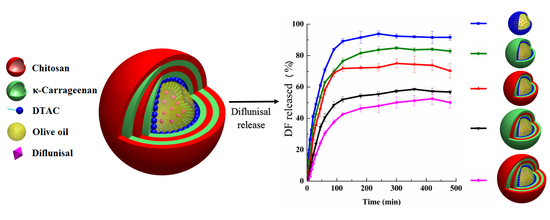
Drug Release Properties of Diflunisal from Layer-By-Layer Self-Assembled κ-Carrageenan/Chitosan Nanocapsules: Effect of Deposited Layers
Abstract
:1. Introduction
2. Materials and Methods
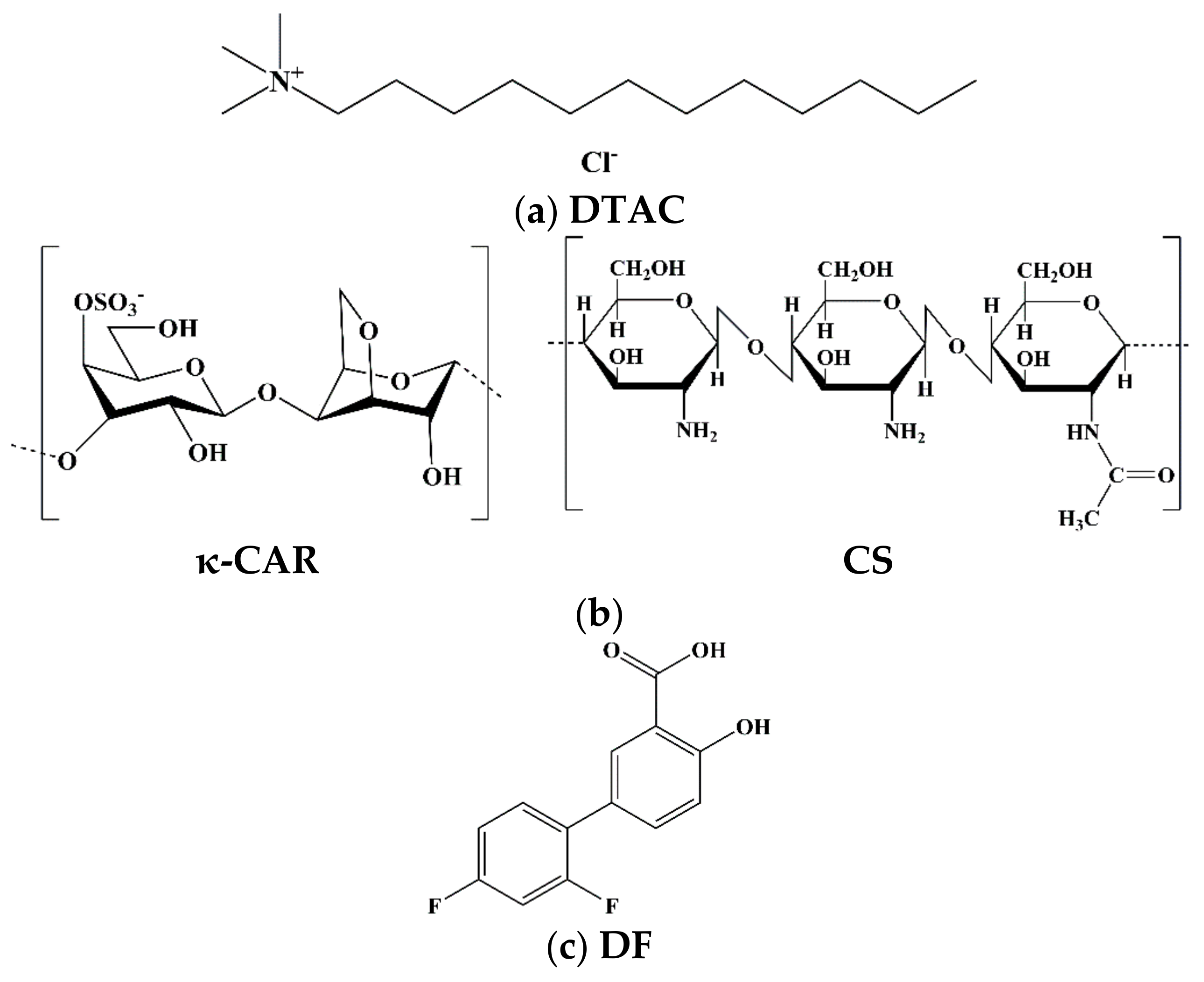
2.1. Materials
2.2. Formulation of Nanoemulsions (NE)
2.3. Preparation of NE(κ-CAR/CS)x Nanocapsules by Layer-By-Layer Self-Assembly
2.4. Characterization of NE and NE(κ-CAR/CS)x Nanocapsules
2.4.1. Particle-Size and Zeta Potential Measurements
2.4.2. Morphological Analyses
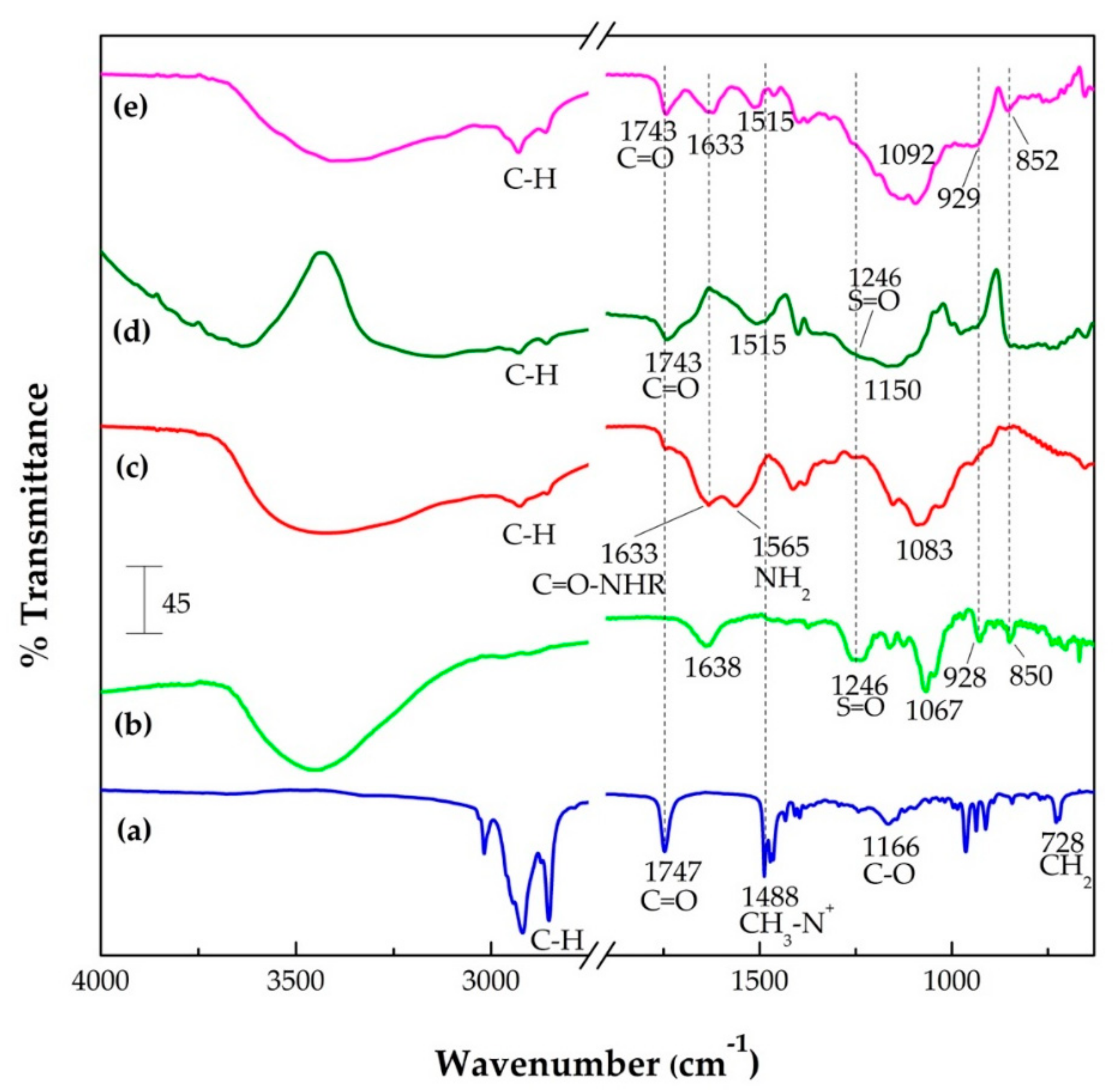
2.4.3. Fourier Transform Infrared (FTIR) Spectroscopy
2.5. Drug Release
2.5.1. Encapsulation Efficiency
2.5.2. In Vitro Drug Release
2.6. Mathematical Modelling
2.6.1. Korsmeyer-Peppas Model
2.6.2. Higuchi Model
2.6.3. First Order Kinetic Model
2.6.4. Zero Order Kinetic Model
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of NE and NE(κ-CAR/CS)x Oil-Core Nanocapsules
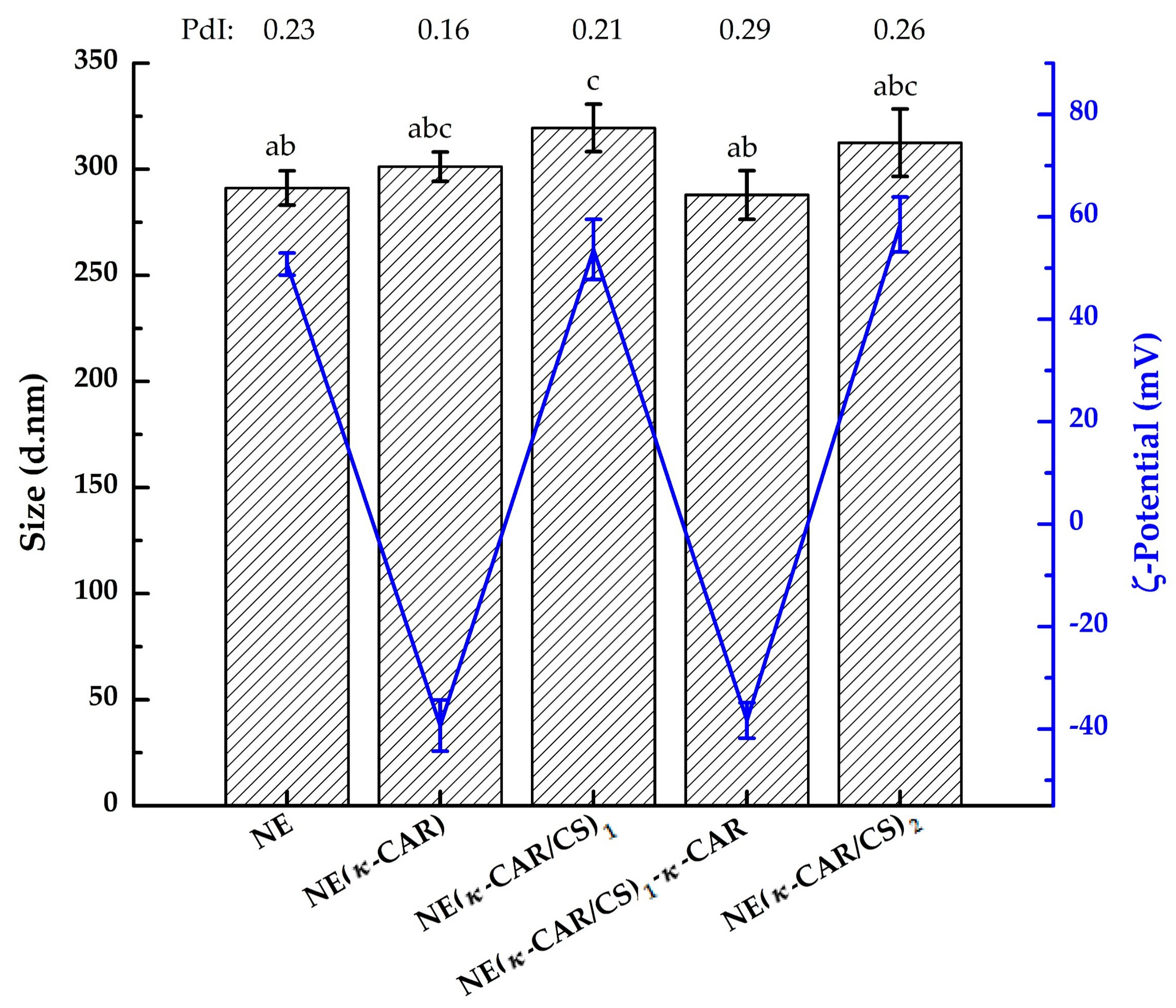
3.1.1. Particle Size
3.1.2. Zeta Potential Measurements
3.1.3. Morphological Analyses
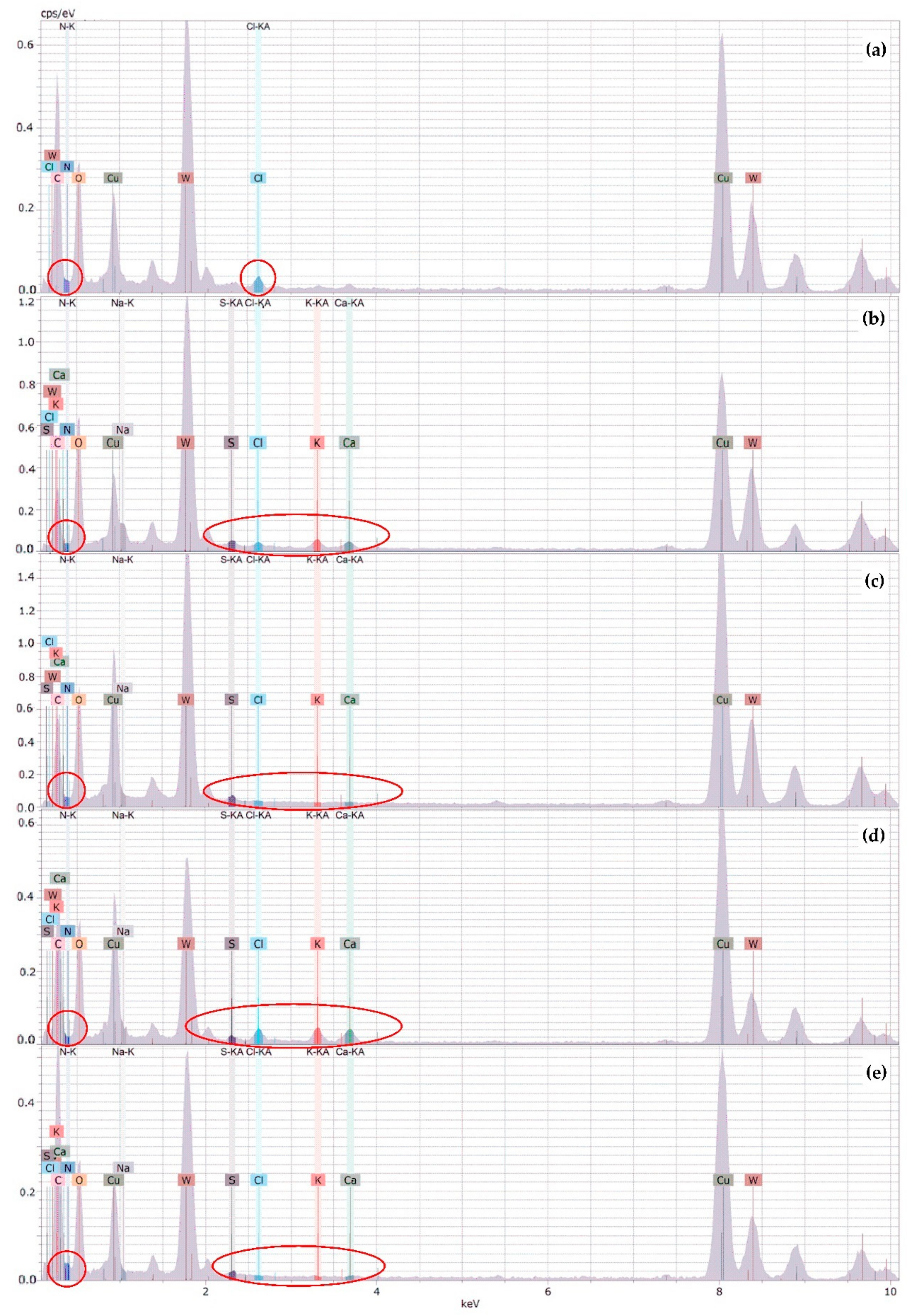
3.1.4. X-ray Energy Dispersive Spectrometry (XEDS) Analysis
3.1.5. Fourier Transform Infrared (FTIR) Spectroscopy
3.2. In Vitro Drug Release
Mathematical Modelling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cuomo, F.; Lopez, F.; Piludu, M.; Miguel, M.G.; Lindman, B.; Ceglie, A. Release of small hydrophilic molecules from polyelectrolyte capsules: Effect of the wall thickness. J. Colloid Interface Sci. 2015, 447, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Xiang, N.; Lyu, Y.; Narsimhan, G. Characterization of fish oil in water emulsion produced by layer by layer deposition of soy β-conglycinin and high methoxyl pectin. Food Hydrocoll. 2016, 52, 678–689. [Google Scholar] [CrossRef]
- Ji, F.; Li, J.; Qin, Z.; Yang, B.; Zhang, E.; Dong, D.; Wang, J.; Wen, Y.; Tian, L.; Yao, F. Engineering pectin-based hollow nanocapsules for delivery of anticancer drug. Carbohydr. Polym. 2017, 177, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Mateos-Maroto, A.; Ruano, M.; Ortega, F.; Rubio, R.G. Layer-by-Layer polyelectrolyte assemblies for encapsulation and release of active compounds. Adv. Colloid Interface Sci. 2017, 249, 290–307. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Wang, C.; Liu, X.; Tong, Z. Multilayer nanocapsules of polysaccharide chitosan and alginate through layer-by-layer assembly directly on PS nanoparticles for release. J. Biomater. Sci. Polym. Ed. 2005, 16, 909–923. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.H.L.; Pereira, S.V.A.; Rabello, R.B.; Rodriguez-Castellón, E.; Beppu, M.M.; Chevallier, P.; Mantovani, D.; Vieira, R.S. Blood protein adsorption on sulfonated chitosan and κ-carrageenan films. Colloids Surfaces B Biointerfaces 2013, 111, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Chopra, M.; Kaur, P.; Bernela, M.; Thakur, R. Surfactant assisted nisin loaded chitosan-carageenan nanocapsule synthesis for controlling food pathogens. Food Control 2014, 37, 158–164. [Google Scholar] [CrossRef]
- Bazylińska, U.; Skrzela, R.; Szczepanowicz, K.; Warszyński, P.; Wilk, K.A. Novel approach to long sustained multilayer nanocapsules: Influence of surfactant head groups and polyelectrolyte layer number on the release of hydrophobic compounds. Soft Matter 2011, 7, 6113. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Hu, Q.; Li, X. From the 2-dimensional unstable polyelectrolyte multilayer to the 3-dimensional stable dry polyelectrolyte capsules. J. Colloid Interface Sci. 2011, 363, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Sato, K.; Sugimoto, K.; Anzai, J.I. Avidin/PSS membrane microcapsules with biotin-binding activity. J. Colloid Interface Sci. 2011, 360, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Xuan, M.; Zhao, J.; Shao, J.; Du, C.; Cui, W.; Duan, L.; Qi, W.; Li, J. Recent progresses in layer-by-layer assembled biogenic capsules and their applications. J. Colloid Interface Sci. 2017, 487, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.Y.; Weiss, J.; Gibis, M.; Choi, M.J.; Hong, G.P. Change of Multiple-Layered Phospholipid Vesicles Produced by Electrostatic Deposition of Polymers during Storage. Int. J. Food Eng. 2016, 12, 763–771. [Google Scholar] [CrossRef]
- Carneiro, T.N.; Novaes, D.S.; Rabelo, R.B.; Celebi, B.; Chevallier, P.; Mantovani, D.; Beppu, M.M.; Vieira, R.S. BSA and fibrinogen adsorption on chitosan/κ-carrageenan polyelectrolyte complexes. Macromol. Biosci. 2013, 13, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Carballo, S.; Lim, S.; Rodriguez, G.; Vila, A.O.; Krueger, C.G.; Gunasekaran, S.; Reed, J.D. Biopolymer coating of soybean lecithin liposomes via layer-by-layer self-assembly as novel delivery system for ellagic acid. J. Funct. Foods 2010, 2, 99–106. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Bourbon, A.I.; Quintas, M.A.C.; Coimbra, M.A.; Vicente, A.A. Κ-Carrageenan/Chitosan Nanolayered Coating for Controlled Release of a Model Bioactive Compound. Innov. Food Sci. Emerg. Technol. 2012, 16, 227–232. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Zhao, Z.; Li, J.; Zhang, R.; Yao, F. Formation and characterization of natural polysaccharide hollow nanocapsules via template layer-by-layer self-assembly. J. Colloid Interface Sci. 2012, 379, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Tao, C.; Cui, Y.; Duan, L.; Yang, Y.; Li, J. Assembly of environmental sensitive microcapsules of PNIPAAm and alginate acid and their application in drug release. J. Colloid Interface Sci. 2009, 332, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.C.; Bourbon, A.I.; Cerqueira, M.A.; Maricato, É.; Nunes, C.; Coimbra, M.A.; Vicente, A.A. Chitosan/fucoidan multilayer nanocapsules as a vehicle for controlled release of bioactive compounds. Carbohydr. Polym. 2015, 115, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Wang, C.; Zhang, J.; Wang, W.; Xiao, L.; Jia, S.; Wang, Z.; Wu, W.; Xiao, J.; Wu, X. New Insight into Polydopamine @ ZIF-8 Nanohybrids: A Zinc-Releasing Container for Potential Anticancer Activity. Polymers 2018, 10. [Google Scholar] [CrossRef]
- Elizarova, I.S.; Luckham, P.F. Fabrication of polyelectrolyte multilayered nano-capsules using a continuous layer-by-layer approach. J. Colloid Interface Sci. 2016, 470, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Fani, A.; Silva, H.D.; Soliva-Fortuny, R.; Martín-Belloso, O.; Vicente, A.A. Formation, stability and antioxidant activity of food-grade multilayer emulsions containing resveratrol. Food Hydrocoll. 2017, 71, 207–215. [Google Scholar] [CrossRef]
- Xiao, F.-X.; Pagliaro, M.; Xu, Y.-J.; Liu, B. Layer-by-layer assembly of versatile nanoarchitectures with diverse dimensionality: A new perspective for rational construction of multilayer assemblies. Chem. Soc. Rev. 2016, 45, 3088–3121. [Google Scholar] [CrossRef] [PubMed]
- Voigt, A.; Lichtenfeld, H.; Sukhorukov, G.B.; Zastrow, H.; Donath, E.; Bäumler, H.; Möhwald, H. Membrane filtration for microencapsulation and microcapsules fabrication by layer-by-layer polyelectrolyte adsorption. Ind. Eng. Chem. Res. 1999, 38, 4037–4043. [Google Scholar] [CrossRef]
- Richardson, J.J.; Liang, K.; Kempe, K.; Ejima, H.; Cui, J.; Caruso, F. Immersive polymer assembly on immobilized particles for automated capsule preparation. Adv. Mater. 2013, 25, 6874–6878. [Google Scholar] [CrossRef] [PubMed]
- Kantak, C.; Beyer, S.; Yobas, L.; Bansal, T.; Trau, D. A “microfluidic pinball” for on-chip generation of Layer-by-Layer polyelectrolyte microcapsules. Lab Chip 2011, 11, 1030. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, G.; Boltyanskiy, R.; Nejati, S.; Thiam, A.R.; Loewenberg, M.; Dufresne, E.R.; Osuji, C.O. Single-step microfluidic fabrication of soft monodisperse polyelectrolyte microcapsules by interfacial complexation. Lab Chip 2014, 14, 3494–3497. [Google Scholar] [CrossRef] [PubMed]
- Machín, R.; Isasi, J.R.; Vélaz, I. Hydrogel matrices containing single and mixed natural cyclodextrins. Mechanisms of drug release. Eur. Polym. J. 2013, 49, 3912–3920. [Google Scholar] [CrossRef]
- Shutava, T.G.; Pattekari, P.P.; Arapov, K.A.; Torchilin, V.P.; Lvov, Y.M. Architectural layer-by-layer assembly of drug nanocapsules with PEGylated polyelectrolytes. Soft Matter 2012, 8, 9418. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Durazo, A.; Lizardi, J.; Higuera-Ciapara, I.; Argüelles-Monal, W.; Goycoolea, F.M. Development and characterization of nanocapsules comprising dodecyltrimethylammonium chloride and κ-carrageenan. Colloids Surfaces B Biointerfaces 2011, 86, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M.C.; Pinheiro, A.C.; Bourbon, A.I.; Cerqueira, M.A.; Vicente, A.A. Hollow chitosan/alginate nanocapsules for bioactive compound delivery. Int. J. Biol. Macromol. 2015, 79, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- MacHín, R.; Isasi, J.R.; Vélaz, I. β-Cyclodextrin hydrogels as potential drug delivery systems. Carbohydr. Polym. 2012, 87, 2024–2030. [Google Scholar] [CrossRef]
- Shu, S.; Sun, L.; Zhang, X.; Wu, Z.; Wang, Z.; Li, C. Polysaccharides-based polyelectrolyte nanoparticles as protein drugs delivery system. J. Nanoparticle Res. 2011, 13, 3657–3670. [Google Scholar] [CrossRef]
- Costa, P.; Lobo, J.M.S. Modelling and Comparison of Dissolution Profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Najib, N.; Suleiman, M.S. The kinetics of drug release from ethylcellulose solid dispersions. DRUG Dev. Ind. Pharm. 1985, 11, 2169–2181. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Kim, B.S.; Choi, J.W. Polyelectrolyte multilayer microcapsules: Self-assembly and toward biomedical applications. Biotechnol. Bioprocess Eng. 2007, 12, 323–332. [Google Scholar] [CrossRef]
- Bazylińska, U.; Skrzela, R.; Piotrowski, M.; Szczepanowicz, K.; Warszyński, P.; Wilk, K.A. Influence of dicephalic ionic surfactant interactions with oppositely charged polyelectrolyte upon the in vitro dye release from oil core nanocapsules. Bioelectrochemistry 2012, 87, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Szczepanowicz, K.; Bazylińska, U.; Pietkiewicz, J.; Szyk-Warszyńska, L.; Wilk, K.A.; Warszyński, P. Biocompatible long-sustained release oil-core polyelectrolyte nanocarriers: From controlling physical state and stability to biological impact. Adv. Colloid Interface Sci. 2015, 222, 678–691. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Sahoo, S.K. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Karangwa, E.; Bashari, M.; Hayat, K.; Hong, X.; Sharif, H.R.; Zhang, X. Fabrication of polymeric nanocapsules from curcumin-loaded nanoemulsion templates by self-assembly. Ultrason. Sonochem. 2015, 23, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Babič, M.; Horák, D.; Jendelová, P.; Glogarová, K.; Herynek, V.; Trchová, M.; Likavčanová, K.; Lesný, P.; Pollert, E.; Hájek, M.; et al. Poly(N,N-dimethylacrylamide)-Coated Maghemite Nanoparticles for Stem Cell Labeling. Bioconjug. Chem. 2009, 20, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Kittitheeranun, P.; Sajomsang, W.; Phanpee, S.; Treetong, A.; Wutikhun, T.; Suktham, K.; Puttipipatkhachorn, S.; Ruktanonchai, U.R. Layer-by-layer engineered nanocapsules of curcumin with improved cell activity. Int. J. Pharm. 2015, 492, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Durazo, A.; Hernández, J.; Lizardi, J.; Higuera-Ciapara, I.; Goycoolea, F.M.; Argüelles-Monal, W. Gelation processes in the non-stoichiometric polylectrolyte–surfactant complex between κ-carrageenan and dodecyltrimethylammonium chloride in KCl. Soft Matter 2011, 7, 2103. [Google Scholar] [CrossRef]
- Grenha, A.; Gomes, M.E.; Rodrigues, M.; Santo, V.E.; Mano, J.F.; Neves, N.M.; Reis, R.L. Development of new chitosan/carrageenan nanoparticles for drug delivery applications. J. Biomed. Mater. Res. Part A 2010, 92, 1265–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Turhan, M.; Gunasekaran, S. Selected properties of pH-sensitive, biodegradable chitosan-poly(vinyl alcohol) hydrogel. Polym. Int. 2004, 53, 911–918. [Google Scholar] [CrossRef]
- Rodrigues, S.; Costa, A.M.R.D.; Grenha, A. Chitosan/carrageenan nanoparticles: Effect of cross-linking with tripolyphosphate and charge ratios. Carbohydr. Polym. 2012, 89, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Sankalia, M.G.; Mashru, R.C.; Sankalia, J.M.; Sutariya, V.B. Stability improvement of alpha-amylase entrapped in kappa-carrageenan beads: Physicochemical characterization and optimization using composite index. Int. J. Pharm. 2006, 312, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.A. Interactions of Triglycerides with Phospholipids: Incorporation into the Bilayer Structure and Formation of Emulsions. Biochemistry 1989, 28, 2514–2520. [Google Scholar] [CrossRef] [PubMed]
- Natrajan, D.; Srinivasan, S.; Sundar, K.; Ravindran, A. Formulation of essential oil-loaded chitosan-alginate nanocapsules. J. Food Drug Anal. 2015, 23, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.S.; Lorenzoni, A.; Ferreira, L.M.; Mattiazzi, J.; Adams, A.I.H.; Denardi, L.B.; Alves, S.H.; Schaffazick, S.R.; Cruz, L. Clotrimazole-loaded Eudragit® RS100 nanocapsules: Preparation, characterization and in vitro evaluation of antifungal activity against Candida species. Mater. Sci. Eng. C 2013, 33, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Bezemer, J.M.; Radersma, R.; Grijpma, D.W.; Dijkstra, P.J.; Feijen, J.; Van Blitterswijk, C.A. Zero-order release of lysozyme from poly(ethylene glycol)/poly(butylene terephthalate) matrices. J. Control. Release 2000, 64, 179–192. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Xu, X.; Wen, N.; Song, R.; Meng, Q.; Guan, Y.; Cheng, S.; Cao, D.; Dong, Y.; Qie, J.; et al. A Drug Carrier for Sustained Zero-Order Release of Peptide Therapeutics. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]


| System | Korsmeyer-Peppas | Higuchi | First Order | Zero Order | |||||
|---|---|---|---|---|---|---|---|---|---|
| kKP × 102 (min−n) | n | R2 | kH × 102 (min−0.5) | R2 | k1 × 102 (min−1) | R2 | k0 × 102 (min−1) | R2 | |
| NE | 7.5 ± 0.4 | 0.56 ± 0.02 | 0.9883 | 9.2 ± 0.1 | 0.9801 | 2.6 ± 0.1 | 0.9177 | 1.7 ± 0.1 | 0.6898 |
| NE-κ-CAR | 3.8 ± 0.1 | 0.74 ± 0.01 | 0.9754 | 8.4 ± 0.1 | 0.8973 | 2.3 ± 0.1 | 0.9879 | 1.6 ± 0.1 | 0.9190 |
| NE(κ-CAR/CS)1 | 2.2 ± 0.1 | 0.89 ± 0.01 | 0.9760 | 7.9 ± 0.3 | 0.8271 | 2.1 ± 0.1 | 0.9791 | 1.5 ± 0.1 | 0.9729 |
| NE(κ-CAR/CS)1-κ-CAR | 1.4 ± 0.2 | 0.97 ± 0.02 | 0.9877 | 7.1 ± 0.1 | 0.8006 | 1.7 ± 0.1 | 0.9717 | 1.3 ± 0.1 | 0.9893 |
| NE(κ-CAR/CS)2 | 1.0 ± 0.1 | 0.99 ± 0.04 | 0.9748 | 6.2 ± 0.5 | 0.7648 | Na | - | 1.0 ± 0.1 | 0.9793 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rochín-Wong, S.; Rosas-Durazo, A.; Zavala-Rivera, P.; Maldonado, A.; Martínez-Barbosa, M.E.; Vélaz, I.; Tánori, J. Drug Release Properties of Diflunisal from Layer-By-Layer Self-Assembled κ-Carrageenan/Chitosan Nanocapsules: Effect of Deposited Layers. Polymers 2018, 10, 760. https://doi.org/10.3390/polym10070760
Rochín-Wong S, Rosas-Durazo A, Zavala-Rivera P, Maldonado A, Martínez-Barbosa ME, Vélaz I, Tánori J. Drug Release Properties of Diflunisal from Layer-By-Layer Self-Assembled κ-Carrageenan/Chitosan Nanocapsules: Effect of Deposited Layers. Polymers. 2018; 10(7):760. https://doi.org/10.3390/polym10070760
Chicago/Turabian StyleRochín-Wong, Sarai, Aarón Rosas-Durazo, Paul Zavala-Rivera, Amir Maldonado, María Elisa Martínez-Barbosa, Itziar Vélaz, and Judith Tánori. 2018. "Drug Release Properties of Diflunisal from Layer-By-Layer Self-Assembled κ-Carrageenan/Chitosan Nanocapsules: Effect of Deposited Layers" Polymers 10, no. 7: 760. https://doi.org/10.3390/polym10070760