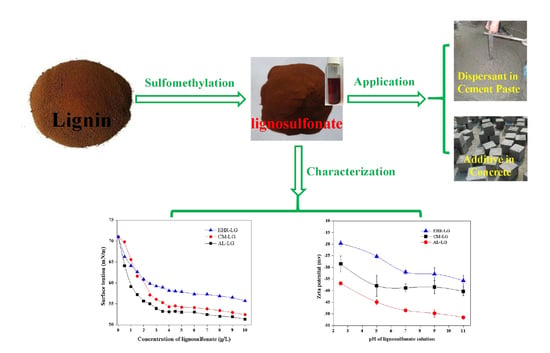
Preparation of Lignosulfonates from Biorefinery Lignins by Sulfomethylation and Their Application as a Water Reducer for Concrete
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
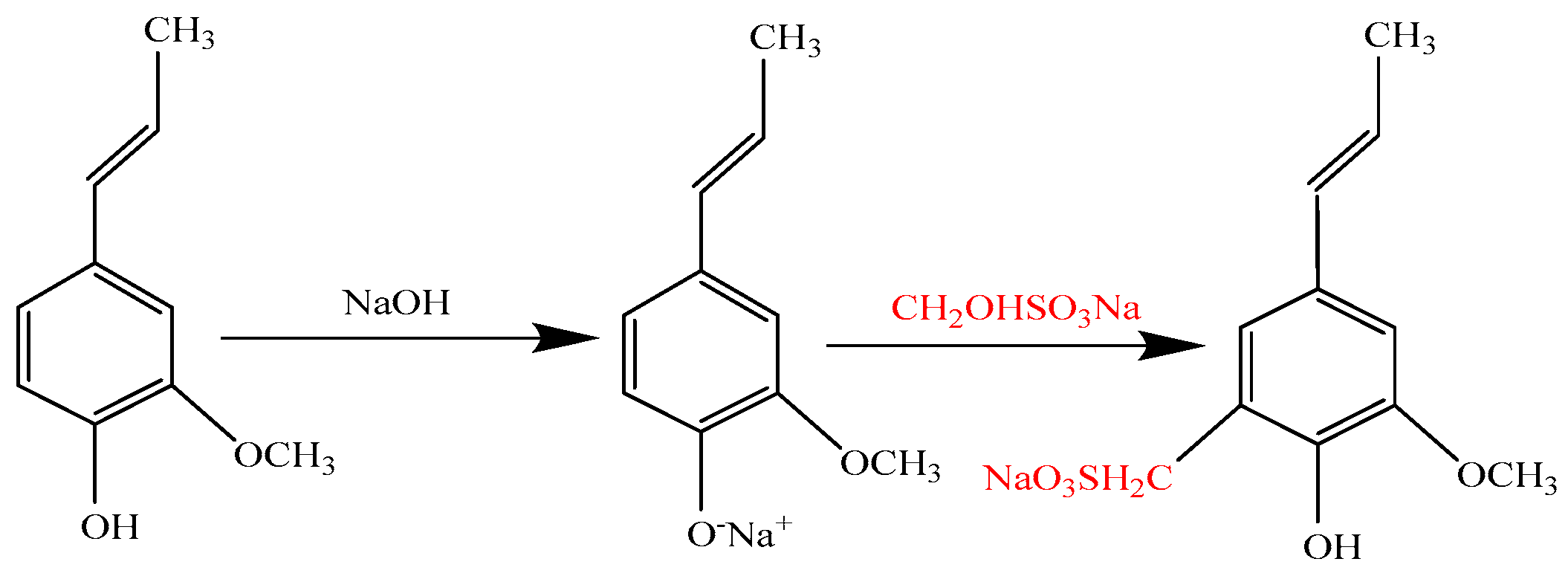
2.2. Sulfomethylation of Alkaline Lignin (AL) and Enzymatic Hydrolysis Residue (EHR)
2.3. Measurement of Molecular Weight
2.4. Sulfonic Group Analysis
2.5. Measurement of Surface Tension
2.6. Zeta Potential Analysis
2.7. Effects of Lignosulfonate on Performance of Cement Paste and Concrete
3. Results and Discussion
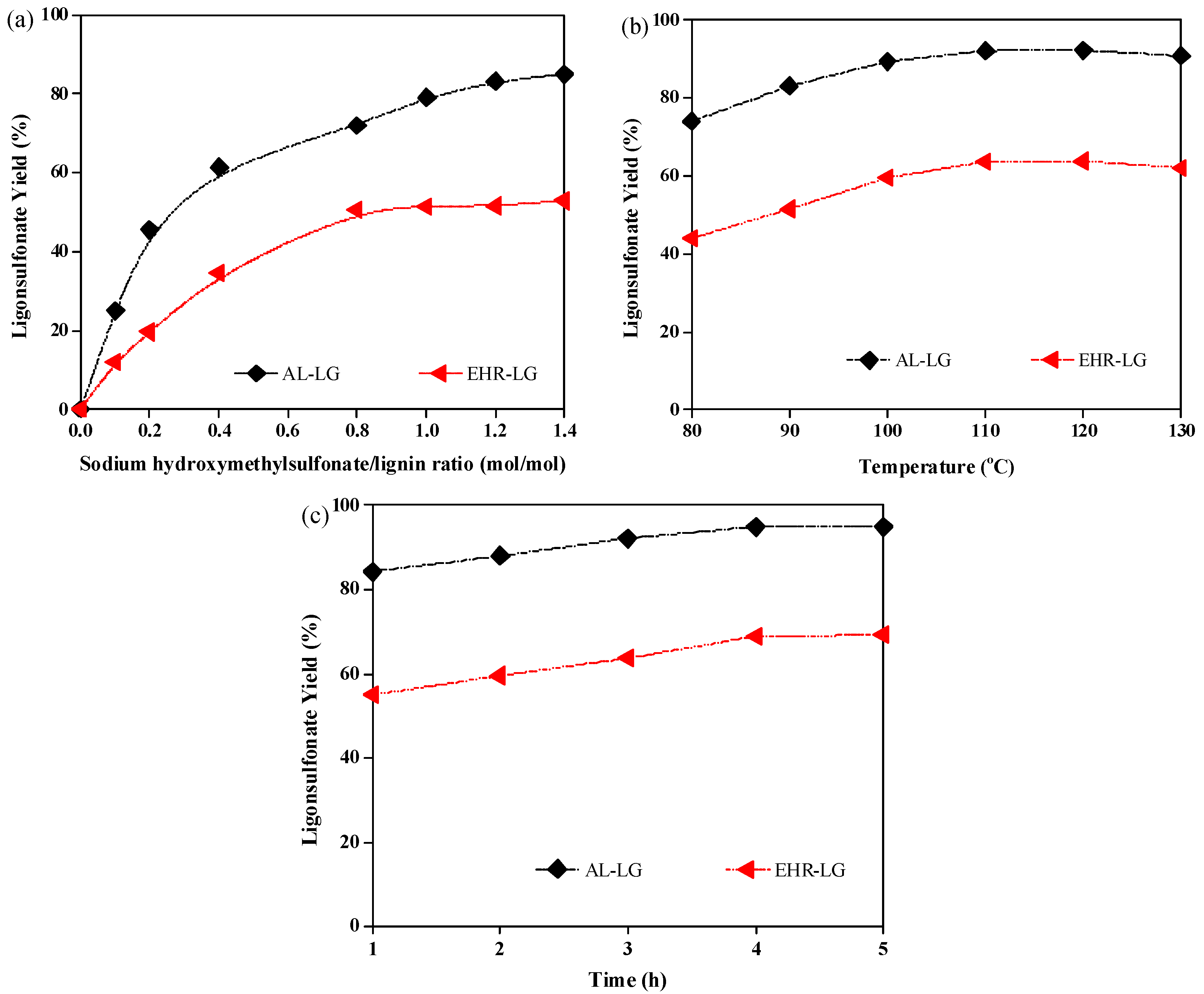
3.1. Effect of Sulfomethylation on Lignosulfonate Production from AL and EHR
3.2. Molecular Weight and Sulfonic Group of Lignosulfonate
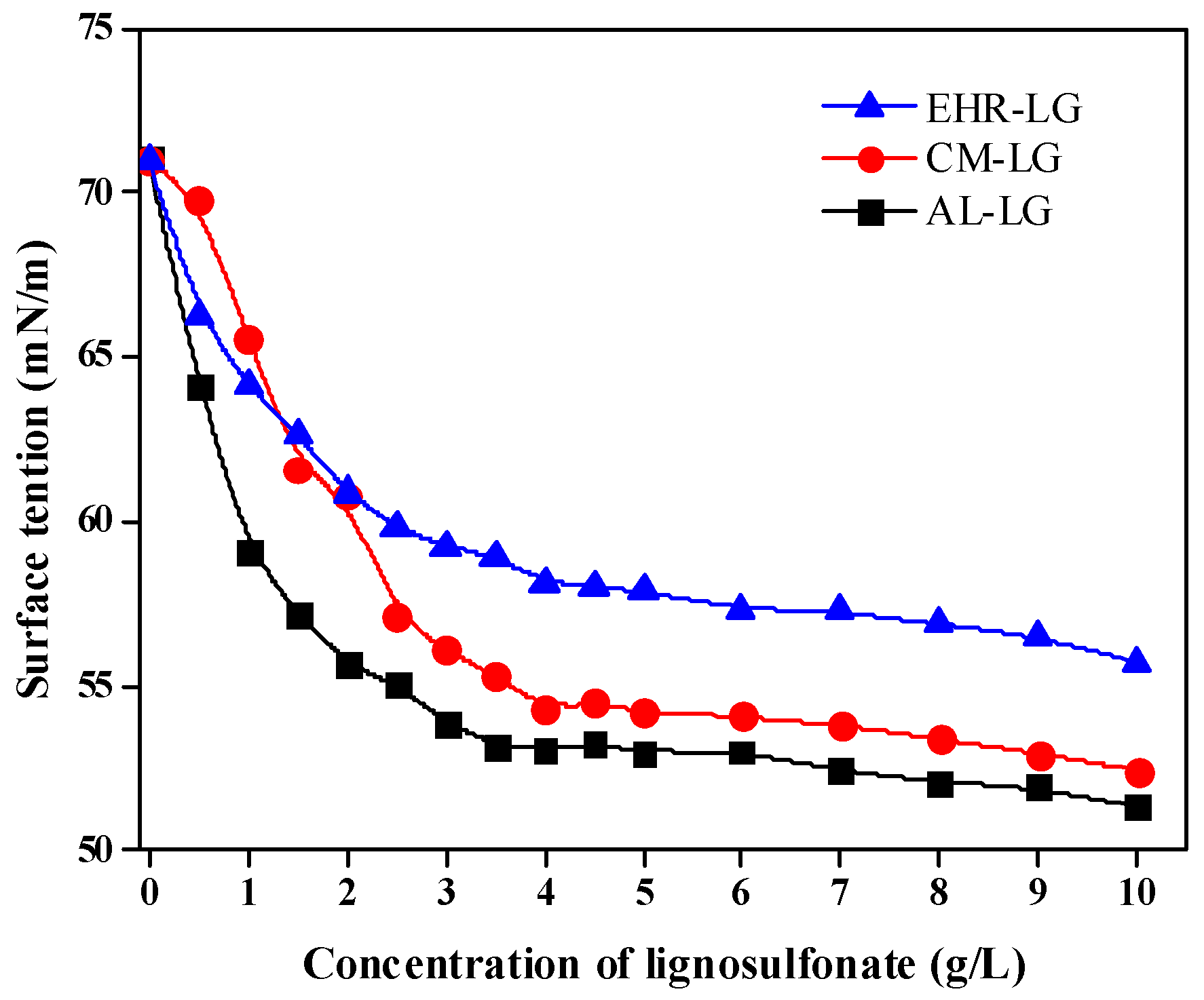
3.3. Surface Tension of Lignosulfonate Solutions
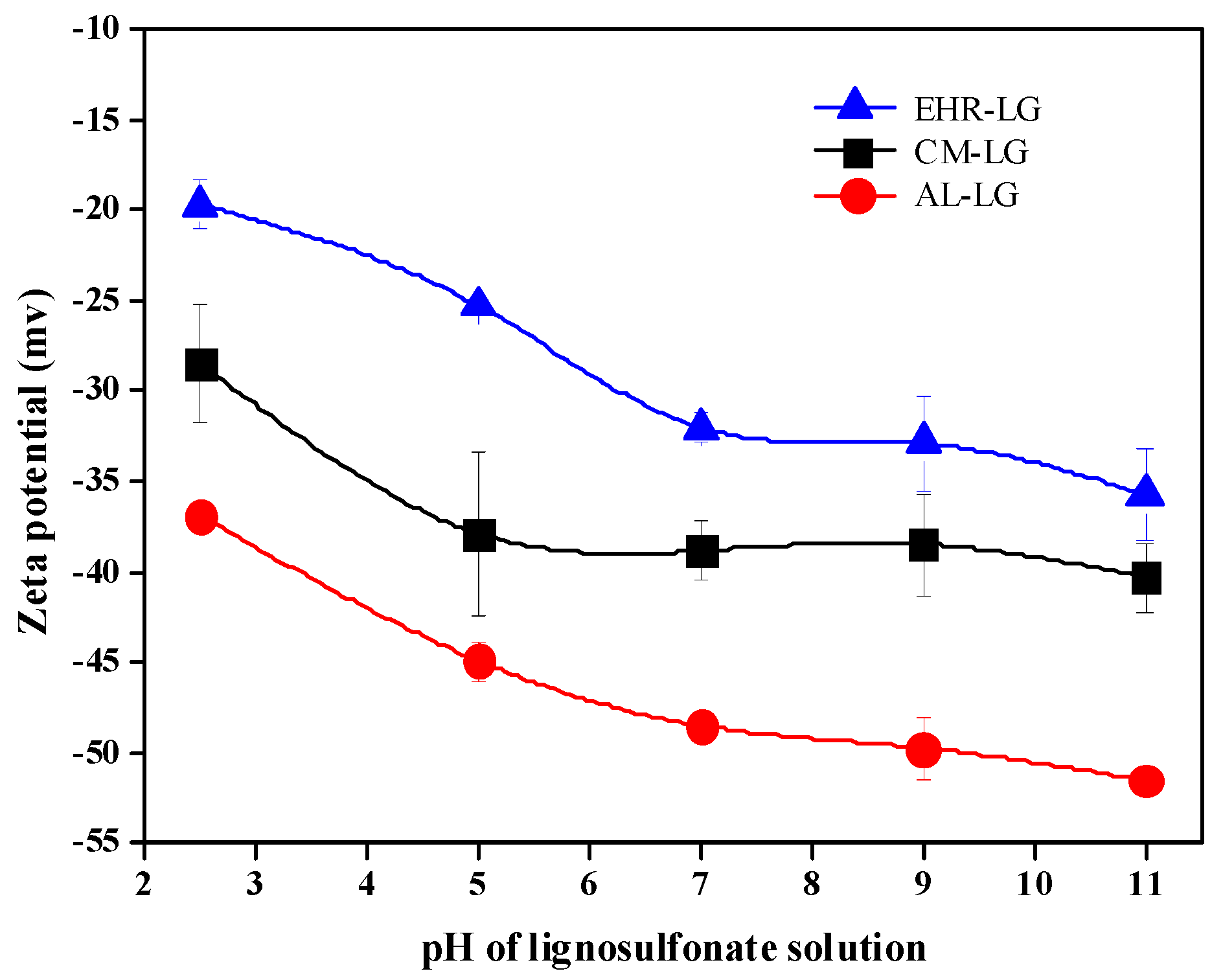
3.4. Zeta Potential of Lignosulfonate Solutions
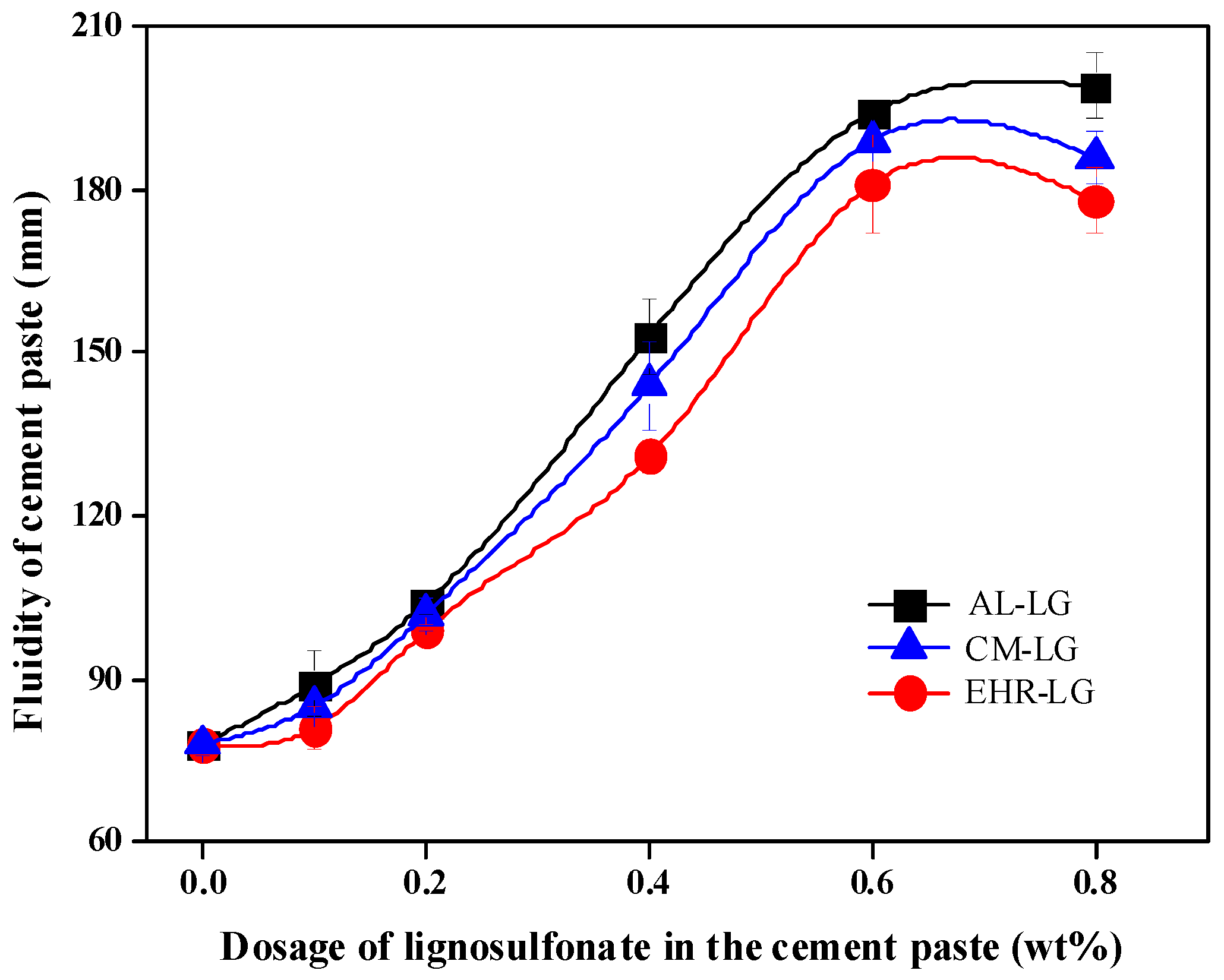
3.5. Evaluation of Lignosulfonates as Cement Paste Dispersants
3.6. Effect of Lignosulfonate on the Concrete Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wen, J.; Chen, T.; Sun, R. Research progress on separation and structural analysis of lignin in lignocellulosic biomass. J. For. Eng. 2017, 2, 76–84. [Google Scholar]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in green polymer composites from lignin for multifunctional applications: A review. ACS Sustain Chem. Eng. 2014, 2, 1072–1092. [Google Scholar]
- Jiang, X.; Liu, J.; Du, X.; Hu, Z.; Chang, H.M.; Jameel, H. Phenolation to improve lignin reactivity toward thermosets application. ACS Sustain Chem. Eng. 2018, 6, 5504–5512. [Google Scholar] [CrossRef]
- Galkin, M.V.; Samec, J.S. Lignin valorization through catalytic lignocellulose fractionation: A fundamental platform for the future biorefinery. ChemSusChem 2016, 9, 1544–1558. [Google Scholar] [PubMed]
- Tian, D.; Hu, J.; Bao, J.; Chandra, R.P.; Saddler, J.N.; Lu, C. Lignin valorization: Lignin nanoparticles as high-value bio-additive for multifunctional nanocomposites. Biotechnol. Biofuels 2017, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Xin, D.L.; Yang, Z.; Liu, F.; Xu, X.R.; Zhang, J.H. Comparison of aqueous ammonia and dilute acid pretreatment of bamboo fractions: Structure properties and enzymatic hydrolysis. Bioresour. Technol. 2015, 175, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; He, J.; Li, X.; Min, D.; Yong, Q. Facilitating the enzymatic saccharification of pulped bamboo residues by degrading the remained xylan and lignin-carbohydrates complexes. Bioresour. Technol. 2015, 192, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Yang, L.F.; Jin, Y.C.; Han, Q.; Chang, H.M.; Jameel, H.; Phillips, R. Green liquor pretreatment for improving enzymatic hydrolysis of corn stover. Bioresour. Technol. 2012, 124, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Hao, X.X.; Hou, X.C.; Zhang, J.H. Effect of alkaline sulfite pretreatment on enzymatic hydrolysis of hybrid China wolftailgrass. J. For. Eng. 2018, 3, 95–101. [Google Scholar]
- Huang, C.; Jeuck, B.; Yong, Q. Using pretreatment and enzymatic saccharification technologies to produce fermentable sugars from agricultural wastes. In Waste Biomass Management-A Holistic Approach; Springer: Cham, Switzerland, 2017; pp. 15–38. [Google Scholar]
- Geng, W.; Jin, Y.; Jameel, H.; Park, S. Strategies to achieve high-solids enzymatic hydrolysis of dilute-acid pretreated corn stover. Bioresour. Technol. 2015, 187, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Bahl, K.; Miyoshi, T.; Jana, S.C. Hybrid fillers of lignin and carbon black for lowering of viscoelastic loss in rubber compounds. Polymer 2014, 55, 3825–3835. [Google Scholar] [CrossRef]
- Upton, B.M.; Kasko, A.M. Strategies for the conversion of lignin to high-value polymeric materials: Review and perspective. Chem. Rev. 2015, 116, 2275–2306. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.X.; Fang, L.Y.; Lai, C.H.; Tang, S.; Liang, C.; Li, X.; Yong, Q. Effects of different pretreatments on antioxidant activities of Moso bamboo lignin. J. For. Eng. 2018, 3, 73–80. [Google Scholar]
- Zhu, J.Y.; Chandra, M.S.; Gu, F.; Gleisner, R.; Reiner, R.; Sessions, J.; Anderson, D. Using sulfite chemistry for robust bioconversion of Douglas-fir forest residue to bioethanol at high titer and lignosulfonate: A pilot-scale evaluation. Bioresour. Technol. 2015, 179, 390–397. [Google Scholar] [PubMed]
- Qin, Y.; Yu, L.; Wu, R.; Yang, D.; Qiu, X.; Zhu, J. Biorefinery lignosulfonates from sulfite-pretreated softwoods as dispersant for graphite. ACS Sustain Chem. Eng. 2016, 4, 2200–2205. [Google Scholar] [CrossRef]
- Konduri, M.K.; Fatehi, P. Production of water-soluble hardwood kraft lignin via sulfomethylation using formaldehyde and sodium sulfite. ACS Sustain Chem. Eng. 2015, 3, 1172–1182. [Google Scholar] [CrossRef]
- He, W.; Fatehi, P. Preparation of sulfomethylated softwood kraft lignin as a dispersant for cement admixture. RSC Adv. 2015, 5, 47031–47039. [Google Scholar] [CrossRef]
- Ouyang, X.; Qiu, X.; Chen, P. Physicochemical characterization of calcium lignosulfonate—A potentially useful water reducer. Colloid Surf. A-Physicochem. Eng. Asp. 2006, 282–283, 489–497. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, X.; Tang, X.; Xu, Y. Process for calcium xylonate production as a concrete admixture derived from in-situ fermentation of wheat straw pre-hydrolysate. Bioresour. Technol. 2018, 261, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Mario, M. Water reducers/retarders. In Concrete Admixtures Handbook (Second Edition); William Andrew Publishing: Norwich, NY, USA, 1996; pp. 286–409. [Google Scholar]
- Yan, M.; Yang, D.; Deng, Y.; Chen, P.; Zhou, H.; Qiu, X. Influence of pH on the behavior of lignosulfonate macromolecules in aqueous solution. Colloid Surf. A-Physicochem. Eng. Asp. 2010, 371, 50–58. [Google Scholar] [CrossRef]
- Ouyang, X.; Ke, L.; Qiu, X.; Guo, Y.; Pang, Y. Sulfonation of alkali lignin and its potential use in dispersant for cement. J. Disper. Sci. Technol. 2009, 30, 1–6. [Google Scholar] [CrossRef]
- Huang, C.; He, J.; Chang, H.M.; Jameel, H.; Yong, Q. Coproduction of ethanol and lignosulfonate from Moso bamboo residues by fermentation and sulfomethylation. Waste Biomass Valori. 2017, 8, 965–974. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP); Technical Report NREL/TP-510-42618; National Renewable Energy Laboratory (NREL), U.S. Dept. of Energy: Colorado, CO, USA, 2011.
- Pang, Y.X.; Qiu, X.Q.; Yang, D.J.; Lou, H.M. Influence of oxidation, hydroxymethylation and sulfomethylation on the physicochemical properties of calcium lignosulfonate. Colloid Surf. A-Physicochem. Eng. Asp. 2008, 312, 154–159. [Google Scholar] [CrossRef]
- Wu, H.; Chen, F.; Feng, Q.; Yue, X. Oxidation and sulfomethylation of alkali-extracted lignin from corn stalk. BioResources 2012, 7, 2742–2751. [Google Scholar]
- Matsushita, Y.; Yasuda, S. Preparation and evaluation of lignosulfonates as a dispersant for gypsum paste from acid hydrolysis lignin. Bioresour. Technol. 2005, 96, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, Y.; Imai, M.; Iwatsuki, A.; Fukushima, K. The relationship between surface tension and the industrial performance of water-soluble polymers prepared from acid hydrolysis lignin, a saccharification by-product from woody materials. Bioresour. Technol. 2008, 99, 3024–3028. [Google Scholar] [PubMed]
- Briscoe, W.H.; Horn, R.G. Direct measurement of surface forces due to charging of solids immersed in a nonpolar liquid. Langmuir 2002, 18, 3945–3956. [Google Scholar] [CrossRef]
- Lewis, J.A.; Matsuyama, H.; Kirby, G.; Morissette, S.; Young, J.F. Polyelectrolyte effects on the rheological properties of concentrated cement suspensions. J. Am. Ceram. Soc. 2000, 83, 1905–1913. [Google Scholar] [CrossRef]
- Felekoğlu, B.; Sarıkahya, H. Effect of chemical structure of polycarboxylate-based superplasticizers on workability retention of self-compacting concrete. Constr. Build. Mater. 2008, 22, 1972–1980. [Google Scholar]
- Jolicoeur, C.; Simard, M.A. Chemical admixture-cement interactions: Phenomenology and physico-chemical concepts. Cement Concrete Comp. 1998, 20, 87–101. [Google Scholar] [CrossRef]
| Lignosulfonate | Mw (g/mol) | Mn (g/mol) | PDI 2 | Sulfonic Group (mmol/g) |
|---|---|---|---|---|
| AL-LG | 15,470 | 6120 | 2.5 | 1.6 |
| EHR-LG | 10,230 | 4440 | 2.3 | 1.0 |
| CM-LG | 12,180 | 3060 | 4.0 | 1.5 |
| Lignosulfonate | Dosage (wt %) 1 | Water Reduction (%) | Air Content (%) | Compressive Strength (Mpa) | ||
|---|---|---|---|---|---|---|
| 7 Days | 14 Days | 28 Days | ||||
| None 2 | / | / | / | 26.7 | 33.4 | 38.4 |
| AL-LG | 0.1 | 5.8 | 2.1 | 28.5 | 36.3 | 40.9 |
| 0.2 | 10.6 | 4.0 | 29.5 | 35.2 | 41.6 | |
| 0.3 | 13.6 | 6.2 | 24.5 | 34.2 | 36.4 | |
| EHR-LG | 0.1 | 3.8 | 2.0 | 28.7 | 36.4 | 39.7 |
| 0.2 | 6.0 | 3.0 | 26.4 | 36.6 | 42.6 | |
| 0.3 | 14.1 | 5.3 | 26.6 | 33.6 | 38.2 | |
| CM-LG | 0.1 | 1.6 | 2.3 | 27.9 | 34.4 | 38.0 |
| 0.2 | 3.9 | 3.0 | 28.0 | 33.9 | 40.9 | |
| 0.3 | 6.8 | 5.0 | 24.2 | 35.3 | 37.8 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Ma, J.; Zhang, W.; Huang, G.; Yong, Q. Preparation of Lignosulfonates from Biorefinery Lignins by Sulfomethylation and Their Application as a Water Reducer for Concrete. Polymers 2018, 10, 841. https://doi.org/10.3390/polym10080841
Huang C, Ma J, Zhang W, Huang G, Yong Q. Preparation of Lignosulfonates from Biorefinery Lignins by Sulfomethylation and Their Application as a Water Reducer for Concrete. Polymers. 2018; 10(8):841. https://doi.org/10.3390/polym10080841
Chicago/Turabian StyleHuang, Caoxing, Junmei Ma, Weiyu Zhang, Guohong Huang, and Qiang Yong. 2018. "Preparation of Lignosulfonates from Biorefinery Lignins by Sulfomethylation and Their Application as a Water Reducer for Concrete" Polymers 10, no. 8: 841. https://doi.org/10.3390/polym10080841