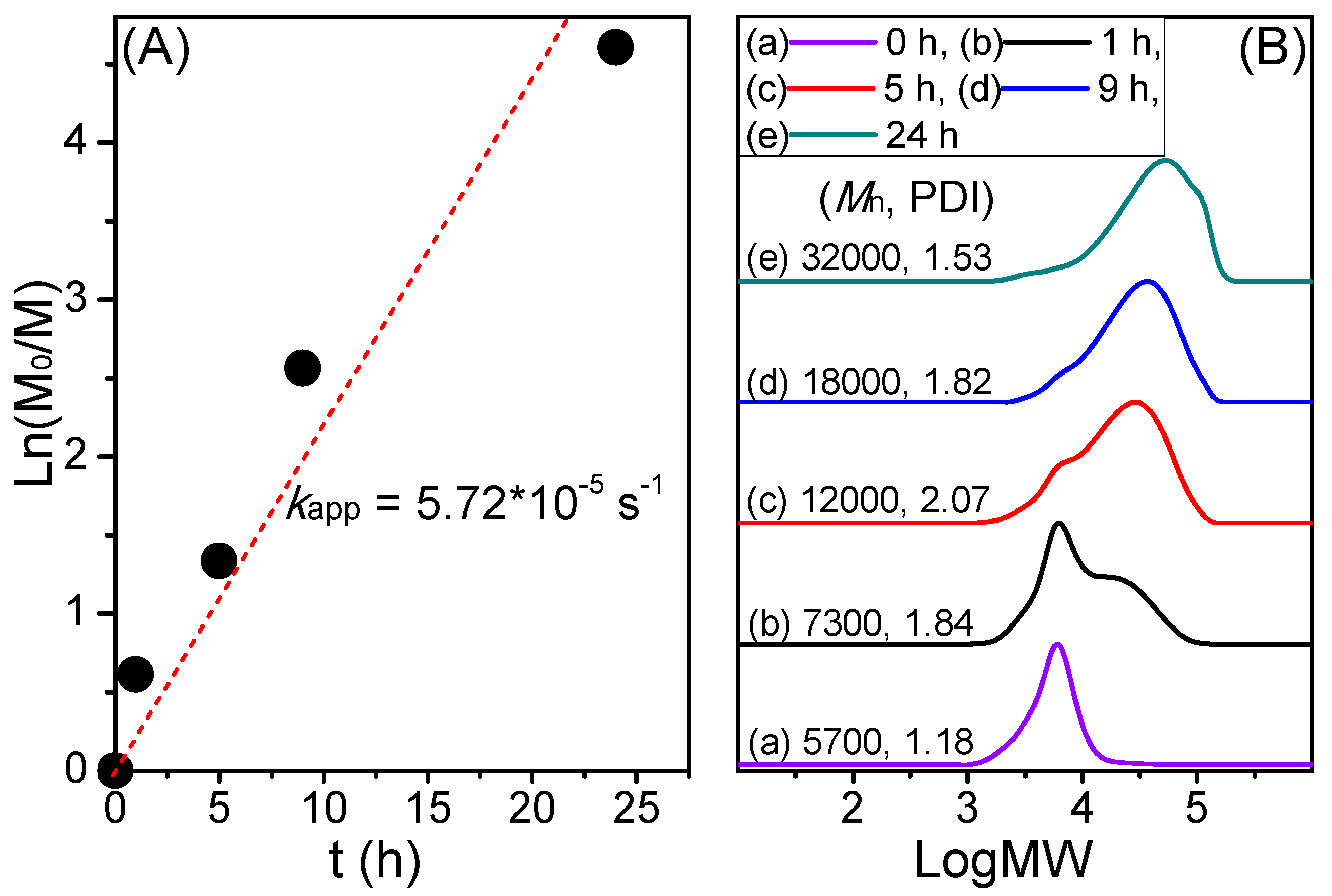
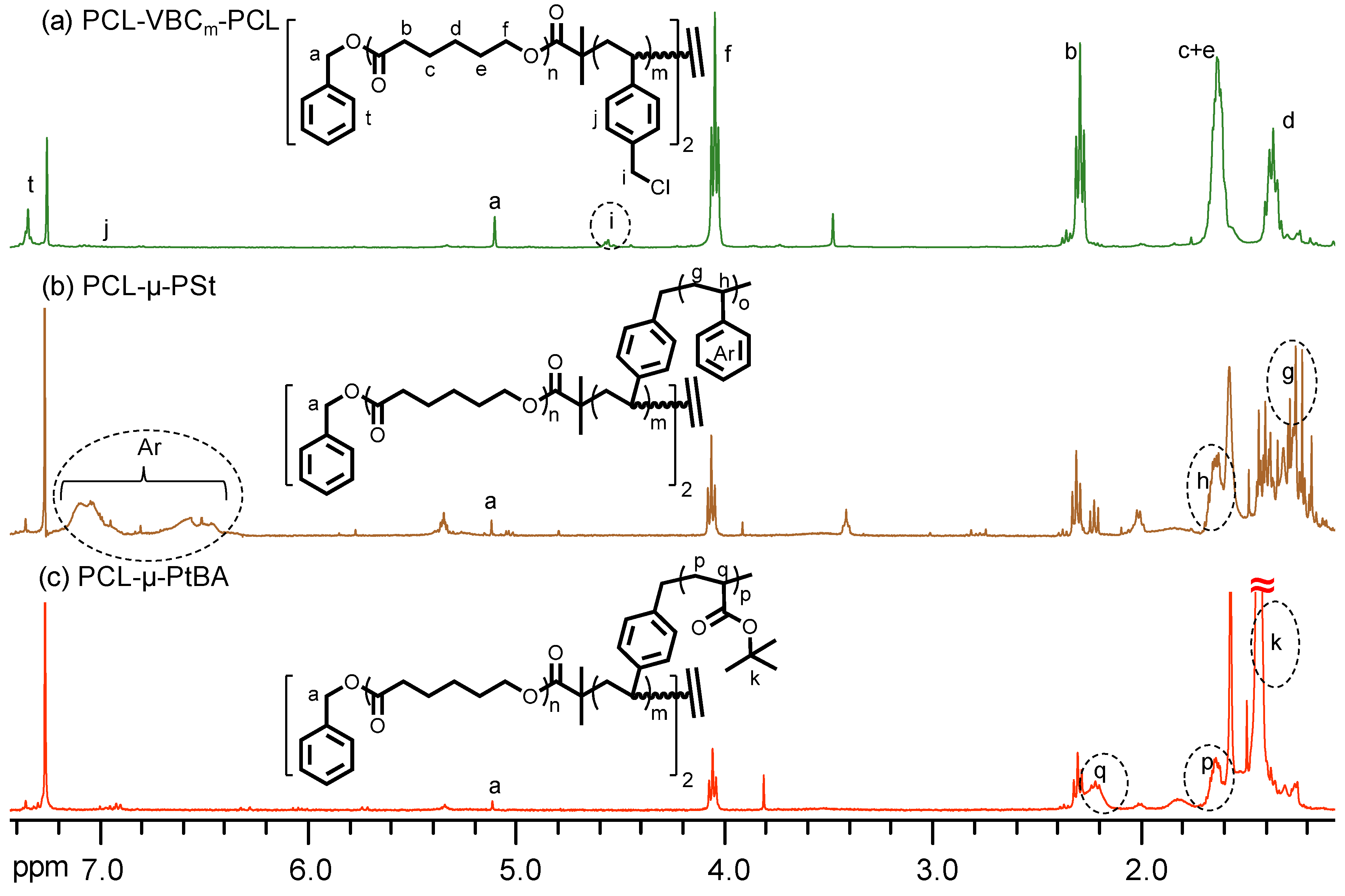
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bielawski, C.W.; Grubbs, R.H. Living ring-opening metathesis polymerization. Prog. Polym. Sci. 2007, 32, 1–29. [Google Scholar] [CrossRef]
- Kamber, N.E.; Jeong, W.; Waymouth, R.M.; Pratt, R.C.; Lohmeijer, B.G.G.; Hedrick, J.L. Organocatalytic ring-opening polymerization. Chem. Rev. 2007, 107, 5813–5840. [Google Scholar] [CrossRef] [PubMed]
- Mecerreyes, D.; Jerome, R.; Dubois, P. Novel macromolecular architectures based on aliphatic polyesters: Relevance of the coordination-insertion ring-opening polymerization. Adv. Polym. Sci. 1999, 147, 1–59. [Google Scholar]
- Braunecker, W.A.; Matyjaszewski, K. Controlled/living radical polymerization: Features, developments, and perspectives. Prog. Polym. Sci. 2007, 32, 93–146. [Google Scholar] [CrossRef]
- Hawker, C.J.; Bosman, A.W.; Harth, E. New polymer synthesis by nitroxide mediated living radical polymerizations. Chem. Rev. 2001, 101, 3661–3688. [Google Scholar] [CrossRef] [PubMed]
- Kamigaito, M.; Ando, T.; Sawamoto, M. Metal-catalyzed living radical polymerization. Chem. Rev. 2001, 101, 3689–3745. [Google Scholar] [CrossRef] [PubMed]
- Moad, G.; Rizzardo, E.; Thang, S.H. Radical addition-fragmentation chemistry in polymer synthesis. Polymer 2008, 49, 1079–1131. [Google Scholar] [CrossRef]
- Sun, H.; Kabb, C.P.; Dai, Y.Q.; Hill, M.R.; Ghiviriga, I.; Bapat, A.P.; Sumerlin, B.S. Macromolecular metamorphosis via stimulus-induced transformations of polymer architecture. Nat. Chem. 2017, 9, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.Q.; Sun, H.; Pal, S.; Zhang, Y.L.; Park, S.; Kabb, C.P.; Wei, W.D.; Sumerlin, B.S. Near-ir-induced dissociation of thermally-sensitive star polymers. Chem. Sci. 2017, 8, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Kabb, C.P.; Sumerlin, B.S. Thermally-labile segmented hyperbranched copolymers: Using reversible-covalent chemistry to investigate the mechanism of self-condensing vinyl copolymerization. Chem. Sci. 2014, 5, 4646–4655. [Google Scholar] [CrossRef]
- Yokozawa, T.; Asai, T.; Sugi, R.; Ishigooka, S.; Hiraoka, S. Chain-growth polycondensation for nonbiological polyamides of defined architecture. J. Am. Chem. Soc. 2000, 122, 8313–8314. [Google Scholar] [CrossRef]
- Yokozawa, T.; Ogawa, M.; Sekino, A.; Sugi, R.; Yokoyama, A. Chain-growth polycondensation for well-defined aramide. Synthesis of unprecedented block copolymer containing aramide with low polydispersity. J. Am. Chem. Soc. 2002, 124, 15158–15159. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Yokoyama, A.; Yokozawa, T. Synthesis of polybenzamide-b-polystyrene block copolymer via combination of chain-growth condensation polymerization and atom transfer radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2948–2954. [Google Scholar] [CrossRef]
- Huang, C.-F.; Chen, M.-J.; Lin, C.-H.; Chiang, Y.-W. Synthesis of well-defined poly(N-H benzamide-co-N-octyl benzamide)s and the study of their blends with nylon 6. Polymers 2017, 9, 172. [Google Scholar] [CrossRef]
- Huang, C.-F.; Ohta, Y.; Yokoyama, A.; Yokozawa, T. Efficient low-temperature atom transfer radical coupling and its application to synthesis of well-defined symmetrical polybenzamides. Macromolecules 2011, 44, 4140–4148. [Google Scholar] [CrossRef]
- Lai, K.-Y.; Huang, Y.-S.; Chu, C.-Y.; Huang, C.-F. Synthesis of poly(N-H benzamide)-b-poly(lauryl methacrylate)-b-poly(N-H benzamide) symmetrical triblock copolymers by combinations of CGCP, SARA ATRP, and SA ATRC. Polymer 2018, 137, 385–394. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pitsikalis, M.; Pispas, S.; Iatrou, H. Polymers with complex architecture by living anionic polymerization. Chem. Rev. 2001, 101, 3747–3792. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Goseki, R.; Ishizone, T.; Hirao, A. Synthesis of well-controlled graft polymers by living anionic polymerization towards exact graft polymers. Polym. Chem. 2014, 5, 5523–5534. [Google Scholar] [CrossRef]
- Sawamoto, M. Modern cationic vinyl polymerization. Prog. Polym. Sci. 1991, 16, 111–172. [Google Scholar] [CrossRef]
- Yagci, Y.; Reetz, I. Externally stimulated initiator systems for cationic polymerization. Prog. Polym. Sci. 1998, 23, 1485–1538. [Google Scholar] [CrossRef]
- Chiefari, J.; Chong, Y.K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living free-radical polymerization by reversible addition–fragmentation chain transfer: The RAFT process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Huang, C.-F.; Hsieh, Y.-A.; Hsu, S.-C.; Matyjaszewski, K. Synthesis of poly(N-vinyl carbazole)-based block copolymers by sequential polymerizations of RAFT-ATRP. Polymer 2014, 55, 6051–6057. [Google Scholar] [CrossRef]
- Huang, C.-F.; Nicolay, R.; Kwak, Y.; Chang, F.-C.; Matyjaszewski, K. Homopolymerization and block copolymerization of N-vinylpyrrolidone by ATRP and RAFT with haloxanthate inifers. Macromolecules 2009, 42, 8198–8210. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Chen, J.-K.; Chen, T.; Huang, C.-F. Synthesis of PNVP-based copolymers with tunable thermosensitivity by sequential reversible addition–fragmentation chain transfer copolymerization and ring-opening polymerization. Polymers 2017, 9, 231. [Google Scholar] [CrossRef]
- Kato, M.; Kamigaito, M.; Sawamoto, M.; Higashimura, T. Polymerization of methyl methacrylate with the carbon tetrachloride/dichlorotris-(triphenylphosphine)ruthenium(II)/methylaluminum bis(2,6-di-tert-butylphenoxide) initiating system: Possibility of living radical polymerization. Macromolecules 1995, 28, 1721–1723. [Google Scholar] [CrossRef]
- Wang, J.-S.; Matyjaszewski, K. Controlled/living radical polymerization. Atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar] [CrossRef]
- Gao, H.F.; Matyjaszewski, K. Synthesis of functional polymers with controlled architecture by CRP of monomers in the presence of cross-linkers: From stars to gels. Prog. Polym. Sci. 2009, 34, 317–350. [Google Scholar] [CrossRef]
- Goseki, R.; Ito, S.; Matsuo, Y.; Higashihara, T.; Hirao, A. Precise synthesis of macromolecular architectures by novel iterative methodology combining living anionic polymerization with specially designed linking chemistry. Polymers 2017, 9, 470. [Google Scholar] [CrossRef]
- Ren, J.M.; McKenzie, T.G.; Fu, Q.; Wong, E.H.H.; Xu, J.T.; An, Z.S.; Shanmugam, S.; Davis, T.P.; Boyer, C.; Qiao, G.G. Star polymers. Chem. Rev. 2016, 116, 6743–6836. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.F.; Matyjaszewski, K. Synthesis of star polymers by a combination of ATRP and the click coupling method. Macromolecules 2006, 39, 4960–4965. [Google Scholar] [CrossRef]
- Whittaker, M.R.; Urbani, C.N.; Monteiro, M.J. Synthesis of 3-miktoarm stars and 1st generation mikto dendritic copolymers by “living” radical polymerization and click chemistry. J. Am. Chem. Soc. 2006, 128, 11360–11361. [Google Scholar] [CrossRef] [PubMed]
- Inglis, A.J.; Sinnwell, S.; Davis, T.P.; Barner-Kowollik, C.; Stenzel, M.H. Reversible addition fragmentation chain transfer (RAFT) and hetero-diels-alder chemistry as a convenient conjugation tool for access to complex macromolecular designs. Macromolecules 2008, 41, 4120–4126. [Google Scholar] [CrossRef]
- Huang, C.-F.; Lee, H.-F.; Kuo, S.-W.; Xu, H.; Chang, F.-C. Star polymers via atom transfer radical polymerization from adamantane-based cores. Polymer 2004, 45, 2261–2269. [Google Scholar] [CrossRef]
- Huang, C.-F.; Kuo, S.-W.; Lin, H.-C.; Chen, J.-K.; Chen, Y.-K.; Xu, H.; Chang, F.-C. Thermal properties, miscibility and specific interactions in comparison of linear and star poly(methyl methacrylate) blend with phenolic. Polymer 2004, 45, 5913–5921. [Google Scholar] [CrossRef]
- Chen, Z.-C.; Chiu, C.-L.; Huang, C.-F. Tuning the solubility of copper complex in atom transfer radical self-condensing vinyl polymerizations to control polymer topology via one-pot to the synthesis of hyperbranched core star polymers. Polymers 2014, 6, 2552–2572. [Google Scholar] [CrossRef] [Green Version]
- Alonzo, J.; Hinestrosa, J.P.; Mays, J.W.; Kilbey, S.M. Kinetics of preferential adsorption of amphiphilic star block copolymers that tether by their corona blocks at the solid/fluid interface. Macromolecules 2014, 47, 4048–4055. [Google Scholar] [CrossRef]
- Ding, H.J.; Park, S.; Zhong, M.J.; Pan, X.C.; Pietrasik, J.; Bettinger, C.J.; Matyjaszewski, K. Facile arm-first synthesis of star block copolymers via ARGET ATRP with ppm amounts of catalyst. Macromolecules 2016, 49, 6752–6760. [Google Scholar] [CrossRef]
- Aimi, J.; Lo, C.-T.; Wu, H.-C.; Huang, C.-F.; Nakanishi, T.; Takeuchi, M.; Chen, W.-C. Phthalocyanine-cored star-shaped polystyrene for nano floating gate in nonvolatile organic transistor memory device. Adv. Electron. Mater. 2016, 2, 1500300. [Google Scholar] [CrossRef]
- Aimi, J.; Wang, P.-H.; Shih, C.-C.; Huang, C.-F.; Nakanishi, T.; Takeuchi, M.; Hsuehe, H.-Y.; Chen, W.-C. A star polymer with a metallo-phthalocyanine core as a tunable charge storage material for nonvolatile transistor memory devices. J. Mater. Chem. C 2018, 6, 2724–2732. [Google Scholar] [CrossRef]
- Grayer, V.; Dormidontova, E.E.; Hadziioannou, G.; Tsitsilianis, C. A comparative experimental and theoretical study between heteroarm star and diblock copolymers in the microphase separated state. Macromolecules 2000, 33, 6330–6339. [Google Scholar] [CrossRef]
- Isono, T.; Otsuka, I.; Kondo, Y.; Halila, S.; Fort, S.; Rochas, C.; Satoh, T.; Borsali, R.; Kakuchi, T. Sub-10 nm nano-organization in AB2- and AB3-type miktoarm star copolymers consisting of maltoheptaose and polycaprolactone. Macromolecules 2013, 46, 1461–1469. [Google Scholar] [CrossRef]
- Isono, T.; Otsuka, I.; Suemasa, D.; Rochas, C.; Satoh, T.; Borsali, R.; Kakuchi, T. Synthesis, self-assembly, and thermal caramelization of maltoheptaose-conjugated polycaprolactones leading to spherical, cylindrical, and lamellar morphologies. Macromolecules 2013, 46, 8932–8940. [Google Scholar] [CrossRef]
- Otsuka, I.; Zhang, Y.; Isono, T.; Rochas, C.; Kakuchi, T.; Satoh, T.; Borsali, R. Sub-10 nm scale nanostructures in self-organized linear di- and triblock copolymers and miktoarm star copolymers consisting of maltoheptaose and polystyrene. Macromolecules 2015, 48, 1509–1517. [Google Scholar] [CrossRef]
- Huang, C.F.; Aimi, J.; Lai, K.Y. Synthesis of novel μ-star copolymers with poly(N-octyl benzamide) and poly(ε-caprolactone) miktoarms through chain-growth condensation polymerization, styrenics-assisted atom transfer radical coupling, and ring-opening polymerization. Macromol. Rapid Commun. 2017, 38, 1600607. [Google Scholar] [CrossRef] [PubMed]
- Bunha, A.K.; Mangadlao, J.; Felipe, M.J.; Pangilinan, K.; Advincula, R. Catenated PS-PMMA block copolymers via supramolecularly templated ATRP initiator approach. Macromol. Rapid Commun. 2012, 33, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Arce, M.M.; Pan, C.W.; Thursby, M.M.; Wu, J.P.; Carnicom, E.M.; Tillman, E.S. Influence of solvent on radical trap-assisted dimerization and cyclization of polystyrene radicals. Macromolecules 2016, 49, 7804–7813. [Google Scholar] [CrossRef]
- Sarbu, T.; Lin, K.Y.; Ell, J.; Siegwart, D.J.; Spanswick, J.; Matyjaszewski, K. Polystyrene with designed molecular weight distribution by atom transfer radical coupling. Macromolecules 2004, 37, 3120–3127. [Google Scholar] [CrossRef]
- Jackson, A.T.; Bunn, A.; Priestnall, I.M.; Borman, C.D.; Irvine, D.J. Molecular spectroscopic characterisation of poly(methyl methacrylate) generated by means of atom transfer radical polymerisation (ATRP). Polymer 2006, 47, 1044–1054. [Google Scholar] [CrossRef]
- Jackson, A.T.; Bunn, A.; Chisholm, M.S. Utilising matrix-assisted laser desorption/ionisation techniques for the generation of structural information from different end-group functionalised poly(methyl methacrylate)s. Polymer 2008, 49, 5254–5261. [Google Scholar] [CrossRef]
- Fischer, H.; Radom, L. Factors controlling the addition of carbon-centered radicals to alkenes-an experimental and theoretical perspective. Angew. Chem. Int. Ed. 2001, 40, 1340–1371. [Google Scholar] [CrossRef]
- Tang, W.; Kwak, Y.; Braunecker, W.; Tsarevsky, N.V.; Coote, M.L.; Matyjaszewski, K. Understanding atom transfer radical polymerization: Effect of ligand and initiator structures on the equilibrium constants. J. Am. Chem. Soc. 2008, 130, 10702–10713. [Google Scholar] [CrossRef] [PubMed]
- Sperling, L.H. Introduction to Physical Polymer Science; Wiley-Interscience: New York, NY, USA, 1986. [Google Scholar]
- Tiptipakorn, S.; Keungputpong, N.; Phothiphiphit, S.; Rimdusit, S. Effects of polycaprolactone molecular weights on thermal and mechanical properties of polybenzoxazine. J. Appl. Polym. Sci. 2015, 132, 41915. [Google Scholar] [CrossRef]
- Mabrouk, M.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Pillay, V. The influence of lyophilized emugel silica microspheres on the physicomechanical properties, in vitro bioactivity and biodegradation of a novel ciprofloxacin-loaded PCL/PAA scaffold. Polymers 2016, 8, 232. [Google Scholar] [CrossRef]

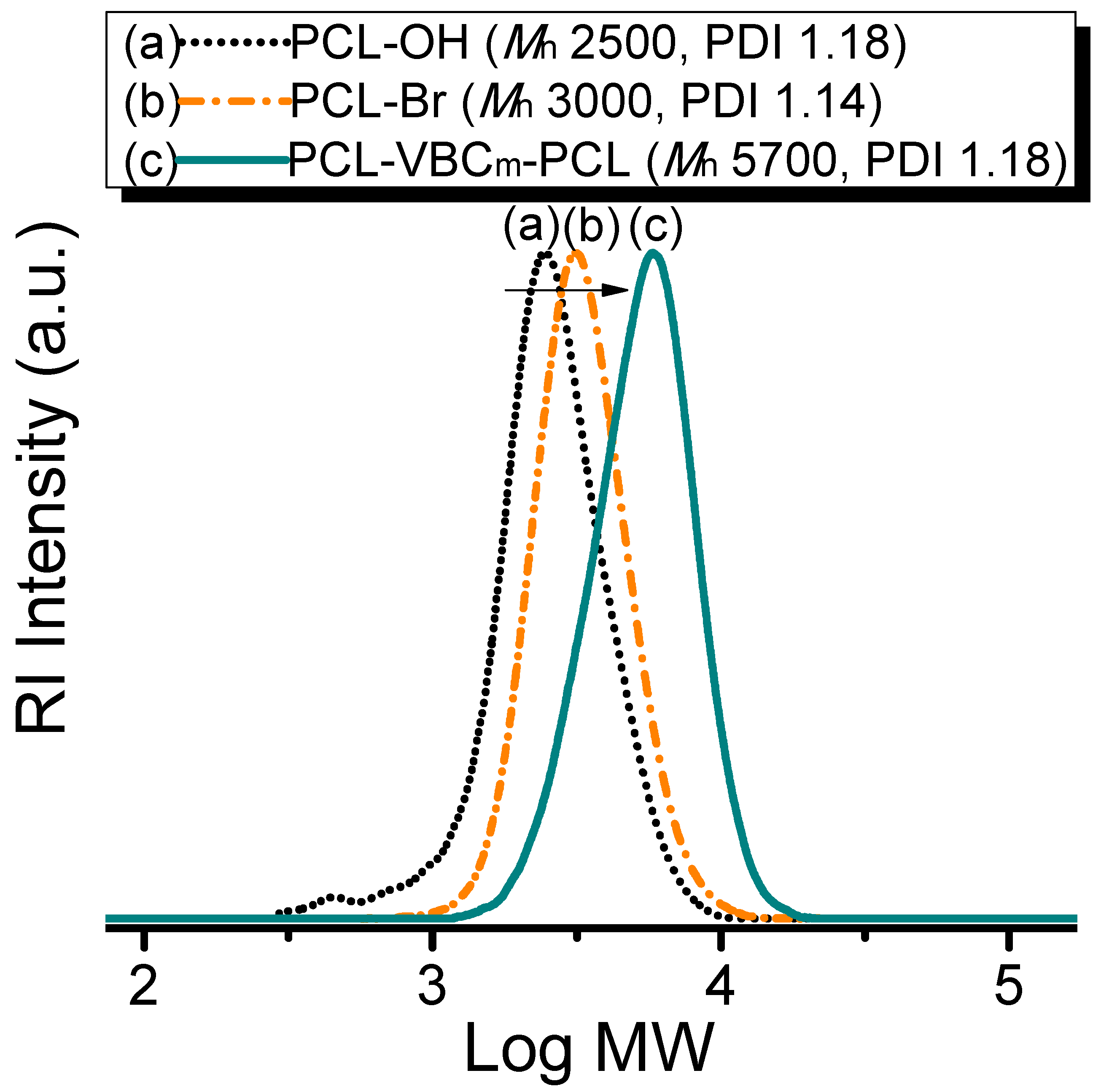
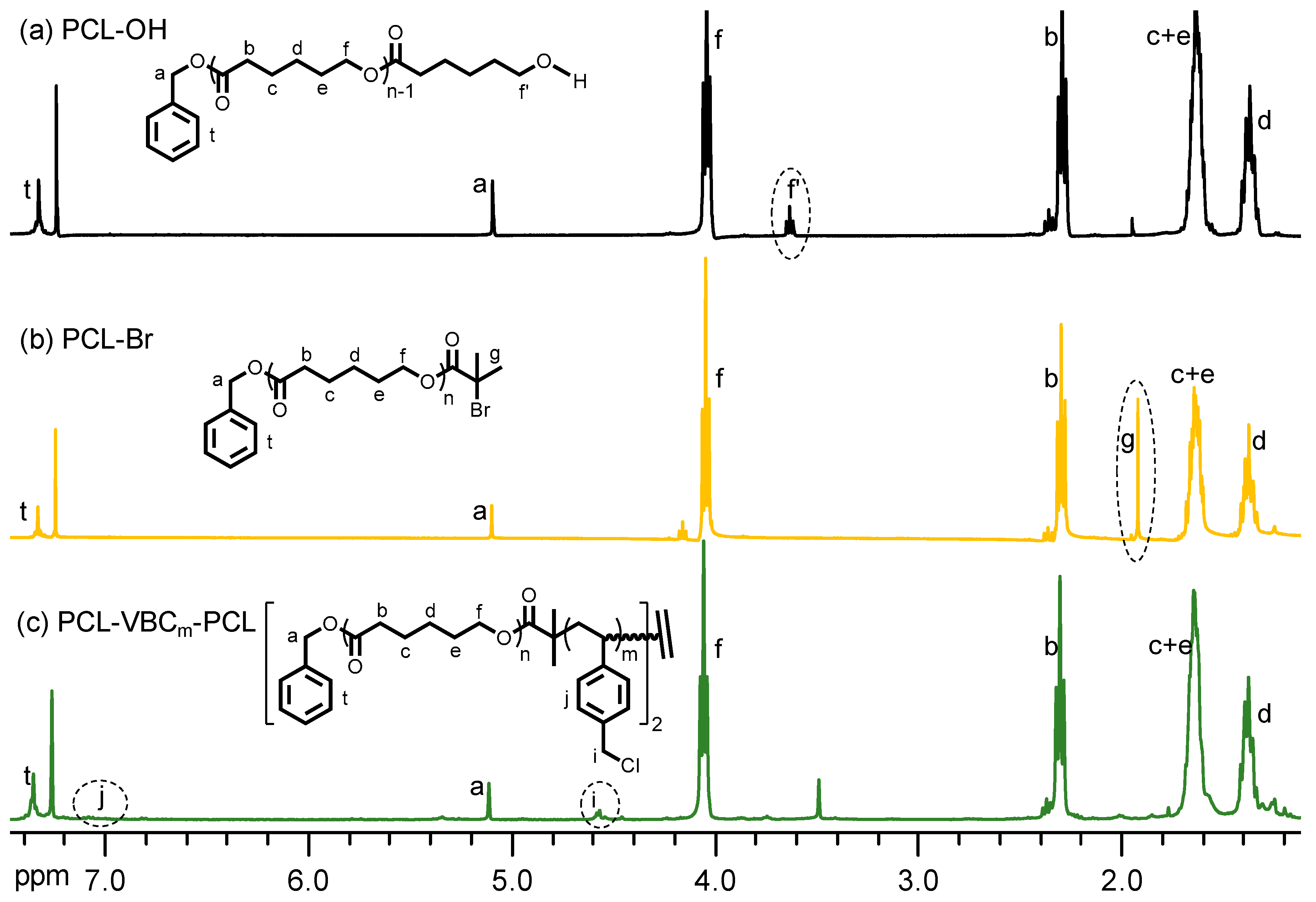
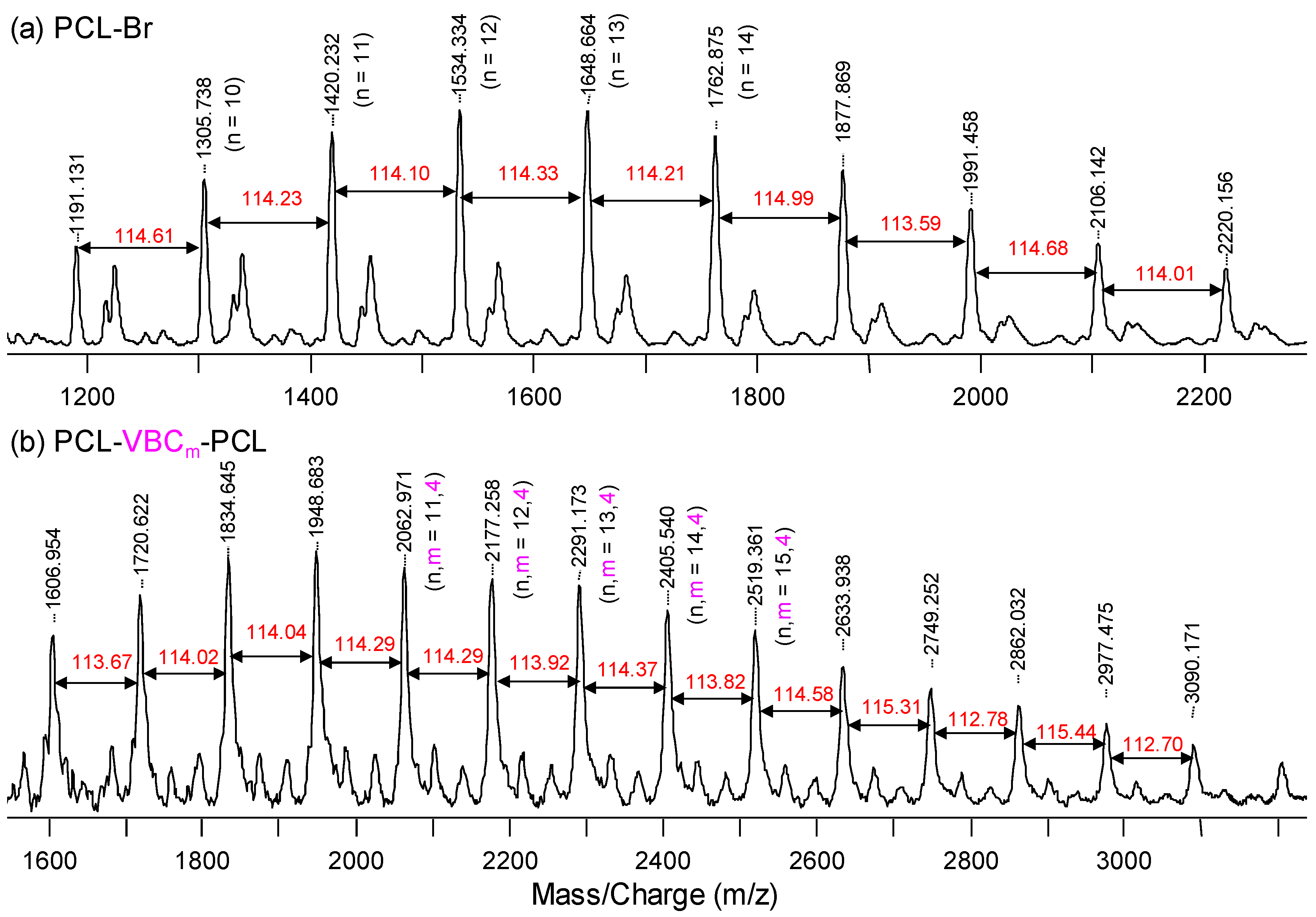

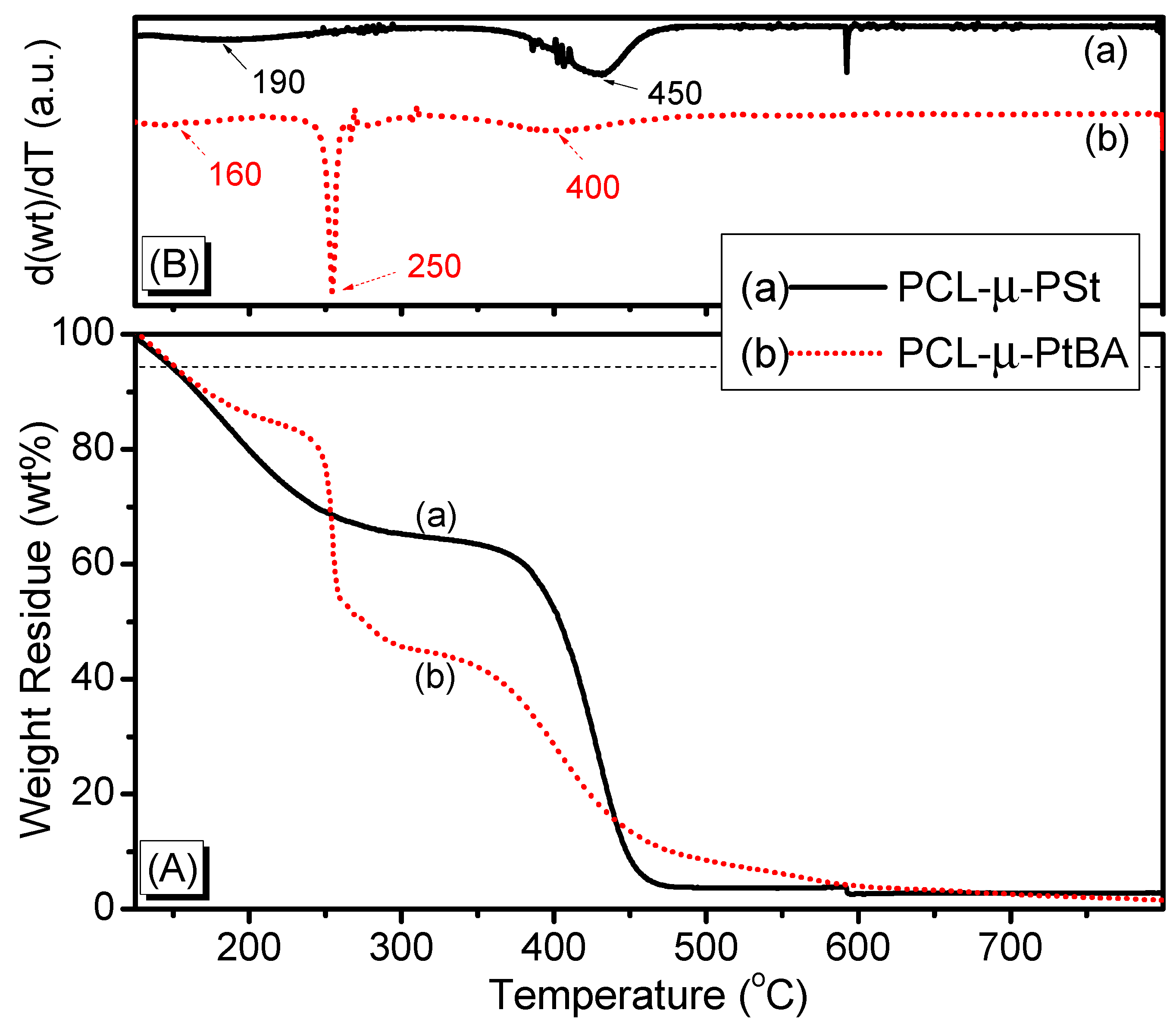
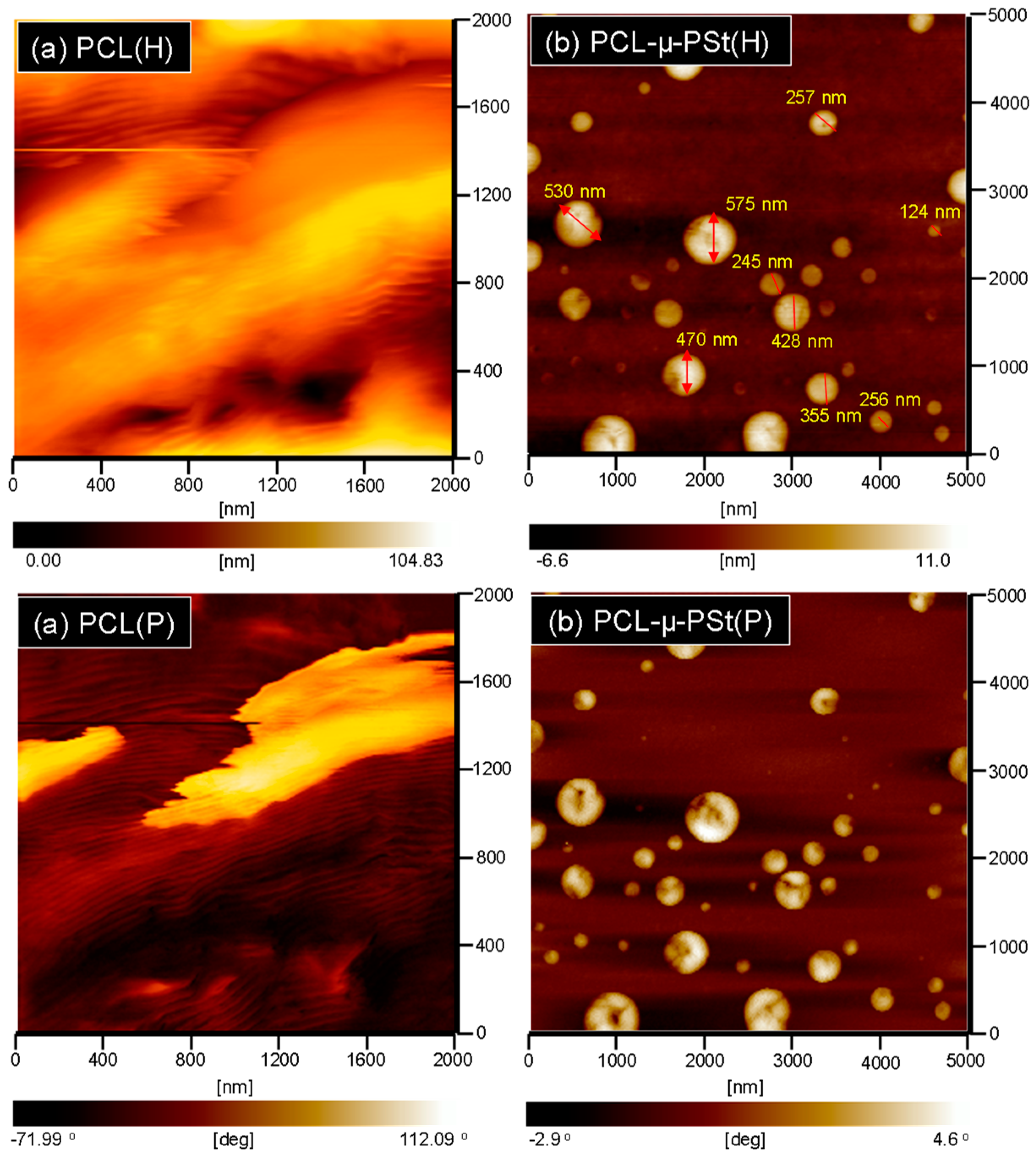
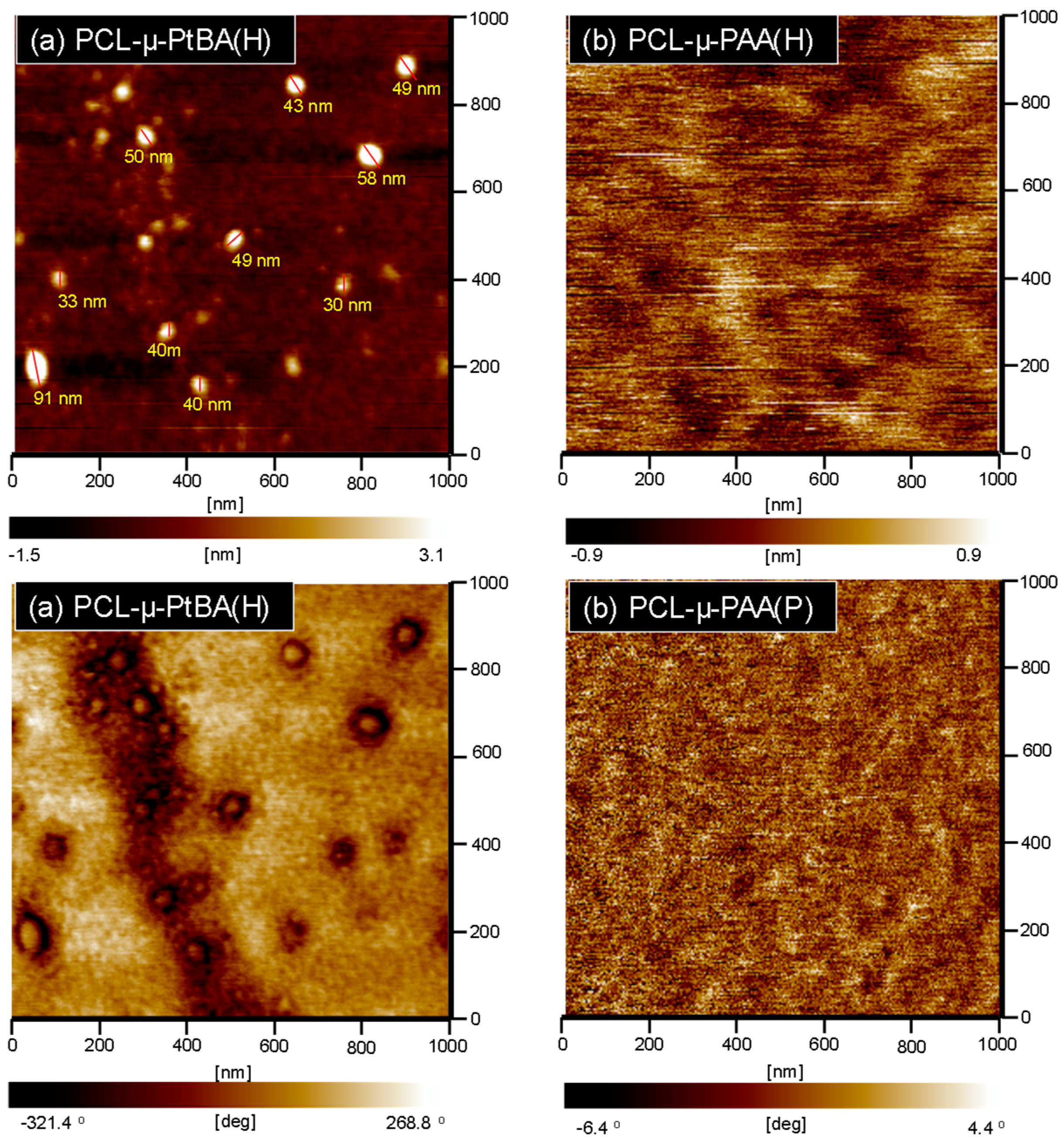
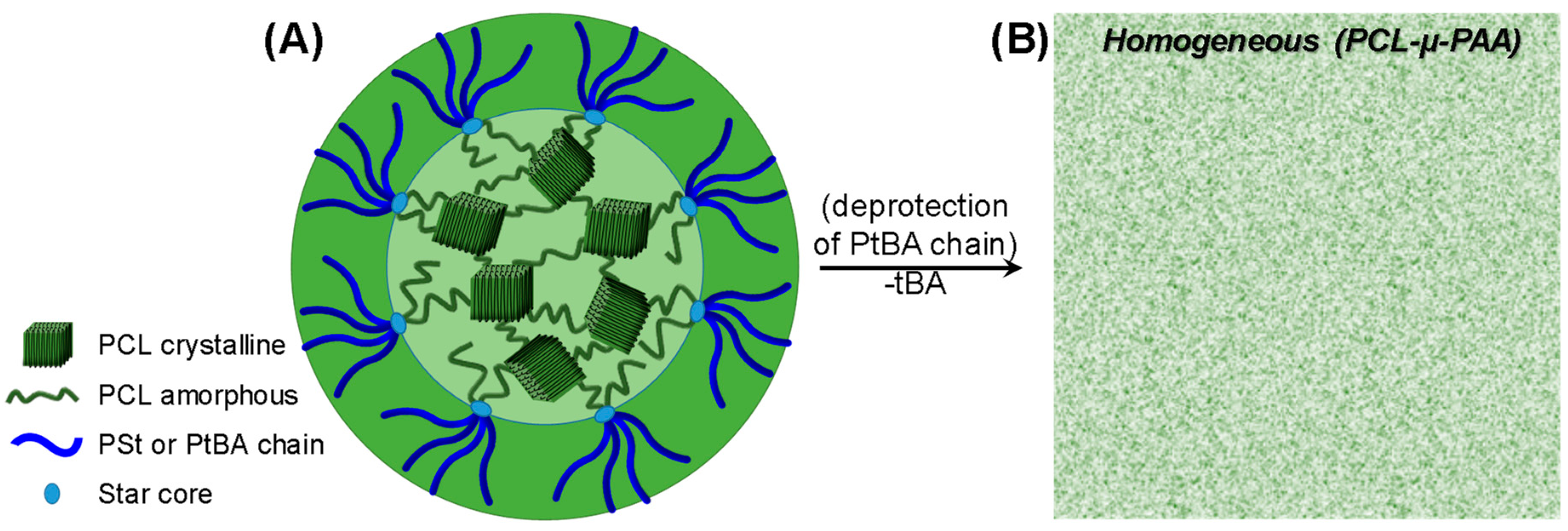
| Sample | Tg (°C) | Tm (°C) | xc (%) a | Td5% (°C) b | Char Yield (wt %) |
|---|---|---|---|---|---|
| First run | |||||
| PCL-μ-PSt | 81 | 60 | 5.5 | 150 | 2.8 |
| PCL-μ-PtBA | 40 | 55 | 2.6 | 151 | 1.2 |
| Second run | |||||
| PCL-μ-PSt | 80 | n.d. c | n.d. | – | – |
| PCL-μ-PtBA | 36 | n.d. | n.d. | – | – |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sathesh, V.; Chen, J.-K.; Chang, C.-J.; Aimi, J.; Chen, Z.-C.; Hsu, Y.-C.; Huang, Y.-S.; Huang, C.-F. Synthesis of Poly(ε-caprolactone)-Based Miktoarm Star Copolymers through ROP, SA ATRC, and ATRP. Polymers 2018, 10, 858. https://doi.org/10.3390/polym10080858
Sathesh V, Chen J-K, Chang C-J, Aimi J, Chen Z-C, Hsu Y-C, Huang Y-S, Huang C-F. Synthesis of Poly(ε-caprolactone)-Based Miktoarm Star Copolymers through ROP, SA ATRC, and ATRP. Polymers. 2018; 10(8):858. https://doi.org/10.3390/polym10080858
Chicago/Turabian StyleSathesh, Venkatesan, Jem-Kun Chen, Chi-Jung Chang, Junko Aimi, Zong-Cheng Chen, Yu-Chih Hsu, Yi-Shen Huang, and Chih-Feng Huang. 2018. "Synthesis of Poly(ε-caprolactone)-Based Miktoarm Star Copolymers through ROP, SA ATRC, and ATRP" Polymers 10, no. 8: 858. https://doi.org/10.3390/polym10080858