Structural Manipulation of the Conjugated Phenyl Moiety in 3-Phenylbenzofulvene Monomers: Effects on Spontaneous Polymerization
Abstract
:1. Introduction
2. Materials and Methods
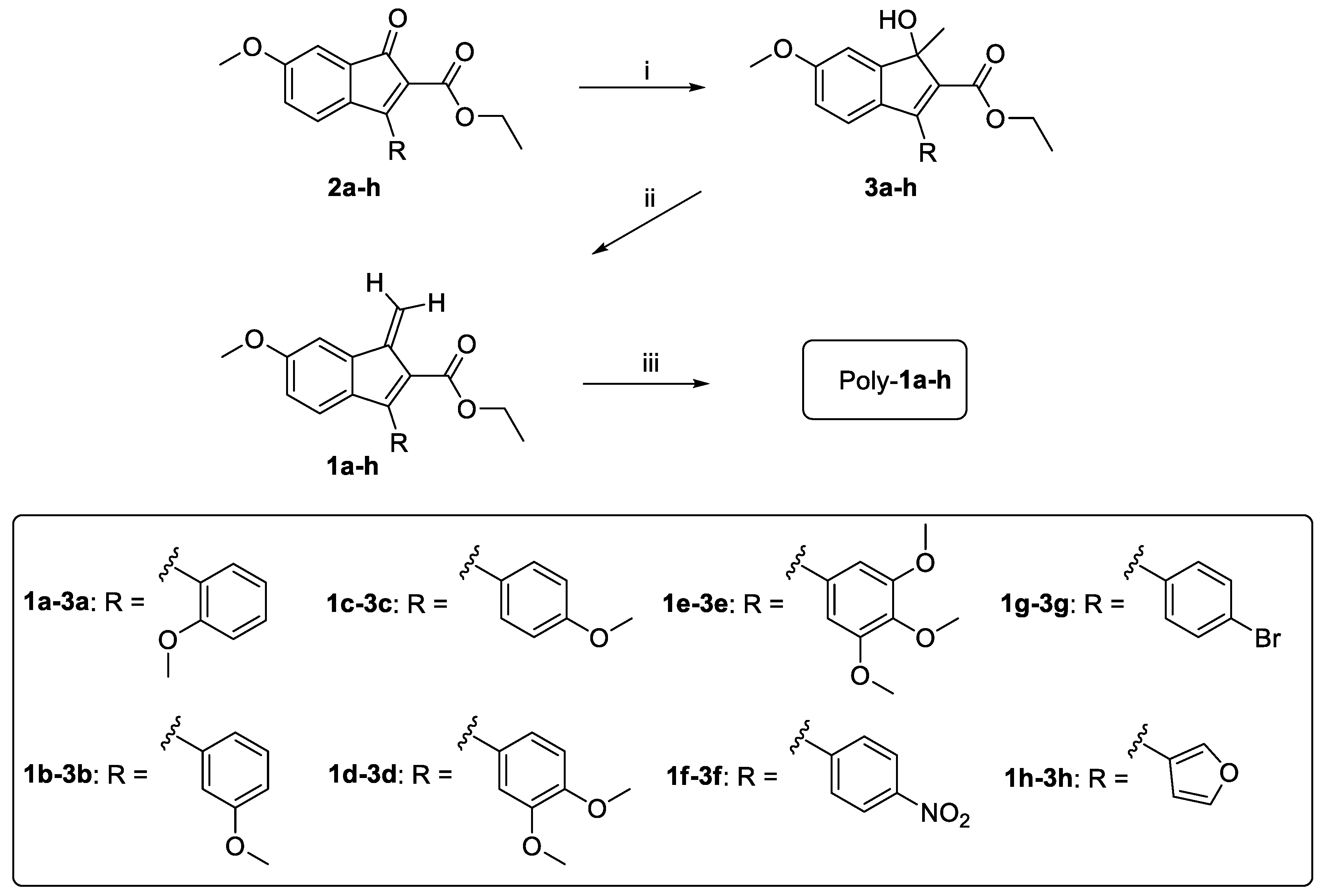
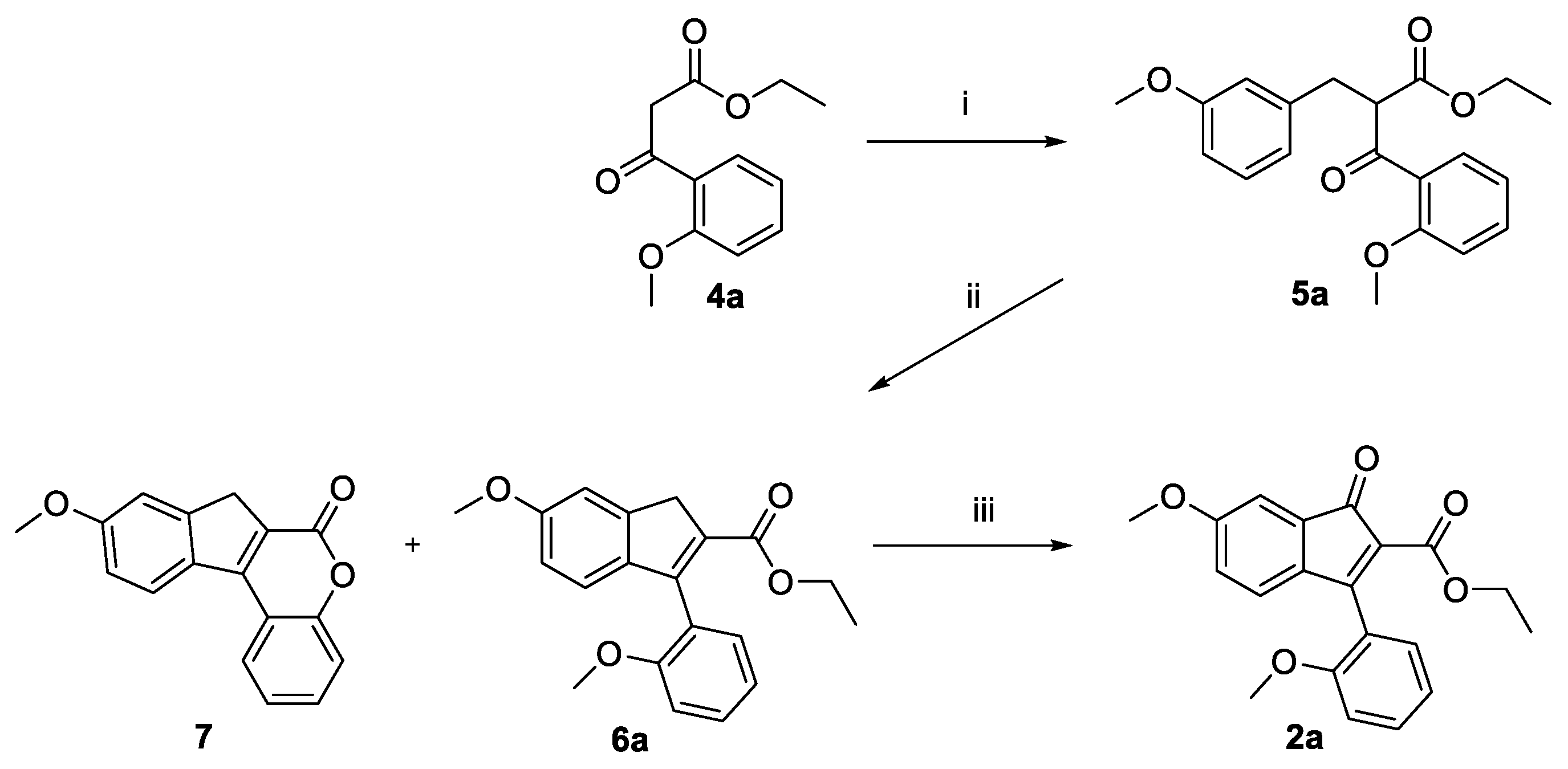
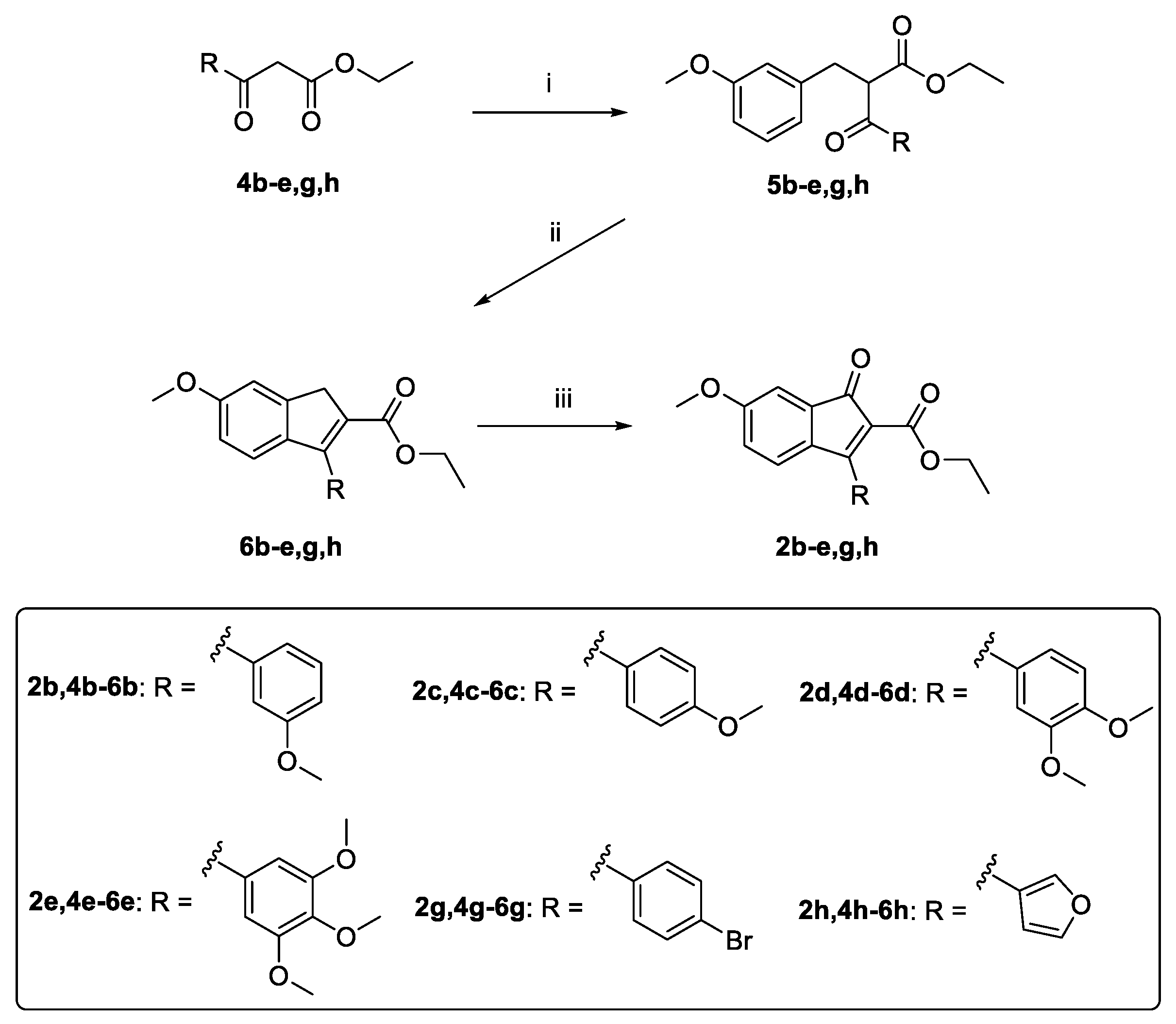
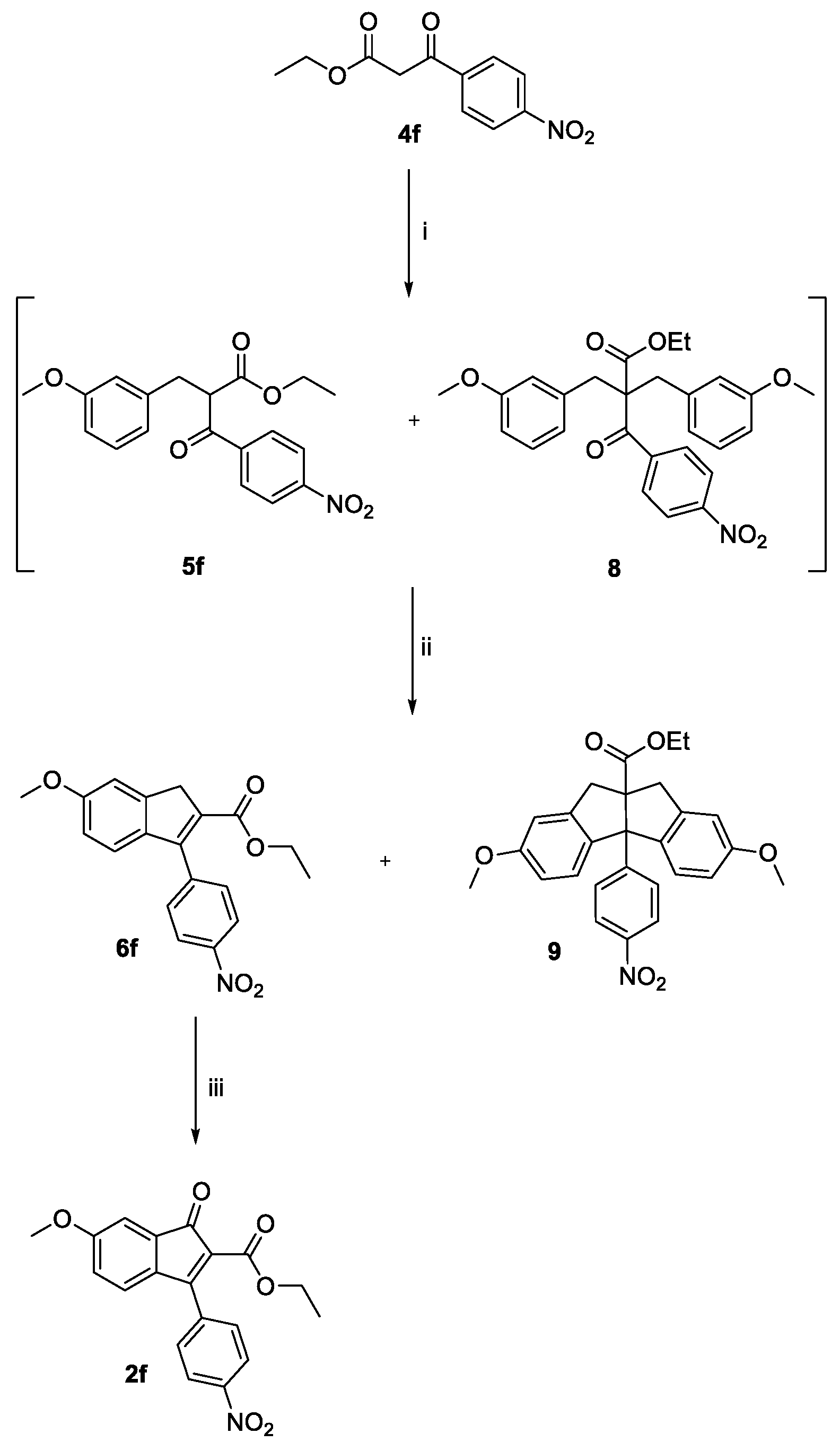
2.1. Synthesis
2.2.1. General Procedure for the Preparation of Solutions of Monomers 1a–h in Chloroform or CDCl3
2.1.2. General Procedure for the Preparation of Polybenzofulvene Derivatives Poly-1b–e,g,h by Spontaneous Polymerization.
2.2. SEC-MALS
2.3. X-ray Crystallography
3. Results
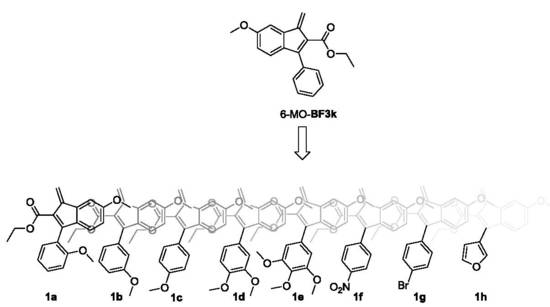
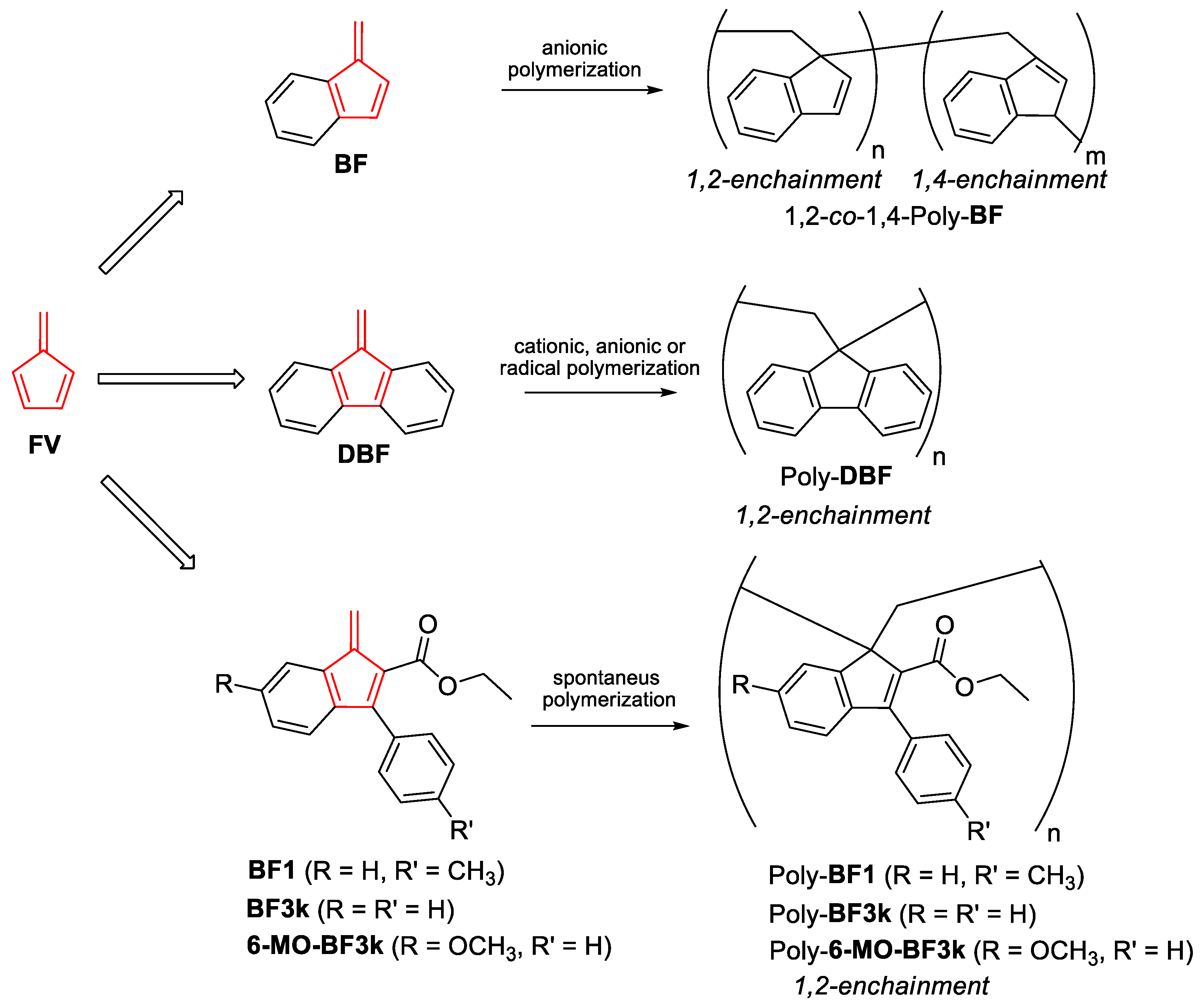
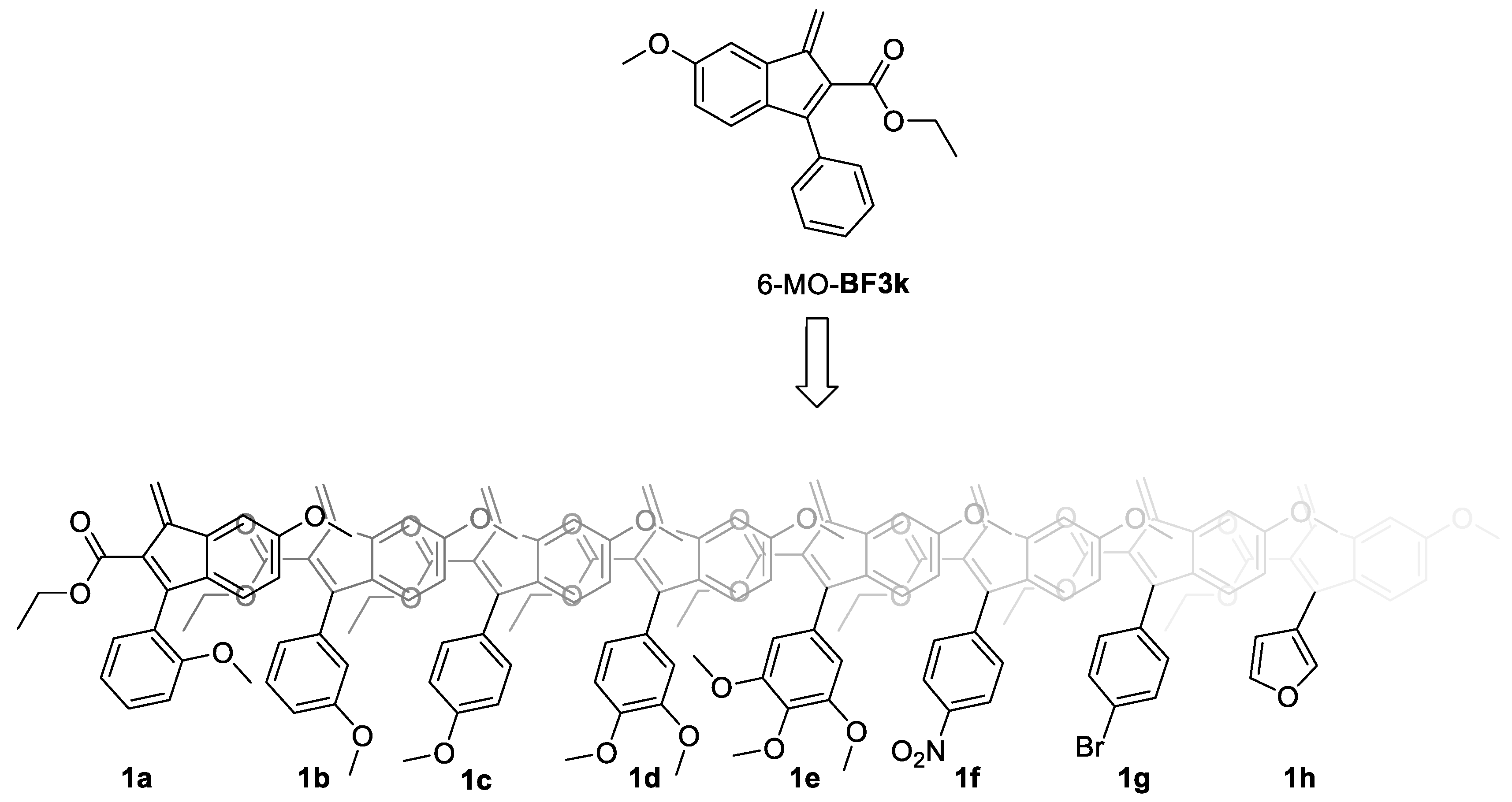
3.1. Design, Synthesis, and Spontaneous Polymerization of Benzofulvene Derivates 1a–h
3.2. Properties of Polybenzofulvene Derivatives Poly-1a–h
3.3. Molecular Weight Distribution of Polybenzofulvene Derivatives Poly-1a–h
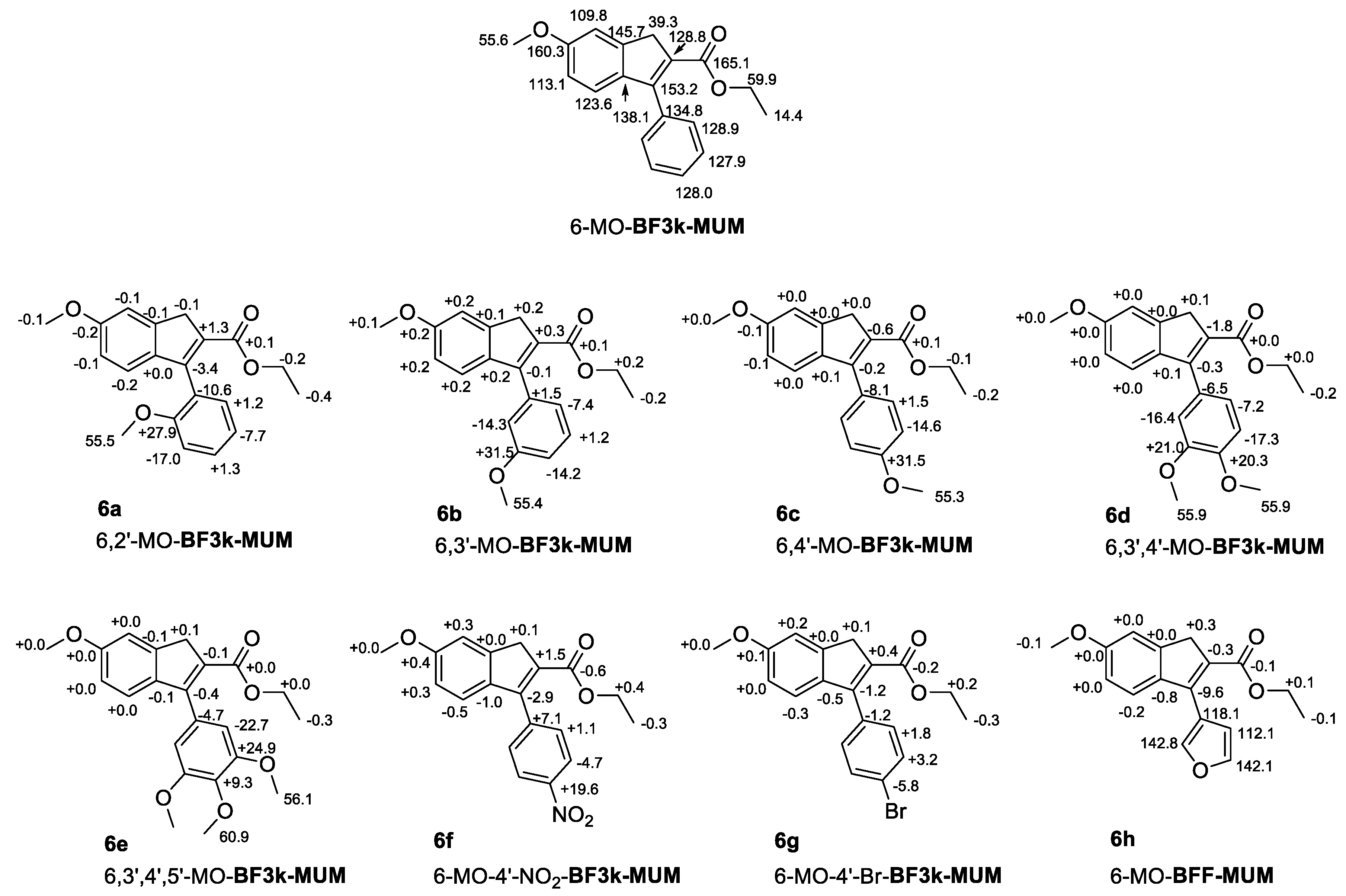
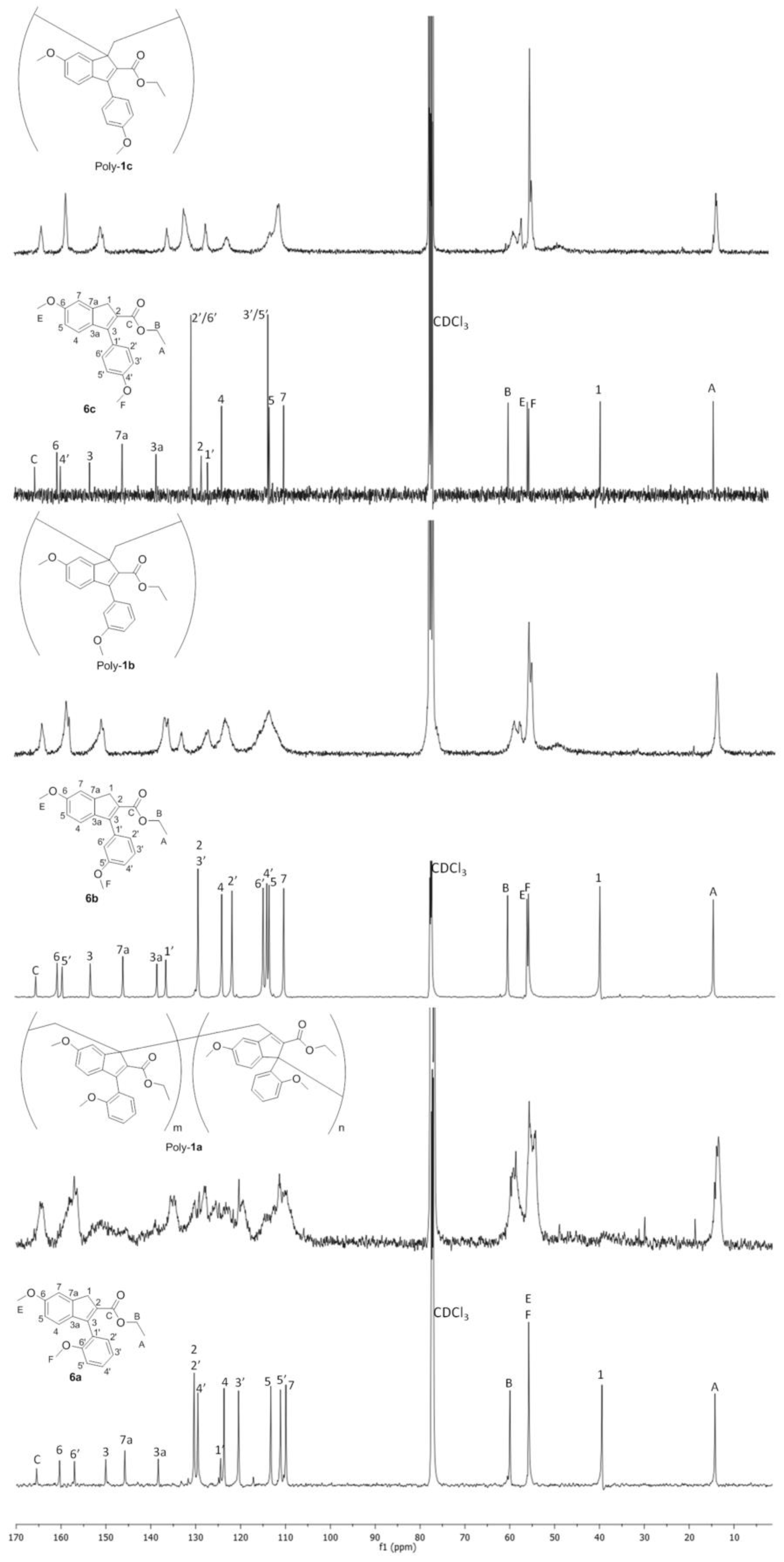
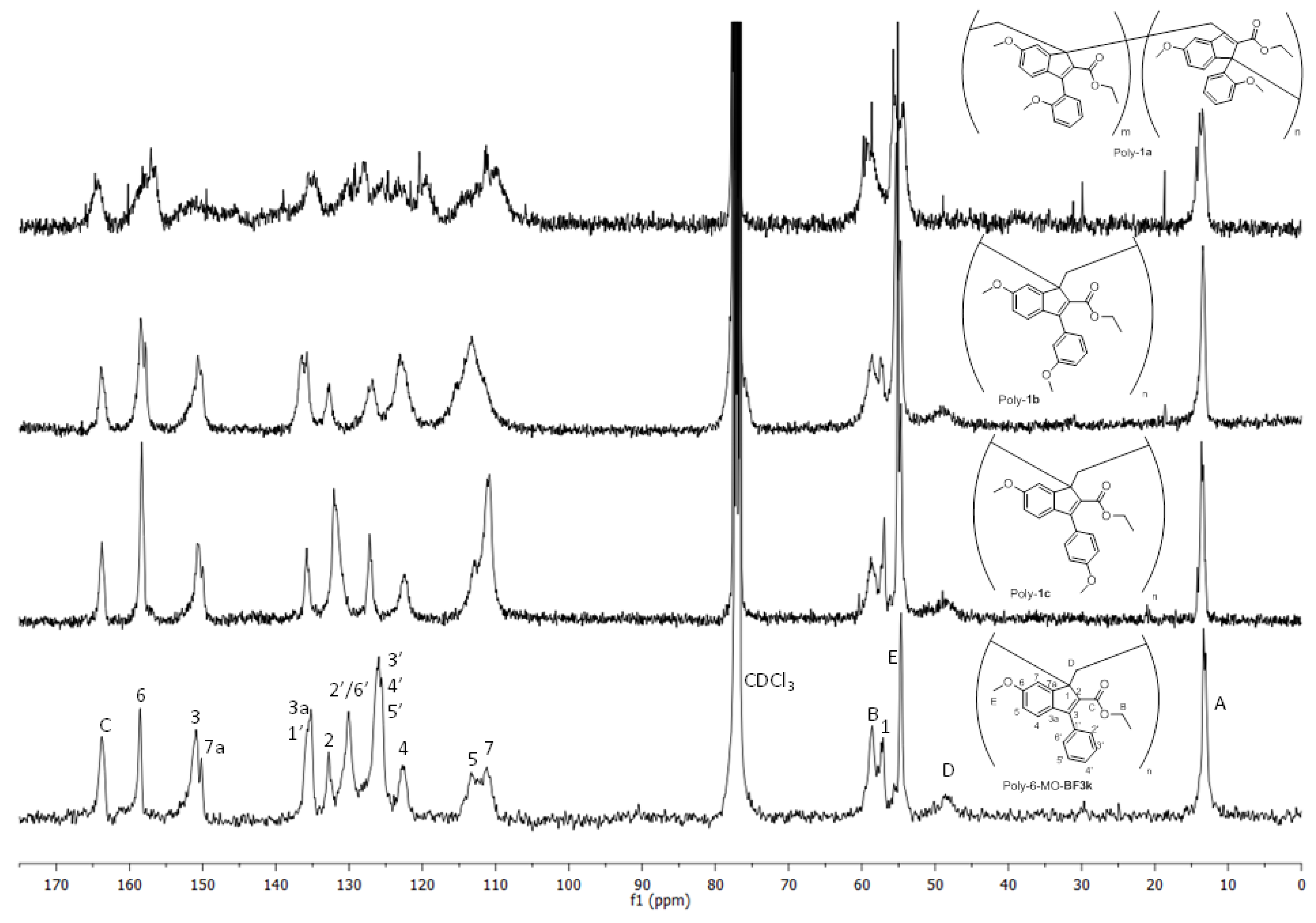
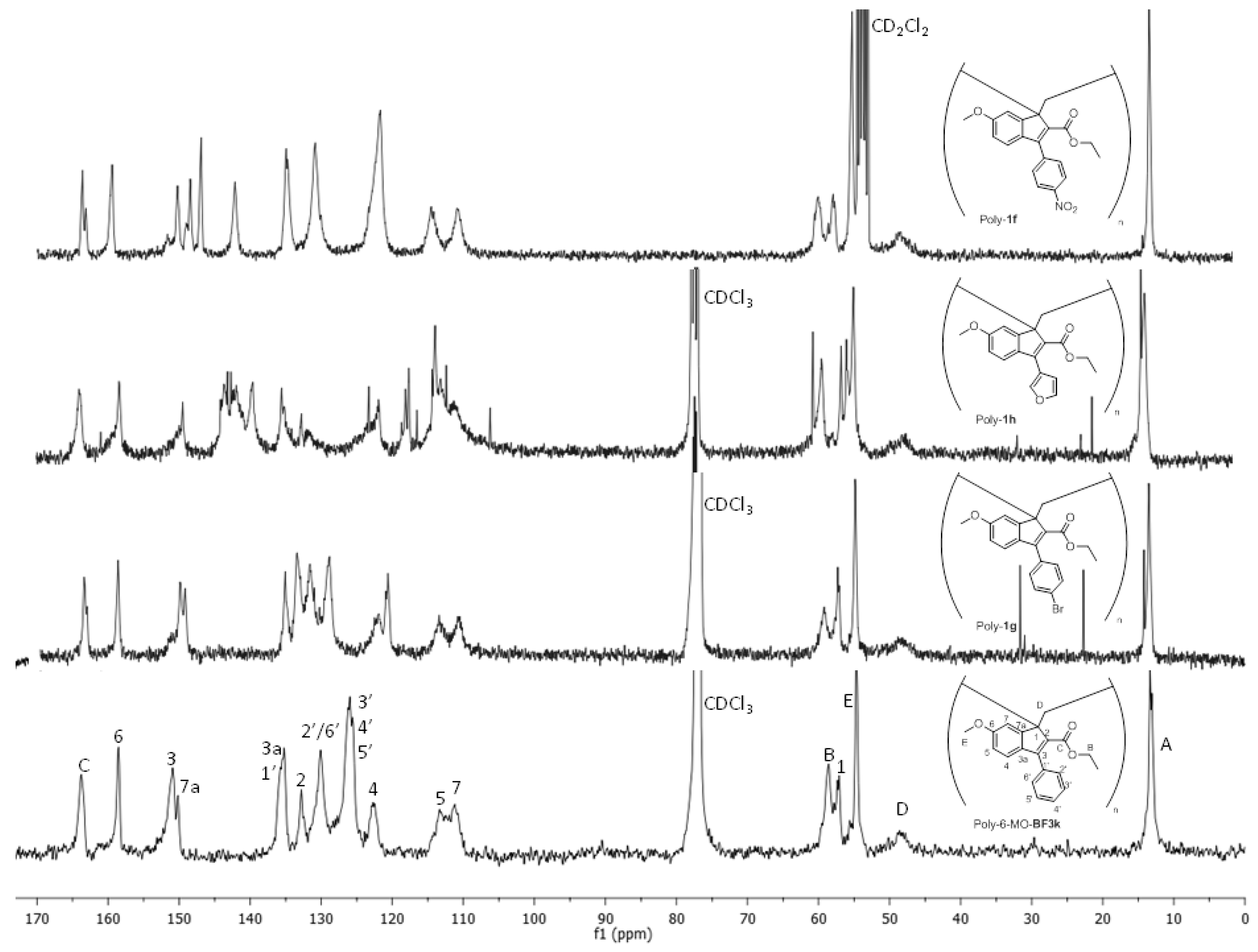
3.4. NMR Studies on Polybenzofulvene Derivatives Poly-1a–h
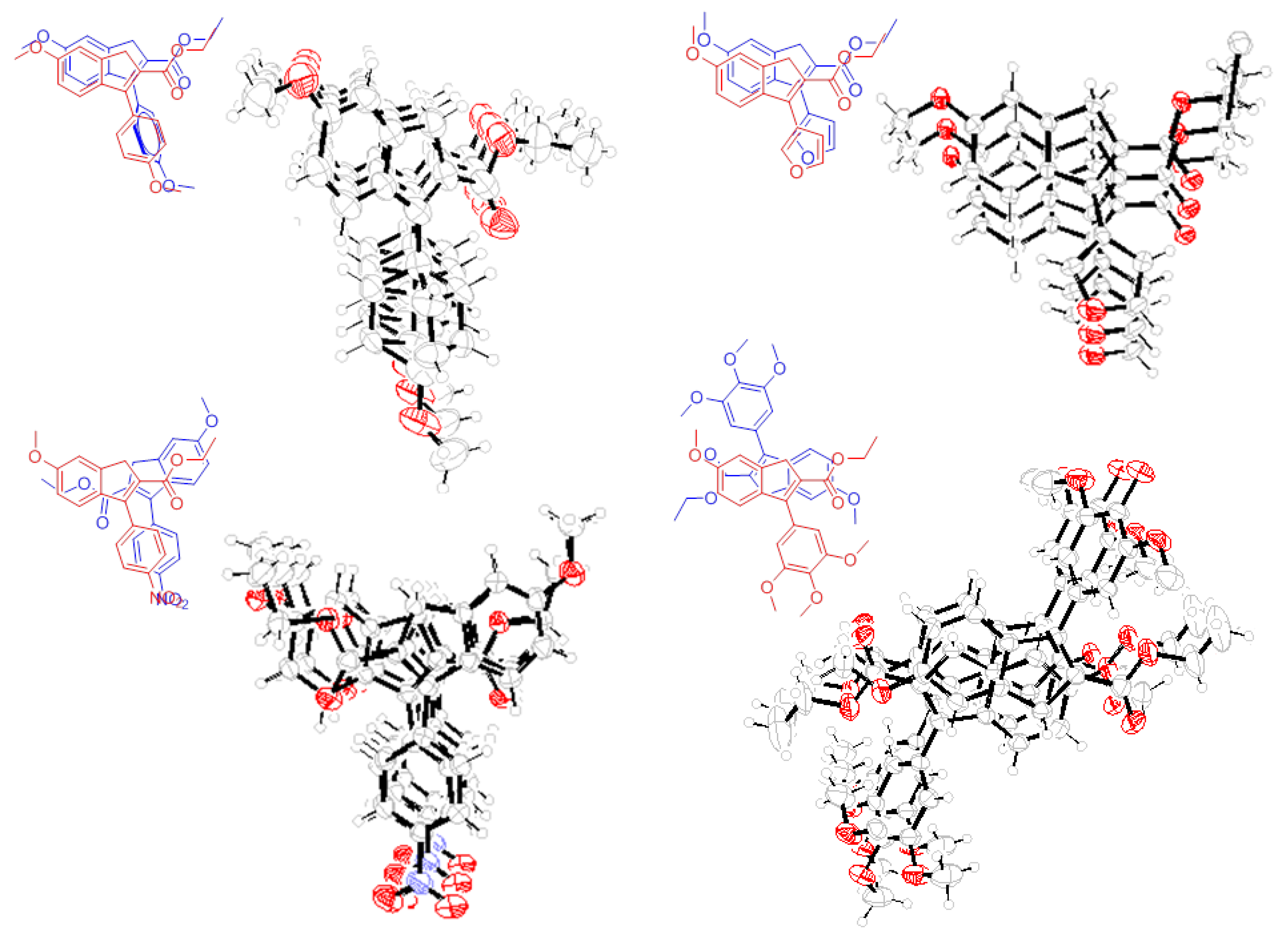
3.5. Single Crystal X-ray Diffraction Studies on the Synthetic Intermediates of Polybenzofulvene Derivatives Poly-1a–h
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Morisaki, Y.; Fernandes, J.A.; Chujo, Y. Synthesis of oligothiophene-layered polymers. Macromol. Rapid Commun. 2009, 47, 2107–2111. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, A.; Paolino, M.; Grisci, G.; Giuliani, G.; Donati, A.; Mendichi, R.; Boccia, A.C.; Botta, C.; Mroz, W.; Samperi, F.; et al. Synthesis and characterization of charge-transporting π-stacked polybenzofulvene derivatives. J. Mater. Chem. 2012, 22, 9611–9623. [Google Scholar] [CrossRef]
- Facchetti, A. π-Conjugated polymers for organic electronics and photovoltaic cell applications. Chem. Mater. 2011, 23, 733–758. [Google Scholar] [CrossRef]
- Guo, X.; Baumgarten, M.; Müllen, K. Designing π-conjugated polymers for organic electronics. Prog. Polym. Sci. 2013, 38, 1832–1908. [Google Scholar] [CrossRef]
- Vohra, V.; Giovanella, U.; Tubino, R.; Murata, H.; Botta, C. Electroluminescence from conjugated polymer electrospun nanofibers in solution processable organic light-emitting diodes. ACS Nano 2011, 5, 5572–5578. [Google Scholar] [CrossRef] [PubMed]
- Evenson, S.J.; Mumm, M.J.; Pokhodnya, K.I.; Rasmussen, S.C. Highly fluorescent dithieno[3,2-b:2′,3′-d]pyrrole-based materials: Synthesis, characterization, and OLED device applications. Macromolecules 2011, 44, 835–841. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Bao, X.; Qiu, M.; Liu, Z.; Wang, N.; Zhang, G.; Yang, R.; Zhang, D. New π-conjugated polymers as acceptors designed for all polymer solar cells based on imide/amide-derivatives. J. Mater. Chem. C 2016, 4, 185–192. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Yang, S.-H.; Hsu, C.-S. Synthesis of conjugated polymers for organic solar cell applications. Chem. Rev. 2009, 109, 5868–5923. [Google Scholar] [CrossRef] [PubMed]
- Sirringhaus, H.; Bird, M.; Richards, T.; Zhao, N. Charge transport physics of conjugated polymer field-effect transistors. Adv. Mater. 2010, 22, 3893–3898. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-K.; Jang, S.-Y.; Pace, G.; Caironi, M.; Park, W.-T.; Khim, D.; Kim, J.; Kim, D.-Y.; Noh, Y.-Y. High-performance organic field-effect transistors with directionally aligned conjugated polymer film deposited from pre-aggregated solution. Chem. Mater. 2015, 27, 8345–8353. [Google Scholar] [CrossRef]
- Morisaki, Y.; Chujo, Y. Through-space conjugated polymers based on cyclophanes. Angew. Chem. 2006, 45, 6430–6437. [Google Scholar] [CrossRef] [PubMed]
- Morisaki, Y.; Hifumi, R.; Lin, L.; Inoshita, K.; Chujo, Y. Through-space conjugated polymers consisting of planar chiral pseudo-ortho-linked [2.2]paracyclophane. Polym. Chem. 2012, 3, 2727–2730. [Google Scholar] [CrossRef]
- Uemura, T.; Uchida, N.; Asano, A.; Saeki, A.; Seki, S.; Tsujimoto, M.; Isoda, S.; Kitagawa, S. Highly photoconducting π-stacked polymer accommodated in coordination nanochannels. J. Am. Chem. Soc. 2012, 134, 8360–8363. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.-H.; Deng, X.-Y.; Chen, L.; Chen, S.-F.; Liu, R.-R.; Hou, X.-Y.; Wong, K.-Y.; Ling, Q.-D.; Huang, W. A π-stacked and conjugated hybrid based on poly(N-vinylcarbazole) postfunctionalized with terfluorene for stable deep-blue hole-transporting materials. J. Polym. Sci. A 2009, 47, 5221–5229. [Google Scholar] [CrossRef]
- Nakano, T. π-Stacked Polymers and Molecules: Theory, Synthesis, and Properties; Springer: Tokyo, Japan, 2014; ISBN 978-4-431-54129-5. [Google Scholar]
- Bergmann, E.D. Fulvenes and substituted fulvenes. Chem. Rev. 1968, 68, 41–84. [Google Scholar] [CrossRef]
- Godman, N.P.; Balaich, G.J.; Iacono, S.T. First preparation of low band gap fulvene-modified polynorbornene via ring-opening metathesis polymerization. Chem. Commun. 2016, 52, 5242–5245. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, Q.; Sun, S.; Ding, Y.; Hu, A. A novel approach toward polyfulvene: Cationic polymerization of enediynes. Macromolecules 2017, 50, 534–541. [Google Scholar] [CrossRef]
- Nakano, T.; Yade, T. Synthesis, structure, and photophysical and electrochemical properties of a π-stacked polymer. J. Am. Chem. Soc. 2003, 125, 15474–15484. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Takewaki, K.; Yade, T.; Okamoto, Y. Dibenzofulvene, a 1,1-diphenylethylene analogue, gives a π-stacked polymer by anionic, free-radical, and cationic catalysts. J. Am. Chem. Soc. 2001, 123, 9182–9183. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Yade, T.; Fukuda, Y.; Yamaguchi, T.; Okumura, S. Free-radical polymerization of dibenzofulvene leading to a π-stacked polymer: Structure and properties of the polymer and proposed reaction mechanism. Macromolecules 2005, 38, 8140–8148. [Google Scholar] [CrossRef]
- Nakano, T. Synthesis, structure and function of π-stacked polymers. Polym. J. 2010, 42, 103–123. [Google Scholar] [CrossRef] [Green Version]
- Nakano, T.; Nakagawa, O.; Yade, T.; Okamoto, Y. Solid-state polymerization of dibenzofulvene leading to a copolymer with oxygen. Macromolecules 2003, 36, 1433–1435. [Google Scholar] [CrossRef]
- Nakano, T.; Yade, T.; Yokoyama, M.; Nagayama, N. Charge transport in a π-stacked poly(dibenzofulvene) film. Chem. Lett. 2004, 33, 296–297. [Google Scholar] [CrossRef]
- Kosaka, Y.; Kitazawa, K.; Inomata, S.; Ishizone, T. Living anionic polymerization of benzofulvene: Highly reactive fixed transoid 1,3-diene. ACS Macro Lett. 2013, 2, 164–167. [Google Scholar] [CrossRef]
- Kosaka, Y.; Kawauchi, S.; Goseki, R.; Ishizone, T. High anionic polymerizability of benzofulvene: New exo-methylene hydrocarbon monomer. Macromolecules 2015, 48, 4421–4430. [Google Scholar] [CrossRef]
- Kosaka, Y.; Goseki, R.; Kawauchi, S.; Ishizone, T. Living anionic polymerization of benzofulvene in hydrocarbon solvent. Macromol. Symp. 2015, 350, 55–66. [Google Scholar] [CrossRef]
- Cappelli, A.; Pericot Mohr, G.; Anzini, M.; Vomero, S.; Donati, A.; Casolaro, M.; Mendichi, R.; Giorgi, G.; Makovec, F. Synthesis and characterization of a new benzofulvene polymer showing a thermoreversible polymerization behavior. J. Org. Chem. 2003, 68, 9473–9476. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, A.; Paolino, M.; Grisci, G.; Giuliani, G.; Donati, A.; Boccia, A.C.; Samperi, F.; Mendichi, R.; Vomero, S. Reversible polymerization techniques leading to π-stacked polymers. In π-Stacked Polymers and Molecules: Theory, Synthesis, and Properties; Nakano, T., Ed.; Springer: Tokyo, Japan, 2014; pp. 51–149. [Google Scholar] [CrossRef]
- Cappelli, A.; Anzini, M.; Vomero, S.; Donati, A.; Zetta, L.; Mendichi, R.; Casolaro, M.; Lupetti, P.; Salvatici, P.; Giorgi, G. New π-stacked benzofulvene polymer showing thermoreversible polymerization: Studies in macromolecular and aggregate structures and polymerization mechanism. J. Polym. Sci. A 2005, 43, 3289–3304. [Google Scholar] [CrossRef]
- Cappelli, A.; Galeazzi, S.; Giuliani, G.; Anzini, M.; Aggravi, M.; Donati, A.; Zetta, L.; Boccia, A.C.; Mendichi, R.; Giorgi, G.; et al. Anionic polymerization of a benzofulvene monomer leading to a thermoreversible π-stacked polymer. Studies in macromolecular and aggregate structure. Macromolecules 2008, 41, 2324–2334. [Google Scholar] [CrossRef]
- Cappelli, A.; Galeazzi, S.; Giuliani, G.; Anzini, M.; Donati, A.; Zetta, L.; Mendichi, R.; Aggravi, M.; Giorgi, G.; Paccagnini, E.; et al. Structural manipulation of benzofulvene derivatives showing spontaneous thermoreversible polymerization. Role of the substituents in the modulation of polymer properties. Macromolecules 2007, 40, 3005–3014. [Google Scholar] [CrossRef]
- Cappelli, A.; Galeazzi, S.; Giuliani, G.; Anzini, M.; Grassi, M.; Lapasin, R.; Grassi, G.; Farra, R.; Dapas, B.; Aggravi, M.; et al. Synthesis and spontaneous polymerization of oligo(ethylene glycol)-conjugated benzofulvene macromonomers. A polymer brush forming a physical hydrogel. Macromolecules 2009, 42, 2368–2378. [Google Scholar] [CrossRef]
- Licciardi, M.; Grassi, M.; Di Stefano, M.; Feruglio, L.; Giuliani, G.; Valenti, S.; Cappelli, A.; Giammona, G. PEG-benzofulvene copolymer hydrogels for antibody delivery. Int. J. Pharm. 2010, 390, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Licciardi, M.; Amato, G.; Cappelli, A.; Paolino, M.; Giuliani, G.; Belmonte, B.; Guarnotta, C.; Pitarresi, G.; Giammona, G. Evaluation of thermoresponsive properties and biocompatibility of polybenzofulvene aggregates for leuprolide delivery. Int. J. Pharm. 2012, 438, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, A.; Grisci, G.; Paolino, M.; Castriconi, F.; Giuliani, G.; Donati, A.; Lamponi, S.; Mendichi, R.; Boccia, A.C.; Samperi, F.; et al. Combining spontaneous polymerization and click reactions for the synthesis of polymer brushes: A ‘grafting onto’ approach. Chem. A Eur. J. 2013, 19, 9710–9721. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, A.; Grisci, G.; Paolino, M.; Razzano, V.; Giuliani, G.; Donati, A.; Bonechi, C.; Mendichi, R.; Boccia, A.C.; Licciardi, M.; et al. Polybenzofulvene derivatives bearing dynamic binding sites as potential anticancer drug delivery systems. J. Mater. Chem. B 2015, 3, 361–374. [Google Scholar] [CrossRef]
- Paolino, M.; Grisci, G.; Giuliani, G.; Zanardi, I.; Andreassi, M.; Travagli, V.; Licciardi, M.; Scialabba, C.; Giammona, G.; Cappelli, A.; et al. π-Stacked polymers in drug delivery applications. J. Drug Deliv. Sci. Technol. 2016, 32, 142–166. [Google Scholar] [CrossRef]
- Cappelli, A.; Paolino, M.; Grisci, G.; Razzano, V.; Giuliani, G.; Donati, A.; Bonechi, C.; Mendichi, R.; Battiato, S.; Samperi, F.; et al. Hyaluronan-coated polybenzofulvene brushes as biomimetic materials. Polym. Chem. 2016, 7, 6529–6544. [Google Scholar] [CrossRef]
- Licciardi, M.; Scialabba, C.; Giammona, G.; Paolino, M.; Razzano, V.; Grisci, G.; Giuliani, G.; Makovec, F.; Cappelli, A. Design and development of hyaluronan-functionalized polybenzofulvene nanoparticles as CD44 receptor mediated drug delivery system. J. Nanopart. Res. 2017, 19, 197. [Google Scholar] [CrossRef]
- Cappelli, A.; Villafiorita-Monteleone, F.; Grisci, G.; Paolino, M.; Razzano, V.; Fabio, G.; Giuliani, G.; Donati, A.; Mendichi, R.; Boccia, A.C.; et al. Highly emissive supramolecular assemblies based on π-stacked polybenzofulvene hosts and a benzothiadiazole guest. J. Mater. Chem. C 2014, 2, 7897–7905. [Google Scholar] [CrossRef]
- Mróz, W.; Villafiorita-Monteleone, F.; Pasini, M.; Grisci, G.; Paolino, M.; Razzano, V.; Cappelli, A.; Botta, C. π-Stacked polybenzofulvene derivatives as hosts for yellow and red emitting OLEDs. Mater. Lett. 2015, 142, 197–200. [Google Scholar] [CrossRef]
- Villafiorita-Monteleone, F.; Cappelli, A.; Paolino, M.; Colombo, M.; Cariati, E.; Mura, A.; Bongiovanni, G.; Botta, C. Aggregation-induced förster resonance energy transfer in polybenzofulvene/dye nanoparticles. J. Phys. Chem. C 2015, 119, 18986–18991. [Google Scholar] [CrossRef]
- Cappelli, A.; Razzano, V.; Paolino, M.; Grisci, G.; Giuliani, G.; Donati, A.; Mendichi, R.; Samperi, F.; Battiato, S.; Boccia, A.C.; et al. Bithiophene-based polybenzofulvene derivatives with high stacking and hole mobility. Polym. Chem. 2015, 6, 7377–7388. [Google Scholar] [CrossRef]
- Cappelli, A.; Razzano, V.; Fabio, G.; Paolino, M.; Grisci, G.; Giuliani, G.; Donati, A.; Mendichi, R.; Mróz, W.; Villafiorita-Monteleone, F.; et al. Side chain engineering in π-stacked polybenzofulvene derivatives bearing electron-rich chromophores for OLED applications. RSC Adv. 2015, 5, 101377–101385. [Google Scholar] [CrossRef]
- Villafiorita-Monteleone, F.; Kozma, E.; Pasini, M.; Paolino, M.; Cappelli, A.; Bongiovanni, G.; Mura, A.; Botta, C. Polybenzofulvenes-based blends with benzothiadiazole and perylene diimide derivatives emitting fromyellow to the deep-red by resonant energy transfer processes. Appl. Phys. Lett. 2017, 110, 183301. [Google Scholar] [CrossRef]
- Villafiorita-Monteleone, F.; Kozma, E.; Giovanella, U.; Catellani, M.; Paolino, M.; Collico, V.; Colombo, M.; Cappelli, A.; Botta, C. Red and deep-red emissive polymeric nanoparticles based on polybenzofulvene and perylenediimide derivatives. Dyes Pigments 2018, 149, 331–335. [Google Scholar] [CrossRef] [Green Version]
- Fabrizi de Biani, F.; Reale, A.; Razzano, V.; Paolino, M.; Giuliani, G.; Donati, A.; Giorgi, G.; Mróz, W.; Piovani, D.; Botta, C.; et al. Electrochemical and optoelectronic properties of terthiophene- and bithiophene-based polybenzofulvene derivatives. RSC Adv. 2018, 8, 10836–10847. [Google Scholar] [CrossRef]
- Cappelli, A.; Paolino, M.; Anzini, P.; Giuliani, G.; Valenti, S.; Aggravi, M.; Donati, A.; Mendichi, R.; Zetta, L.; Boccia, A.C.; et al. Structure-property relationships in densely grafted π-stacked polymers. J. Polym. Sci. A 2010, 48, 2446–2461. [Google Scholar] [CrossRef]
- Cappelli, A.; Paolino, M.; Grisci, G.; Giuliani, G.; Donati, A.; Mendichi, R.; Boccia, A.C.; Samperi, F.; Battiato, S.; Paccagnini, E.; et al. A click chemistry-based ‘‘grafting through’’ approach to the synthesis of a biorelevant polymer brush. Polym. Chem. 2011, 2, 2518–2527. [Google Scholar] [CrossRef]
- Guckian, K.M.; Schweitzer, B.A.; Ren, R.X.-F.; Sheils, C.J.; Paris, P.L.; Tahmassebi, D.C.; Kool, E.T. Experimental measurement of aromatic stacking affinities in the context of duplex DNA. J. Am. Chem. Soc. 1996, 118, 8182–8183. [Google Scholar] [CrossRef] [PubMed]
- Makwana, K.M.; Mahalakshmi, R. Implications of aromatic-aromatic interactions: From protein structures to peptide models. Protein Sci. 2015, 24, 1920–1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paolino, M.; Ennen, F.; Komber, H.; Cernescu, M.; Cappelli, A.; Brutschy, B.; Voit, B.; Appelhans, D. Self-assembly of poly(propylene imine) glycodendrimers: Role of aromatic interactions in the formation of necklace- and donut-like nanostructures. Polym. Chem. 2012, 3, 3239–3242. [Google Scholar] [CrossRef]
- Reczek, J.J.; Iverson, B.L. Using aromatic donor acceptor interactions to affect macromolecular assembly. Macromolecules 2006, 39, 5601–5603. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, K.; Kumar, A.; Wüthrich, K.; Ernst, R.R. Experimental techniques of two-dimensional correlated spectroscopy. J. Magn. Reson. 1980, 40, 321–334. [Google Scholar] [CrossRef]
- Jeener, J.; Meier, B.H.; Bachmann, P.; Ernst, R.R. Investigation of exchange processes by two-dimensional NMR spectroscopy. J. Chem. Phys. 1979, 71, 4546–4553. [Google Scholar] [CrossRef]
- Bax, A.D.; Griffey, R.H.; Hawkins, B.L. Correlation of proton and nitrogen-15 chemical shifts by multiple quantum NMR. J. Magn. Reson. 1983, 55, 301–315. [Google Scholar] [CrossRef]
- Bax, A.D.; Summers, M.F. Proton and carbon-13 assignments from sensitivity-enhanced detection of heteronuclear multiple-bond connectivity by 2D multiple quantum NMR. J. Am. Chem. Soc. 1986, 108, 2093–2094. [Google Scholar] [CrossRef]
| Polymer | Mp (kg/mol) | Mw (kg/mol) | PDI | Rg (nm) | K (nm) | α |
|---|---|---|---|---|---|---|
| poly-1a | 44.1 | 38.8 | 1.5 | 7.7 | 0.0104 | 0.61 |
| poly-1b | 365 | 400 | 1.9 | 22.4 | 0.0261 | 0.51 |
| poly-1c | 754 | 782 | 2.0 | 32.3 | 0.0117 | 0.56 |
| poly-1d | 181 | 191 | 1.8 | 13.0 | 0.0212 | 0.51 |
| poly-1e | 748 | 831 | 3.0 | 31.0 | 0.0253 | 0.50 |
| poly-1f | nd a | nd a | nd a | nd a | nd a | nd a |
| poly-1g | 2950 | 2269 | 2.8 | 58.5 | 0.0433 | 0.48 |
| poly-1h | 66.8 | 59.3 | 2.4 | 8.7 | 0.1670 | 0.35 |
| poly-BF3k | 1900 | 1506 | 3.4 | 49.9 | 0.0066 | 0.60 |
| poly-6-MO-BF3k | 312 | 347 | 4.3 | 19.4 | 0.0057 | 0.61 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paolino, M.; Grisci, G.; Reale, A.; Razzano, V.; Giuliani, G.; Donati, A.; Mendichi, R.; Piovani, D.; Boccia, A.C.; Grillo, A.; et al. Structural Manipulation of the Conjugated Phenyl Moiety in 3-Phenylbenzofulvene Monomers: Effects on Spontaneous Polymerization. Polymers 2018, 10, 752. https://doi.org/10.3390/polym10070752
Paolino M, Grisci G, Reale A, Razzano V, Giuliani G, Donati A, Mendichi R, Piovani D, Boccia AC, Grillo A, et al. Structural Manipulation of the Conjugated Phenyl Moiety in 3-Phenylbenzofulvene Monomers: Effects on Spontaneous Polymerization. Polymers. 2018; 10(7):752. https://doi.org/10.3390/polym10070752
Chicago/Turabian StylePaolino, Marco, Giorgio Grisci, Annalisa Reale, Vincenzo Razzano, Germano Giuliani, Alessandro Donati, Raniero Mendichi, Daniele Piovani, Antonella C. Boccia, Alessandro Grillo, and et al. 2018. "Structural Manipulation of the Conjugated Phenyl Moiety in 3-Phenylbenzofulvene Monomers: Effects on Spontaneous Polymerization" Polymers 10, no. 7: 752. https://doi.org/10.3390/polym10070752