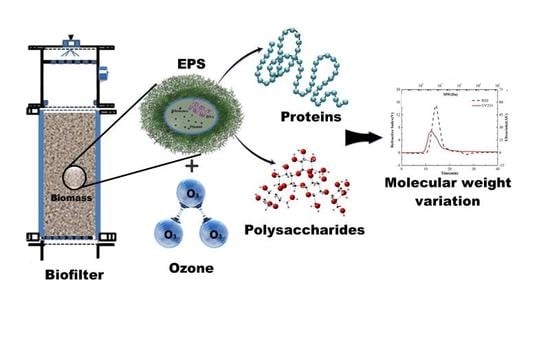
Ozonation and Depolymerization of Extracellular Polymeric Substances (EPS) Extracted from a Biofilter Treating Gaseous Toluene
Abstract
:1. Introduction
2. Material and Methods
2.1. Experimental Set-Up and Operation
2.2. EPS Collection and Isolation
2.3. Ozone Experiment Setup
2.4. Analytical Methods
2.4.1. Analysis of Polysaccharides, Protein and TOC
2.4.2. Analysis of the EEM Spectra of EPS
2.4.3. Analysis of Molecular Weight Distribution of EPS
2.4.4. Analysis of VOCs from the Ozone Reactor
3. Results and Discussions
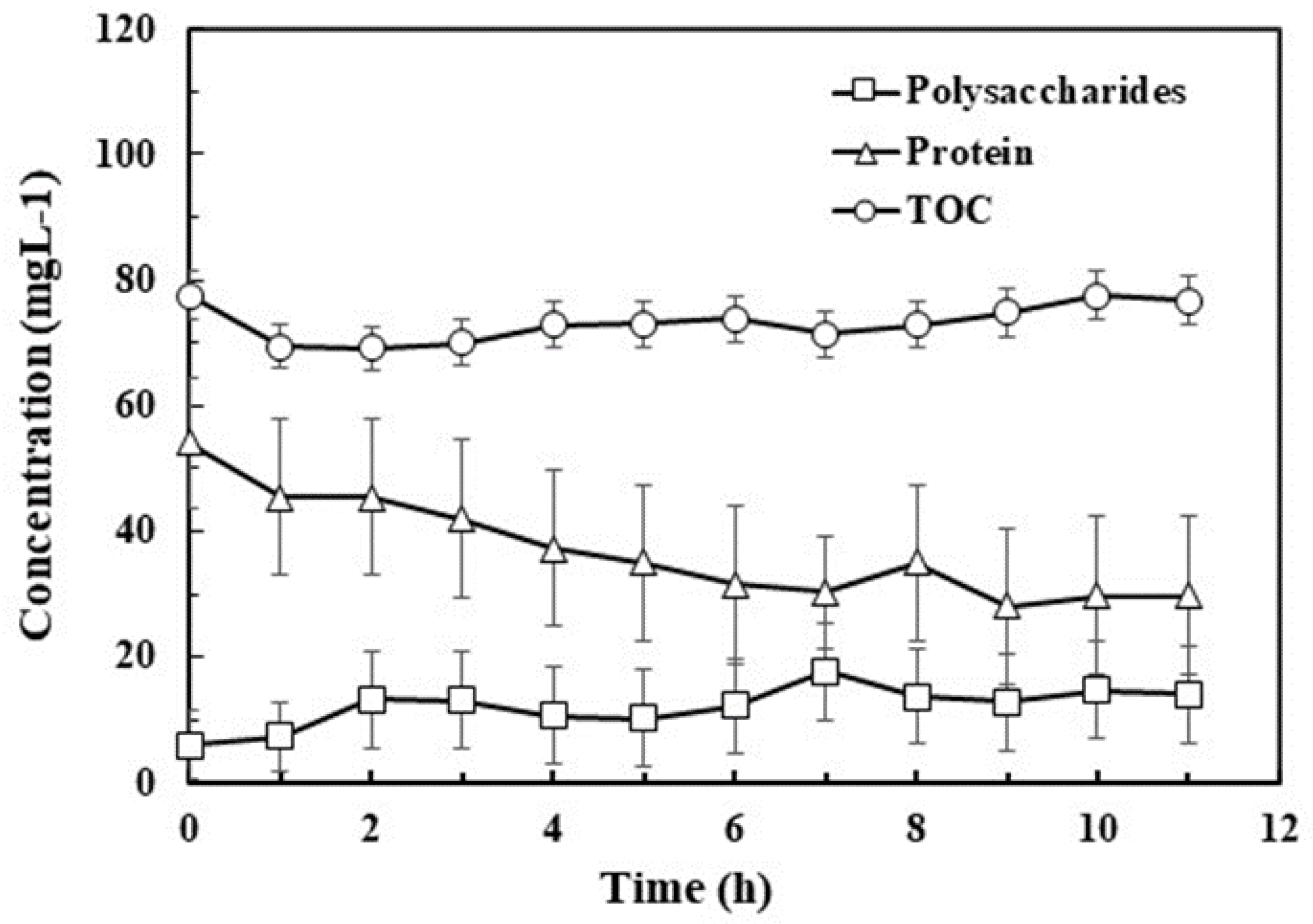
3.1. EPS Components Profile
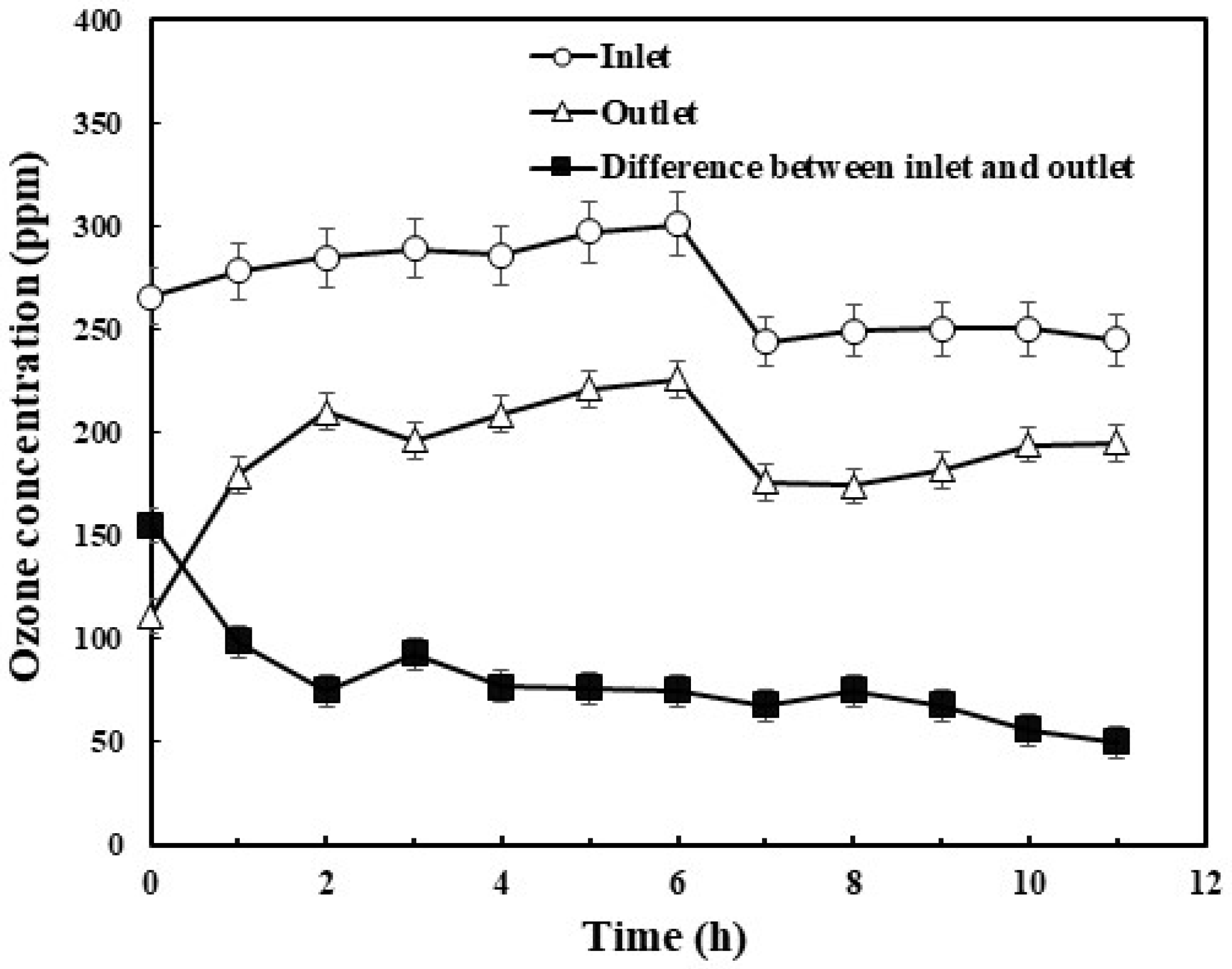
3.2. Utilization of Ozone
3.3. Effect of Ozone on Components of EPS
3.4. Influence of the Ozonation Process on Molecular Weight (MW) Distributions and Functional Groups of EPS
3.4.1. Molecular Weight (MW) Distribution
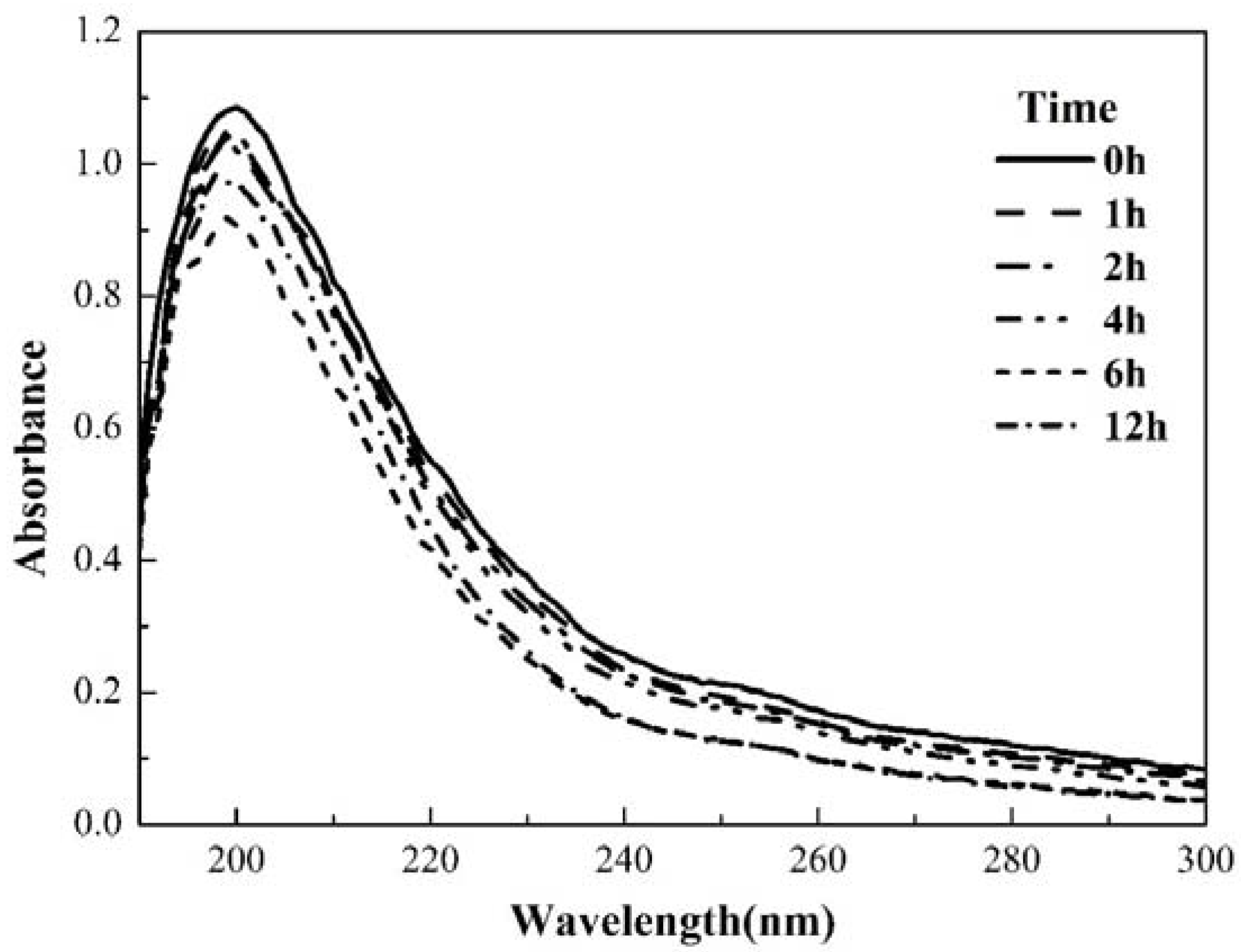
3.4.2. UV-Vis Absorption Spectra of EPS
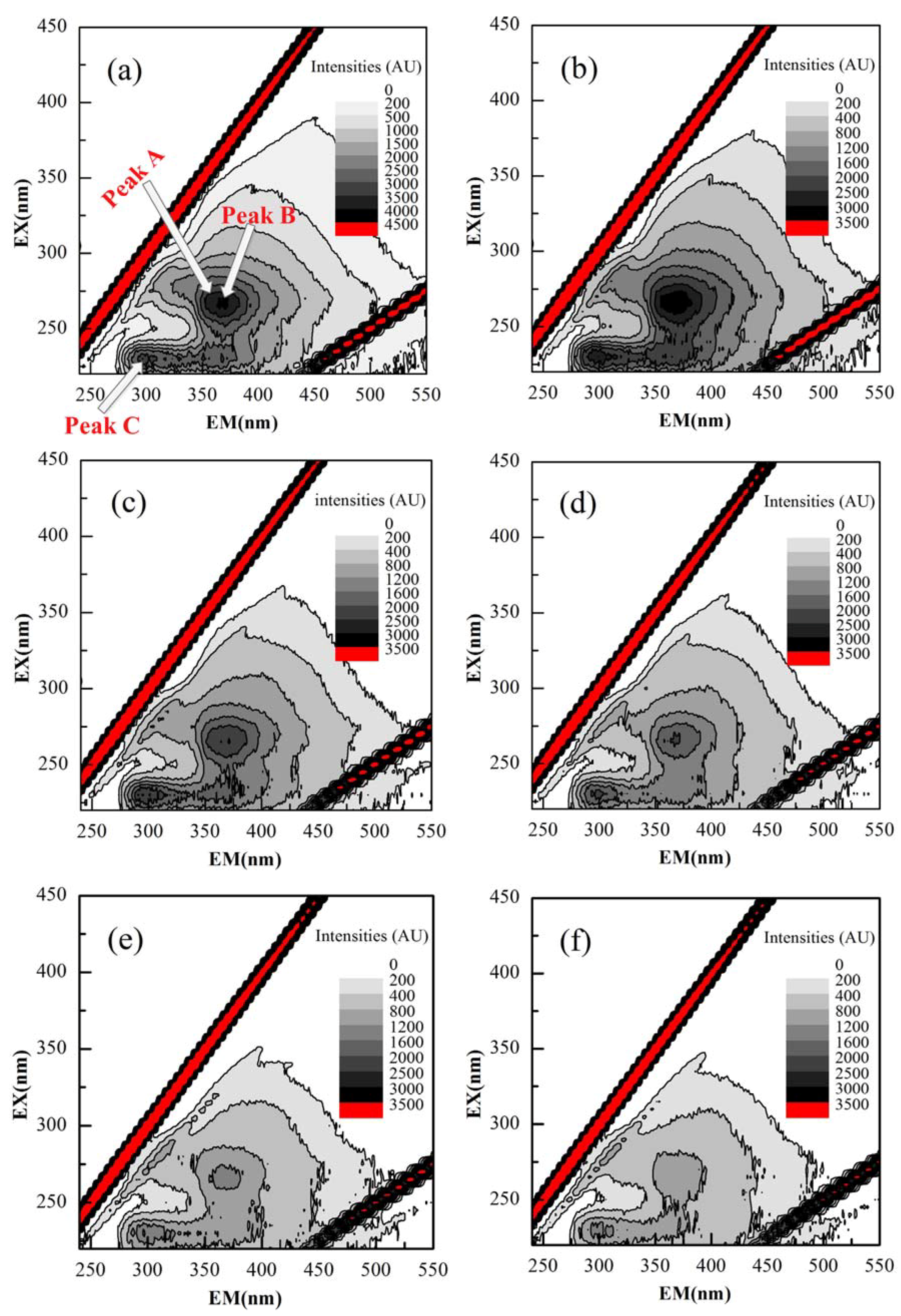
3.4.3. EEM Spectra of EPS
3.4.4. VOC Production under Ozone Exposure
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Malakar, S.; Saha, P.D.; Baskaran, D.; Rajamanickam, R. Microbial biofilter for toluene removal: Performance evaluation, transient operation and theoretical prediction of elimination capacity. Sustain. Environ. Res. 2018, 28, 121–127. [Google Scholar] [CrossRef]
- Oliver, J.P.; Janni, K.A.; Schilling, J.S. Bait and scrape: An approach for assessing biofilm microbial communities on organic media used for gas-phase biofiltration. Ecol. Eng. 2016, 91, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Bishop, P.L. Spatial distribution of extracellular polymeric substances in biofilms. J. Environ. Eng. 2001, 127, 850–856. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. Relevance of microbial extracellular polymeric substances (EPSs)-Part I: Structural and ecological aspects. Water Sci. Technol. 2001, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Gao, J.-M.; Chen, Y.-P.; Yan, P.; Dong, Y.; Shen, Y.; Guo, J.-S.; Zeng, N.; Zhang, P. Composition and aggregation of extracellular polymeric substances (EPS) in hyperhaline and municipal wastewater treatment plants. Sci. Rep. 2016, 6, 26721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, P.H.; Jahn, A.; Palmgren, R. Conceptual model for production and composition of exopolymers in biofilms. Water Sci. Technol. 1997, 36, 11–19. [Google Scholar] [CrossRef]
- Pannard, A.; Pédrono, J.; Bormans, M.; Briand, E.; Claquin, P.; Lagadeuc, Y. Production of exopolymers (EPS) by cyanobacteria: Impact on the carbon-to-nutrient ratio of the particulate organic matter. Aquat. Ecol. 2016, 50, 29–44. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Xi, J.; Yeung, M. Degradation of extracellular polymeric substances (EPS) extracted from activated sludge by low-concentration ozonation. Chemosphere 2016, 147, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, M.; Wang, Z.; She, Z.; Jin, C.; Zhao, Y.; Li, Z. The effects of divalent copper on performance, extracellular polymeric substances and microbial community of an anoxic-aerobic sequencing batch reactor. RSC Adv. 2015, 5, 30737–30747. [Google Scholar] [CrossRef]
- Hernández, J.; Gómez-Cuervo, S.; Omil, F. EPS and SMP as stability indicators during the biofiltration of diffuse methane emissions. Water Air Soil Pollut. 2015, 226, 343. [Google Scholar] [CrossRef]
- Dignac, M.-F.; Urbain, V.; Rybacki, D.; Bruchet, A.; Snidaro, D.; Scribe, P. Chemical description of extracellular polymers: Implication on activated sludge floc structure. Water Sci. Technol. 1998, 38, 45–53. [Google Scholar] [CrossRef]
- Park, C.; Novak, J.T. Characterization of activated sludge exocellular polymers using several cation-associated extraction methods. Water Res. 2007, 41, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. Relevance of microbial extracellular polymeric substances (EPSs)-Part II: Technical aspects. Water Sci. Technol. 2001, 43, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wu, J. Improvement of membrane filterability of the mixed liquor in a membrane bioreactor by ozonation. J. Membr. Sci. 2008, 318, 210–216. [Google Scholar] [CrossRef]
- García-Pérez, T.; Aizpuru, A.; Arriaga, S. By-passing acidification limitations during the biofiltration of high formaldehyde loads via the application of ozone pulses. J. Hazard. Mater. 2013, 262, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Diaz, G.; Arriaga, S. Biofiltration of high formaldehyde loads with ozone additions in long-term operation. Appl. Microbiol. Biotechnol. 2015, 99, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Saingam, P.; Gu, F.; Hu, H.-Y.; Zhao, X. Effect of continuous ozone injection on performance and biomass accumulation of biofilters treating gaseous toluene. Appl. Microbiol. Biotechnol. 2015, 99, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Braguglia, C.; Gianico, A.; Mininni, G. Comparison between ozone and ultrasound disintegration on sludge anaerobic digestion. J. Environ. Manag. 2012, 95, S139–S143. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-S.; Zhang, P.; Chen, Y.-P.; Shen, Y.; Hu, X.; Yan, P.; Yang, J.-X.; Fang, F.; Li, C.; Gao, X. Microbial attachment and adsorption–desorption kinetic of tightly bound extracellular polymeric substances on model organic surfaces. Chem. Eng. J. 2015, 279, 516–521. [Google Scholar] [CrossRef]
- Xu, Y.; Saingam, P.; Gu, F.; Xi, J.; Hu, H.-Y. The effect of injected ozone on the microbial metabolic characteristics in biofilters treating gaseous toluene. Ecol. Eng. 2016, 94, 174–181. [Google Scholar] [CrossRef]
- Frølund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 1996, 30, 1749–1758. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Chabaliná, L.D.; Pastor, M.R.; Rico, D.P. Characterization of soluble and bound EPS obtained from 2 submerged membrane bioreactors by 3D-EEM and HPSEC. Talanta 2013, 115, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.-M.; Ni, B.-J.; Seviour, T.; Sheng, G.-P.; Yu, H.-Q. Characterization of autotrophic and heterotrophic soluble microbial product (SMP) fractions from activated sludge. Water Res. 2012, 46, 6210–6217. [Google Scholar] [CrossRef] [PubMed]
- Hoigné, J.; Bader, H. Rate constants of reactions of ozone with organic and inorganic compounds in water—I: Non-dissociating organic compounds. Water Res. 1983, 17, 173–183. [Google Scholar] [CrossRef]
- Pan, G.; Chen, C.; Chang, H.; Gratzl, J. Model Experiments on the Splitting of Glycosidic Bonds by Ozone. In Proceedings of the International Symposium on Wood and Pulping Chemistry, Stockholm, Sweden, 9–12 June 1981; pp. 132–144. [Google Scholar]
- Wang, Y.; Hollingsworth, R.I.; Kasper, D.L. Ozonolysis for selectively depolymerizing polysaccharides containing β-d-aldosidic linkages. Proc. Natl. Acad. Sci. USA 1998, 95, 6584–6589. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.-Y.; Wang, X.-M.; Li, X.-Y. Effect of biopolymer clusters on the fouling property of sludge from a membrane bioreactor (MBR) and its control by ozonation. Process Biochem. 2011, 46, 162–167. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.K.; Graham, N.J. Oxidation of amino acids, peptides and proteins by ozone: A review. Ozone Sci. Eng. 2010, 32, 81–90. [Google Scholar] [CrossRef]
- Pryor, W.A.; Giamalva, D.H.; Church, D.F. Kinetics of ozonation. 2. Amino acids and model compounds in water and comparisons to rates in nonpolar solvents. J. Am. Chem. Soc. 1984, 106, 7094–7100. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation—Emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef] [PubMed]
- Le Lacheur, R.M.; Glaze, W.H. Reactions of ozone and hydroxyl radicals with serine. Environ. Sci. Technol. 1996, 30, 1072–1080. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Ziółek, M.; Nawrocki, J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl. Catal. B Environ. 2003, 46, 639–669. [Google Scholar] [CrossRef]
- Świetlik, J.; Dąbrowska, A.; Raczyk-Stanisławiak, U.; Nawrocki, J. Reactivity of natural organic matter fractions with chlorine dioxide and ozone. Water Res. 2004, 38, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Katai, A.A.; Schuerch, C. Mechanism of ozone attack on α-methyl glucoside and cellulosic materials. J. Polym. Sci. Part A 1966, 4, 2683–2703. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baig, Z.T.; Meng, L.; Saingam, P.; Xi, J. Ozonation and Depolymerization of Extracellular Polymeric Substances (EPS) Extracted from a Biofilter Treating Gaseous Toluene. Polymers 2018, 10, 763. https://doi.org/10.3390/polym10070763
Baig ZT, Meng L, Saingam P, Xi J. Ozonation and Depolymerization of Extracellular Polymeric Substances (EPS) Extracted from a Biofilter Treating Gaseous Toluene. Polymers. 2018; 10(7):763. https://doi.org/10.3390/polym10070763
Chicago/Turabian StyleBaig, Zenab Tariq, Lu Meng, Prakit Saingam, and Jinying Xi. 2018. "Ozonation and Depolymerization of Extracellular Polymeric Substances (EPS) Extracted from a Biofilter Treating Gaseous Toluene" Polymers 10, no. 7: 763. https://doi.org/10.3390/polym10070763