Dopamine-Dyed and Functionally Finished Silk with Rapid Oxidation Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. The Preparation of PDA–Fe3+–Silk Fabric
2.3. Characterization and Measurements
2.3.1. Color Measurement
2.3.2. The Color Fastness Test
2.3.3. The Tensile Fracture Strength Test
2.3.4. SEM Analysis
2.3.5. AFM Analysis
2.3.6. XPS Study
2.3.7. The UV-Protection Factor of Colored Silk Fabric
2.3.8. Water Contact Angle Measurement
3. Results and Discussion
3.1. Effect of Reaction Conditions on the Dyeing of PDA Silk
3.2. The Color Fastness Test
3.3. The Fabric Strength Test
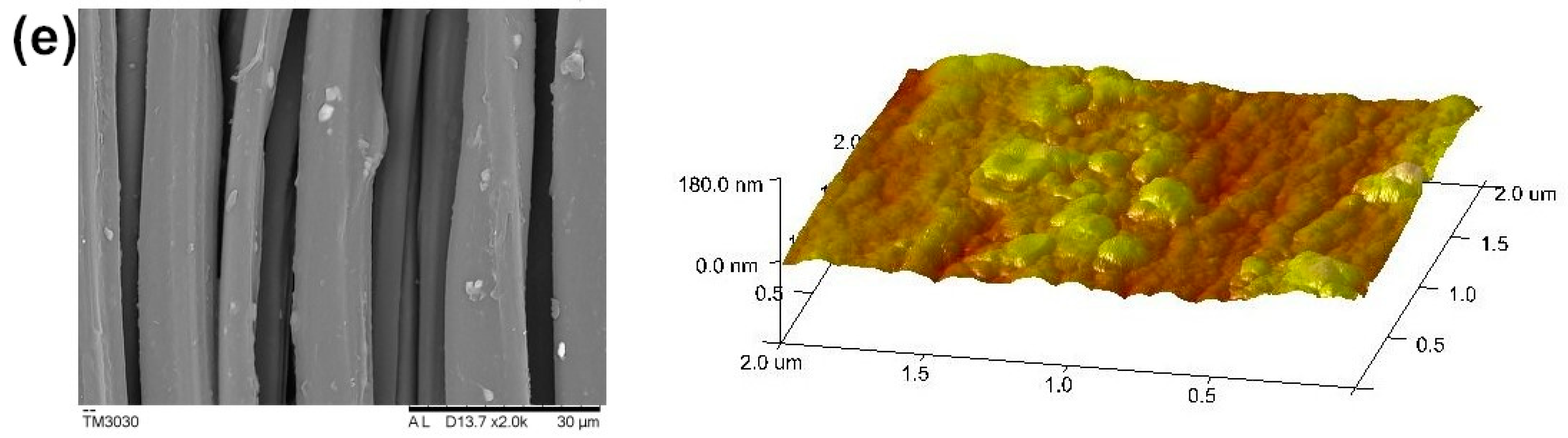
3.4. Surface Morphology Analysis
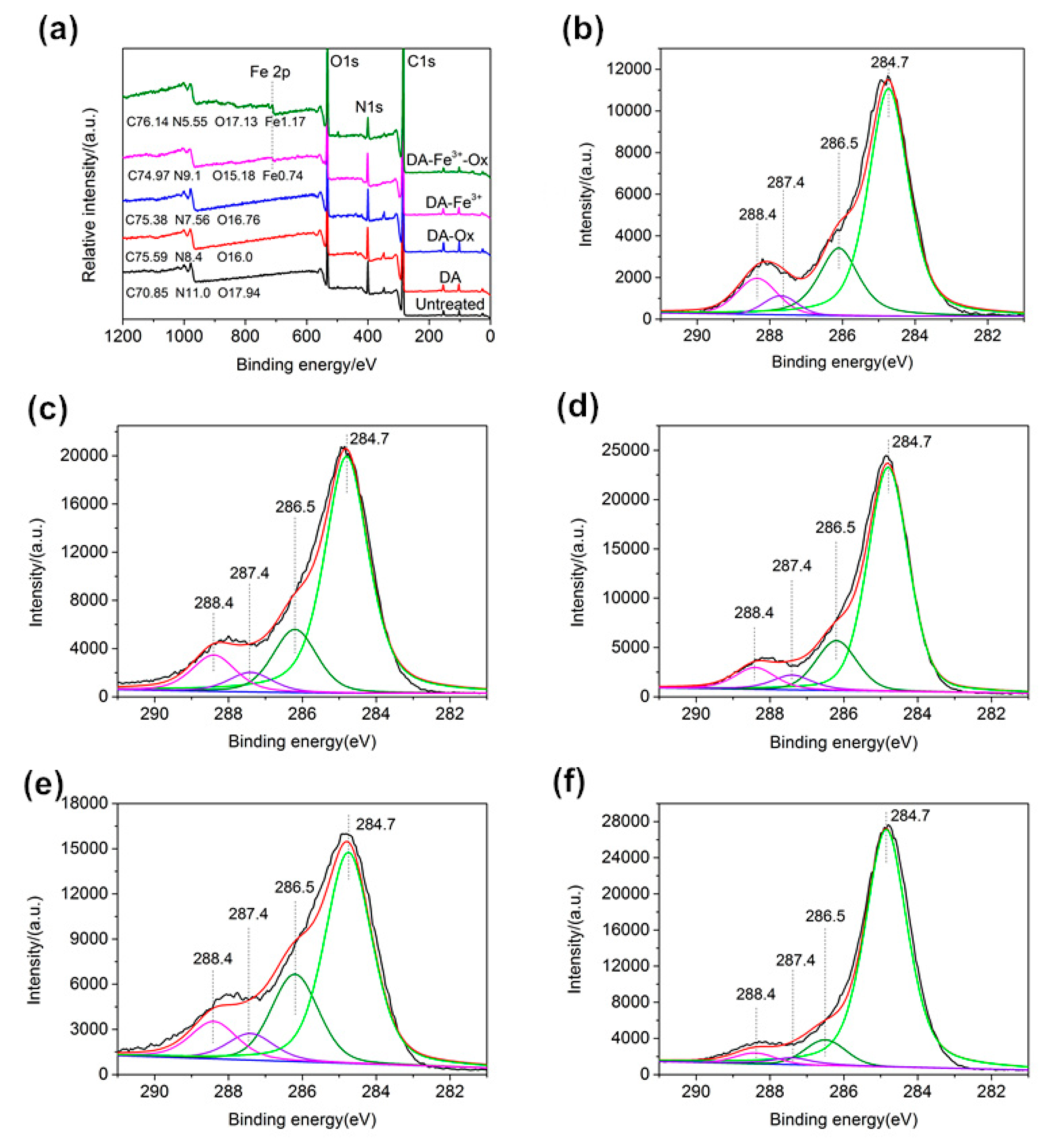
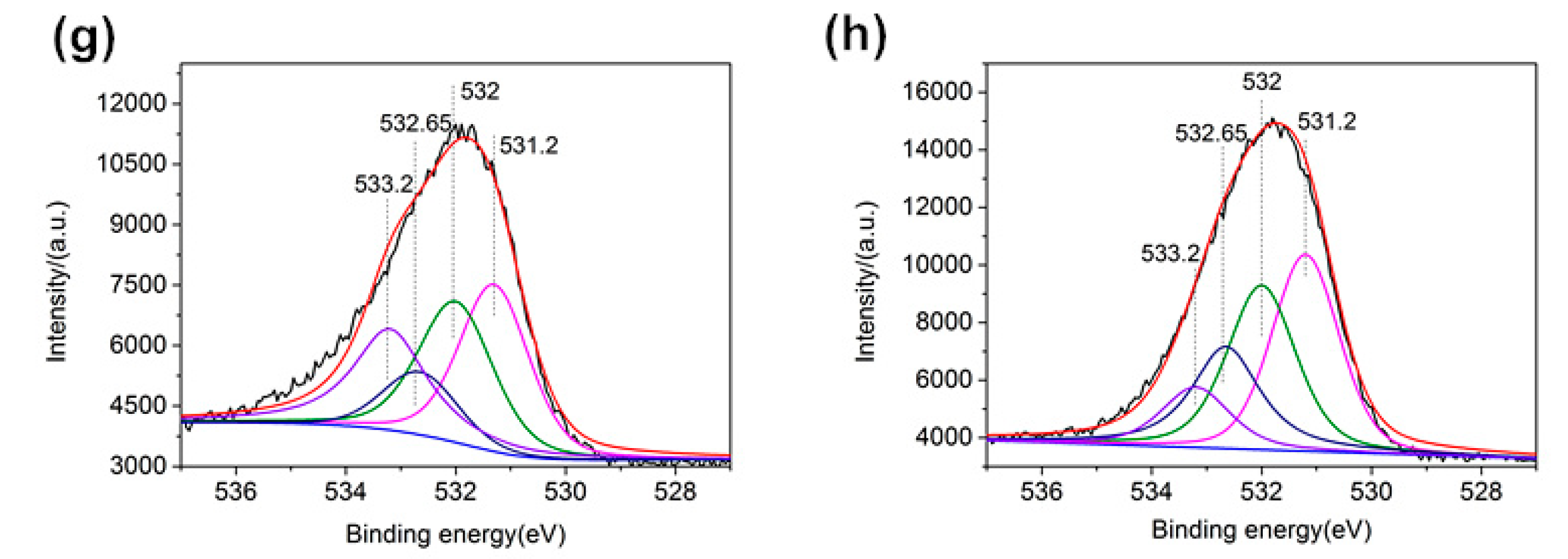
3.5. XPS Analysis
3.6. Anti-Ultraviolet and Hydrophobic Properties Test
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tang, G.; Zhao, P.; Wang, Y.; Gao, W.; Cheng, M.; Xin, J.; Li, X.; Wang, Y. Mortality and air pollution in Beijing: The long-term relationship. Atmos. Environ. 2017, 150, 238–243. [Google Scholar] [CrossRef]
- Currell, M.J.; Han, D. The global drain: Why China’s water pollution problems should matter to the rest of the world. Environ. Sci. Policy Sustain. Dev. 2017, 59, 16–29. [Google Scholar] [CrossRef]
- Ito, T.; Adachi, Y.; Yamanashi, Y. Long–term natural remediation process in textile dye–polluted river sediment driven by bacterial community changes. Water. Res. 2016, 100, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Hou, A.; Shi, Y.; Yu, Y. Preparation of the cellulose/silica hybrid containing cationic group by sol–gel crosslinking process and its dyeing properties. Carbohydr. Polym. 2009, 77, 201–205. [Google Scholar] [CrossRef]
- Zheng, L.; Zheng, H.; Du, B.; Wei, J.; Gao, S.; Zhang, J. Dyeing procedures of polyester fiber in supercritical carbon dioxide using a special dyeing frame. J. Eng. Fiber Fabr. 2015, 10, 37–46. [Google Scholar]
- Ferrero, F.; Periolatto, M. Ultrasound for low temperature dyeing of wool with acid dye. Ultrason. Sonochem. 2012, 19, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Hussain, T.; Nawaz, R. Optimization of alkaline extraction of natural dye from Henna leaves and its dyeing on cotton by exhaust method. J. Clean. Prod. 2009, 17, 61–66. [Google Scholar] [CrossRef]
- Platzek, T.; Lang, C.; Grohmann, G.; Gi, U.S.; Baltes, W. Formation of a carcinogenic aromatic amine from an azo dye by human skin bacteria in vitro. Hum. Exp. Toxicol. 1999, 18, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, T.; Mussak, R.; Mahmudali, A.; Ganglberger, E.; Geissler, S. Extraction of natural dyes for textile dyeing from coloured plant wastes released from the food and beverage industry. J. Sci. Food. Agric. 2010, 86, 233–242. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, Y.; Zhang, Y.; Guo, Z.; Zhang, H.; Xin, J.; Zhang, L. Bioinspired superhydrophobic Fe3O4@polydopamine@Ag hybrid nanoparticles for liquid marble and oil spill. Adv. Mater. Interfaces 2015, 2, 1–10. [Google Scholar] [CrossRef]
- Yang, J.; Xu, H.; Zhang, L.; Zhong, Y.; Sui, X.; Mao, Z. Lasting superhydrophobicity and antibacterial activity of Cu nanoparticles immobilized on the surface of dopamine modified cotton fabrics. Surf. Coat. Technol. 2017, 309, 149–154. [Google Scholar] [CrossRef]
- Lu, Z.; Xiao, J.; Wang, Y.; Meng, M. In situ synthesis of silver nanoparticles uniformly distributed on polydopamine-coated silk fibers for antibacterial application. J. Colloid Interface Sci. 2015, 452, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Miyazaki, K.; Hori, T. Fabrication of polydopamine-coated superhydrophobic fabrics for oil/water separation and self-cleaning. Appl. Surf. Sci. 2016, 370, 243–251. [Google Scholar] [CrossRef]
- Son, H.Y.; Ryu, J.H.; Lee, H.; Nam, Y. Silver-Polydopamine Hybrid Coatings of Electrospun Poly(vinyl alcohol) Nanofibers. Macromol. Mater. Eng. 2013, 298, 547–554. [Google Scholar] [CrossRef]
- Peng, Y.; Yu, Z.; Li, F.; Chen, Q.; Yin, D.; Min, X. A novel reduced graphene oxide-based composite membrane prepared via a facile deposition method for multifunctional applications: Oil/water separation and cationic dyes removal. Sep. Purif. Technol. 2018, 200, 130–140. [Google Scholar] [CrossRef]
- Cheng, D.; He, M.; Ran, J.; Cai, G.; Wu, J.; Wang, X. In situ reduction of TiO2, nanoparticles on cotton fabrics through polydopamine templates for photocatalysis and UV protection. Cellulose 2017, 25, 1413–1424. [Google Scholar] [CrossRef]
- Ran, J.; He, M.; Li, W.; Cheng, D.; Wang, X. Growing ZnO nanoparticles on polydopamine-templated cotton fabrics for durable antimicrobial activity and UV protection. Polymers 2018, 10, 495. [Google Scholar] [CrossRef]
- Fu, J.; Xin, Q.; Wu, X.; Chen, Z.; Yan, Y.; Liu, S.; Wang, M.; Xu, Q. Selective adsorption and separation of organic dyes from aqueous solution on polydopamine microspheres. J. Colloid Interfaces Sci. 2016, 461, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, Z.; He, Y.; Fan, Y.; Ma, J.; Yin, X. Facile way in fabricating a cotton fabric membrane for switchable oil/water separation and water purification. Appl. Surf. Sci. 2018, 441, 500–507. [Google Scholar] [CrossRef]
- He, L.; So, V.L.L.; Xin, J.H. Dopamine polymerization-induced surface colouration of various materials. RSC Adv. 2014, 4, 20317–20322. [Google Scholar] [CrossRef]
- Im, K.M.; Kim, T.W.; Jeon, J.R. Metal-Chelation-Assisted Deposition of Polydopamine on Human Hair: A Ready-to-Use Eumelanin-Based Hair Dyeing Methodology. ACS Biomater. Sci. Eng. 2017, 3, 628–636. [Google Scholar] [CrossRef]
- Jia, W.; Wang, Q.; Fan, X.; Dong, A.; Yu, Y.; Wang, P. Laccase-mediated in situ oxidation of dopa for bio-inspired coloration of silk fabric. RSC Adv. 2017, 7, 12977–12983. [Google Scholar] [CrossRef] [Green Version]
- Mao, Y.; Zhu, M.; Wang, W.; Yu, D. Well-defined silver conductive pattern fabricated on polyester fabric by screen printing a dopamine surface modifier followed by electroless plating. Soft Matter 2018, 14, 1260–1269. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhang, Z.; Yang, M.; Wang, W.; Men, X.; Liu, W. POSS grafted hybrid-fabric composites with a biomimic middle layer for simultaneously improved UV resistance and tribological properties. Compos. Sci. Technol. 2018, 160, 69–78. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Elucidating the structure of poly(dopamine). Langmuir 2012, 28, 6428–6435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Z.; Zhang, X.; Li, Y.; Wang, H.; Wang, J.; Zhu, Y. High-adhesive superhydrophobic litchi-like coatings fabricated by in-situ growth of nano-silica on polyethersulfone surface. Chem. Eng. J. 2018, 343, 699–707. [Google Scholar] [CrossRef]
- Holten-Andersen, N.; Harrington, M.J.; Birkedal, H.; Lee, B.P.; Messersmith, P.B.; Lee, K.Y.C.; Waite, J.H. pH-induced metal-ligand cross-links inspired by mussel yield self-healing polymer networks with near-covalent elastic moduli. Proc. Natl. Acad. Sci. USA 2011, 108, 2651–2655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Li, H. Dopamine deposited rapidly on the surface of PET fabric by UV radiation and electroless nickel plating. Mater. Res. Innov. 2015, 19, 174–179. [Google Scholar] [CrossRef]
- Xu, C.X.; Huang, K.J.; Fan, Y.; Wu, Z.W.; Li, J.; Gan, T. Simultaneous electrochemical determination of dopamine and tryptophan using a TiO-graphene/poly(4-aminobenzenesulfonic acid) composite film based platform. Mater. Sci. Eng. C Mater. 2012, 32, 969–974. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Tang, L.; Lu, J.; Li, J. Application of graphene-modified electrode for selective detection of dopamine. Electrochem. Commun. 2009, 11, 889–892. [Google Scholar] [CrossRef]
- Fan, L.; Li, B.; Zhang, J. Antibioadhesive Superhydrophobic Syringe Needles Inspired by Mussels and Lotus Leafs. Adv. Mater. Interfaces 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.; Purich, D.; Wu, C.; Wu, Y.; Chen, T.; Cui, C.; Zhang, L.; Cansiz, S.; Hou, W.; Wang, Y.; et al. Ionic Functionalization of Hydrophobic Colloidal Nanoparticles to Form Ionic Nanoparticles with Enzymelike Properties. J. Am. Chem. Soc. 2015, 137, 14952–14958. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, Y.M.; Velikov, K.P.; Kegel, W.K. Colloidal stability and chemical reactivity of complex colloids containing Fe3+. Food. Chem. 2014, 155, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Dai, F.; Ren, Y.; Liu, H.; Chen, L.; Yang, P.; Liu, Y.; Li, X.; Wang, W.; Xiang, H. Comparative transcriptome analyses on silk glands of six silkmoths imply the genetic basis of silk structure and coloration. BMC Genom. 2015, 16, 203. [Google Scholar]

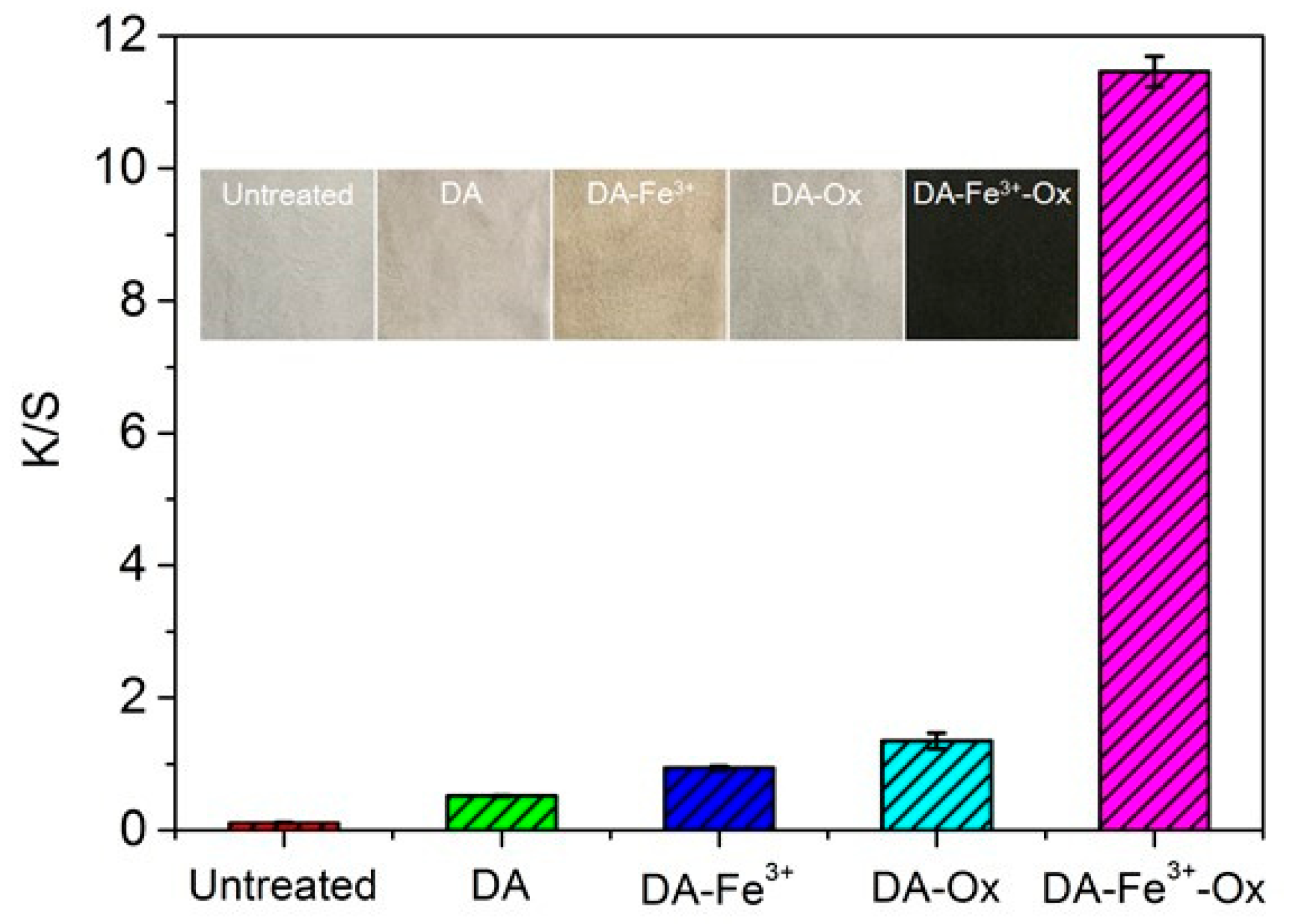
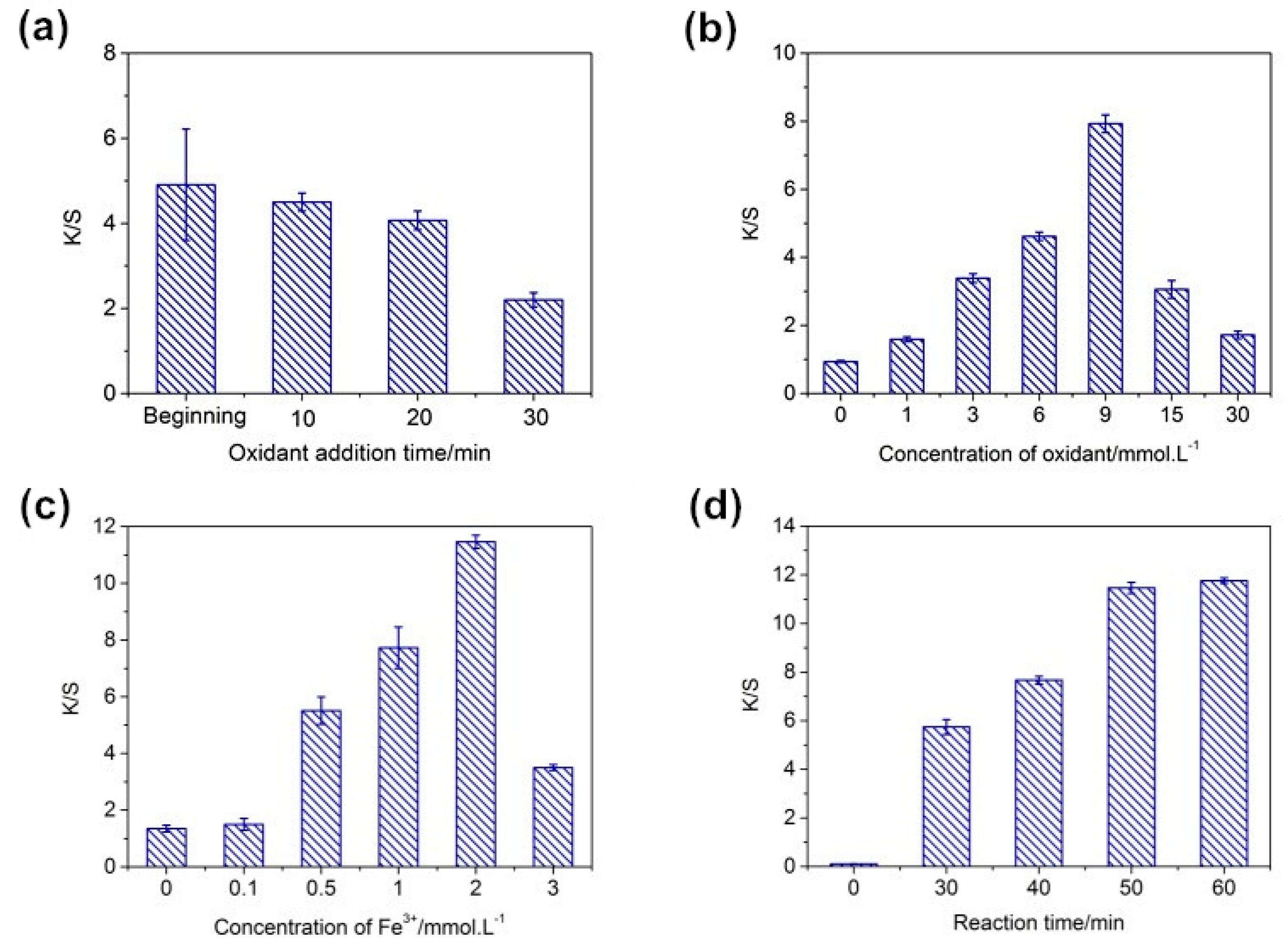
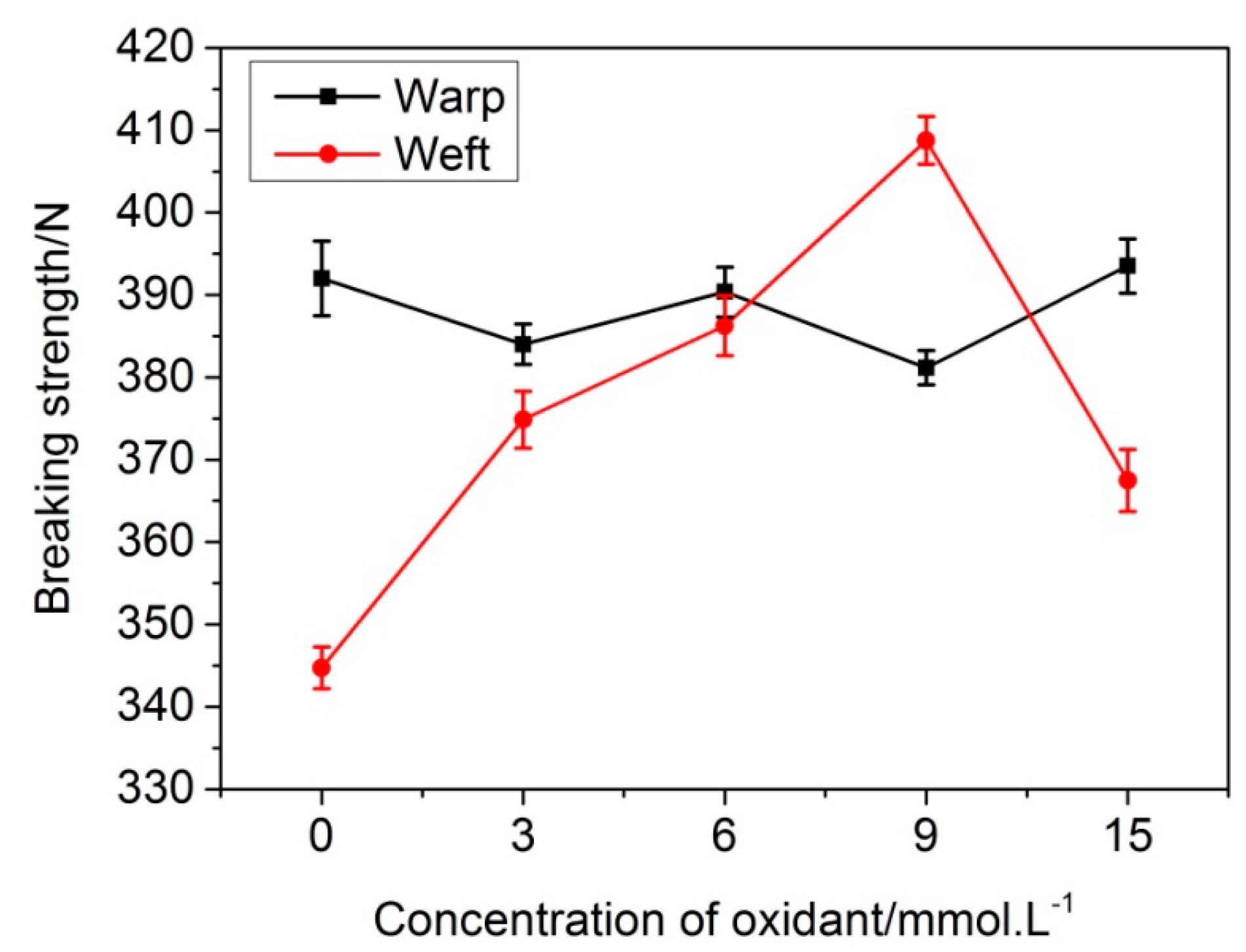
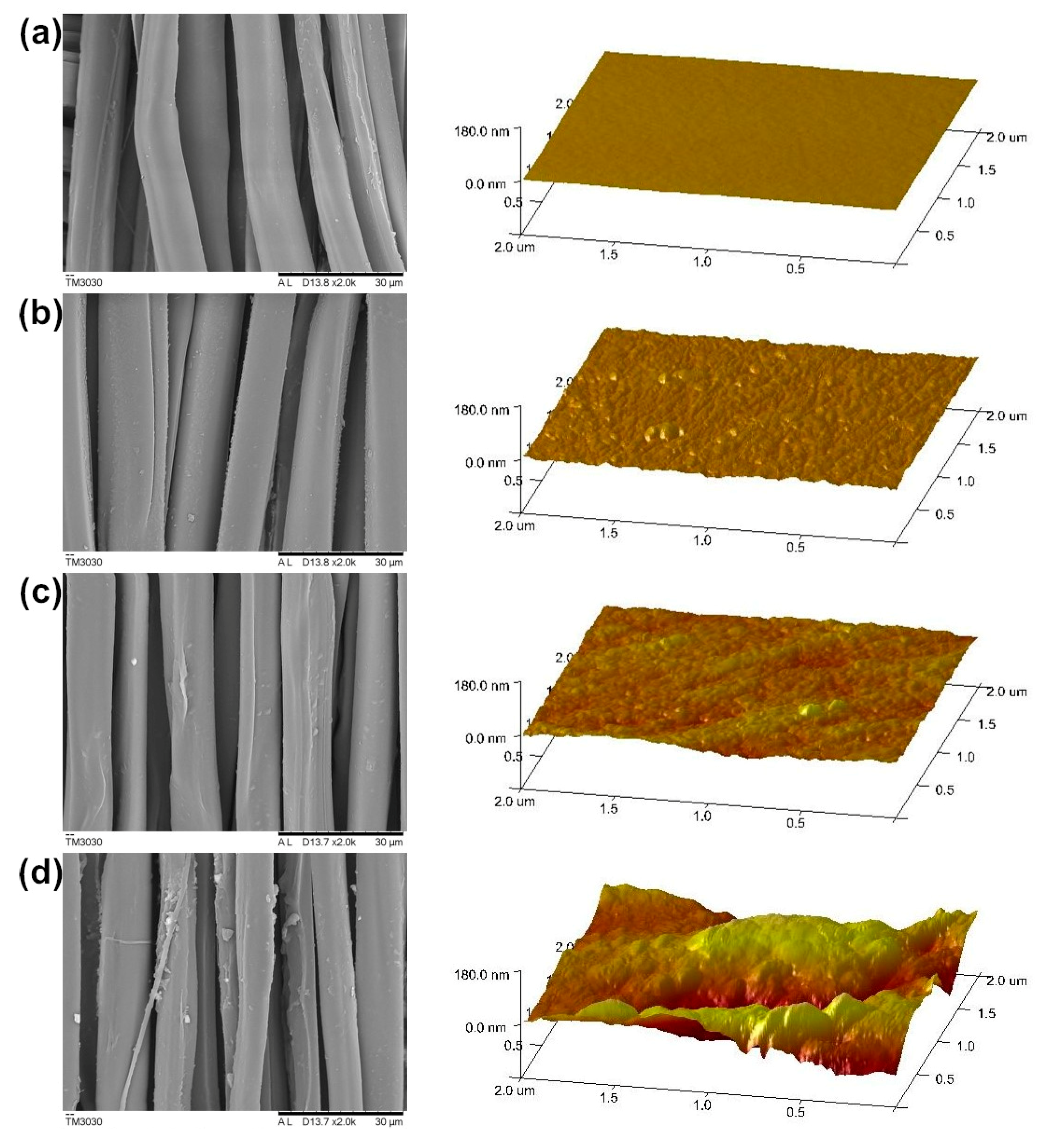
| Samples | L* | a* | b* | c* | h |
|---|---|---|---|---|---|
| Untreated | 95.16 | −0.26 | 2.23 | 2.24 | 96.65 |
| DA | 76.07 | 0.42 | 1.5 | 1.56 | 74.35 |
| DA–Fe3+ | 73.79 | 1.04 | 5.48 | 5.58 | 79.23 |
| DA–Ox | 64.43 | 0.58 | 5.16 | 5.19 | 83.59 |
| DA–Fe3+–Ox | 25.59 | 1.24 | −1.33 | 1.82 | 313.09 |
| Samples | Washing Fastness | Rubbing Fastness | Light Fastness | |||
|---|---|---|---|---|---|---|
| Fading | Cotton Staining | Silk Staining | Dry | Wet | ||
| DA | 4 | 4–5 | 4–5 | 5 | 4 | 4 |
| DA–Fe3+ | 4 | 4 | 4–5 | 4–5 | 4 | 3 |
| DA–Ox | 5 | 5 | 5 | 5 | 4 | 4 |
| DA–Fe3+–Ox | 4 | 4 | 4 | 4 | 3–4 | 4 |
| Samples | Transmittance/% | UPF | Water Contact Angle (°) | ||
|---|---|---|---|---|---|
| UVA | UVB | ||||
| untreated | 40.67% | 18.77% | 3.84 | 0 |  |
| DA | 28.31% | 13.37% | 5.61 | 0 |  |
| DA–Fe3+ | 22.65% | 12.43% | 6.38 | 126.5 ± 1.3 |  |
| DA–Ox | 15.82% | 8.62% | 9.26 | 26.7 ± 0.8 |  |
| DA–Fe3+–Ox | 3.73% | 2.85% | 33.16 | 142.1 ± 1.5 |  |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, B.; Zhou, Q.; Xing, T.; Chen, G. Dopamine-Dyed and Functionally Finished Silk with Rapid Oxidation Polymerization. Polymers 2018, 10, 728. https://doi.org/10.3390/polym10070728
Yan B, Zhou Q, Xing T, Chen G. Dopamine-Dyed and Functionally Finished Silk with Rapid Oxidation Polymerization. Polymers. 2018; 10(7):728. https://doi.org/10.3390/polym10070728
Chicago/Turabian StyleYan, Biaobiao, Qingqing Zhou, Tieling Xing, and Guoqiang Chen. 2018. "Dopamine-Dyed and Functionally Finished Silk with Rapid Oxidation Polymerization" Polymers 10, no. 7: 728. https://doi.org/10.3390/polym10070728





