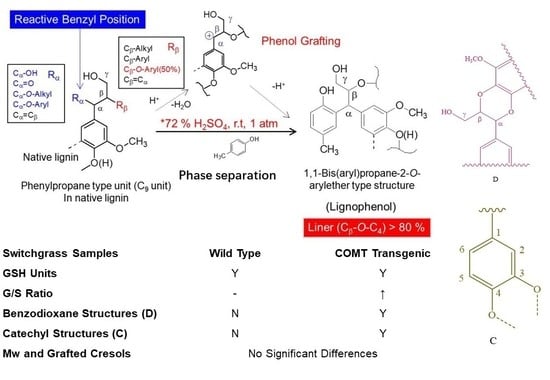
Structural Characterization of Lignocresols from Transgenic and Wild-Type Switchgrass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
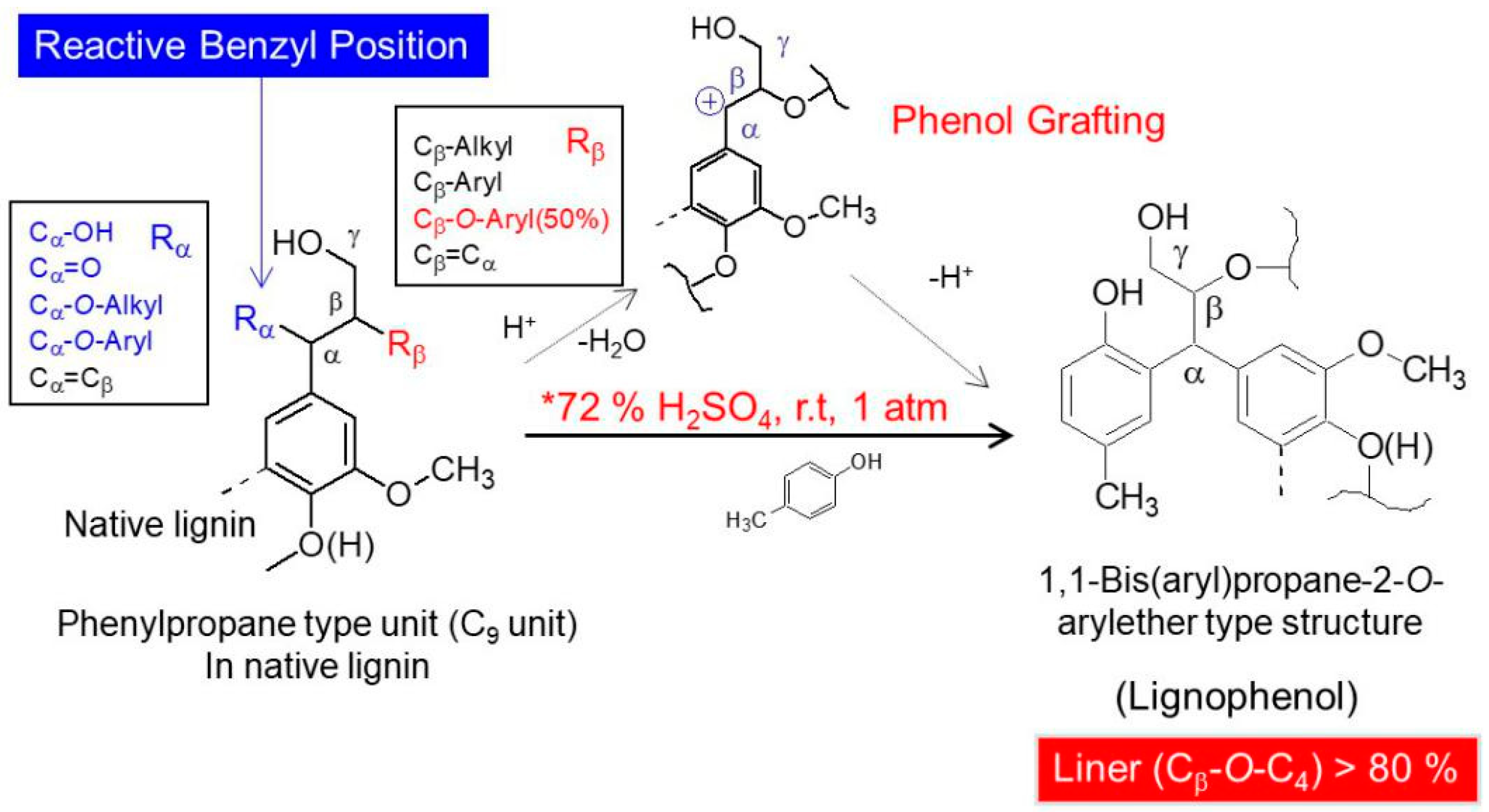
2.2. Phase Separation Procedure
2.3. Characterization of LCs
3. Results and Discussions
3.1. Yields of LCs from Switchgrass through Phase Separation
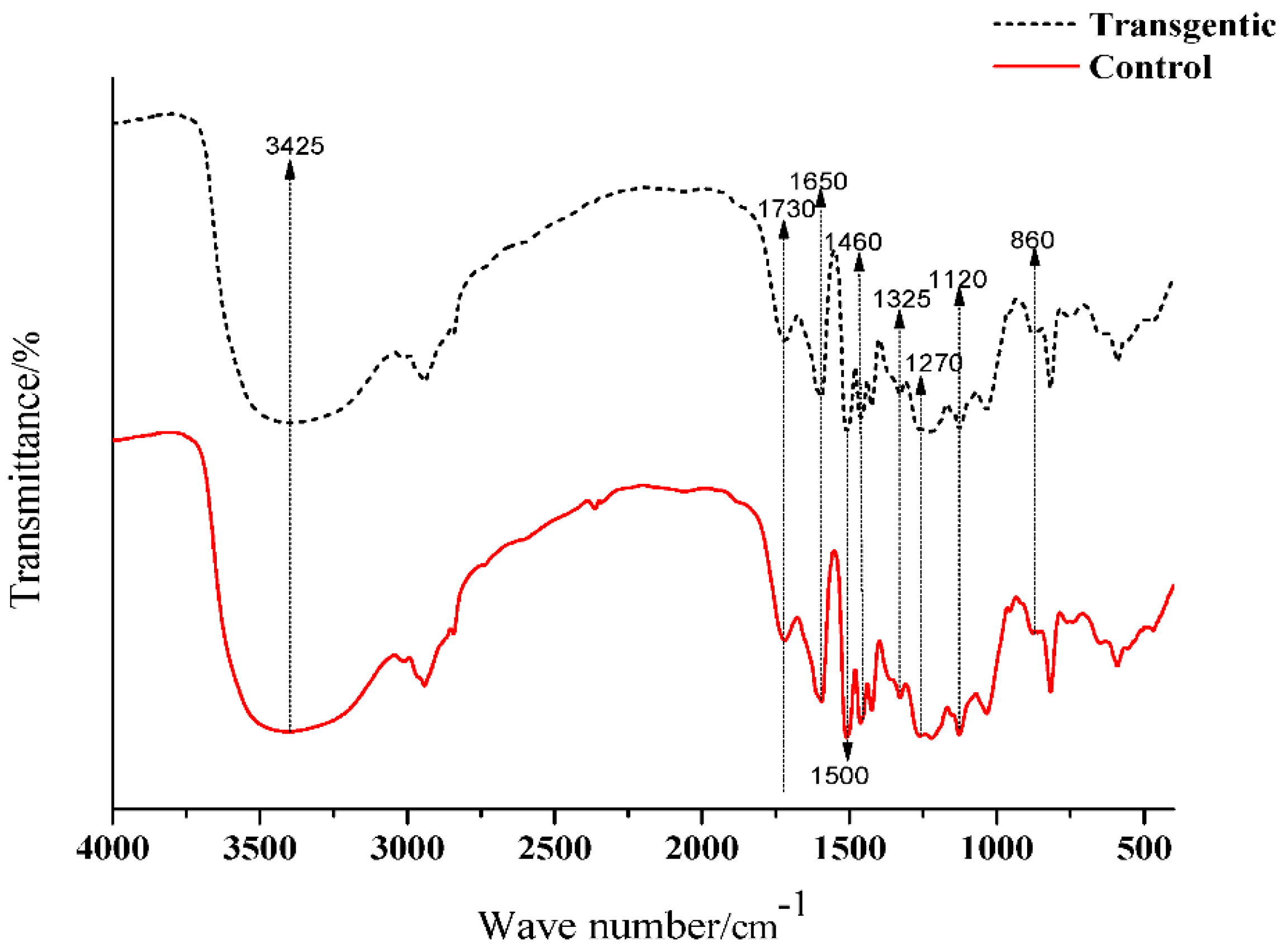
3.2. FTIR Spectra of Switchgrass LCs
3.3. Molecular Weights of LCs and Amounts of Incorporated p-Cresols
3.4. Elemental Analysis and Methoxyl Content
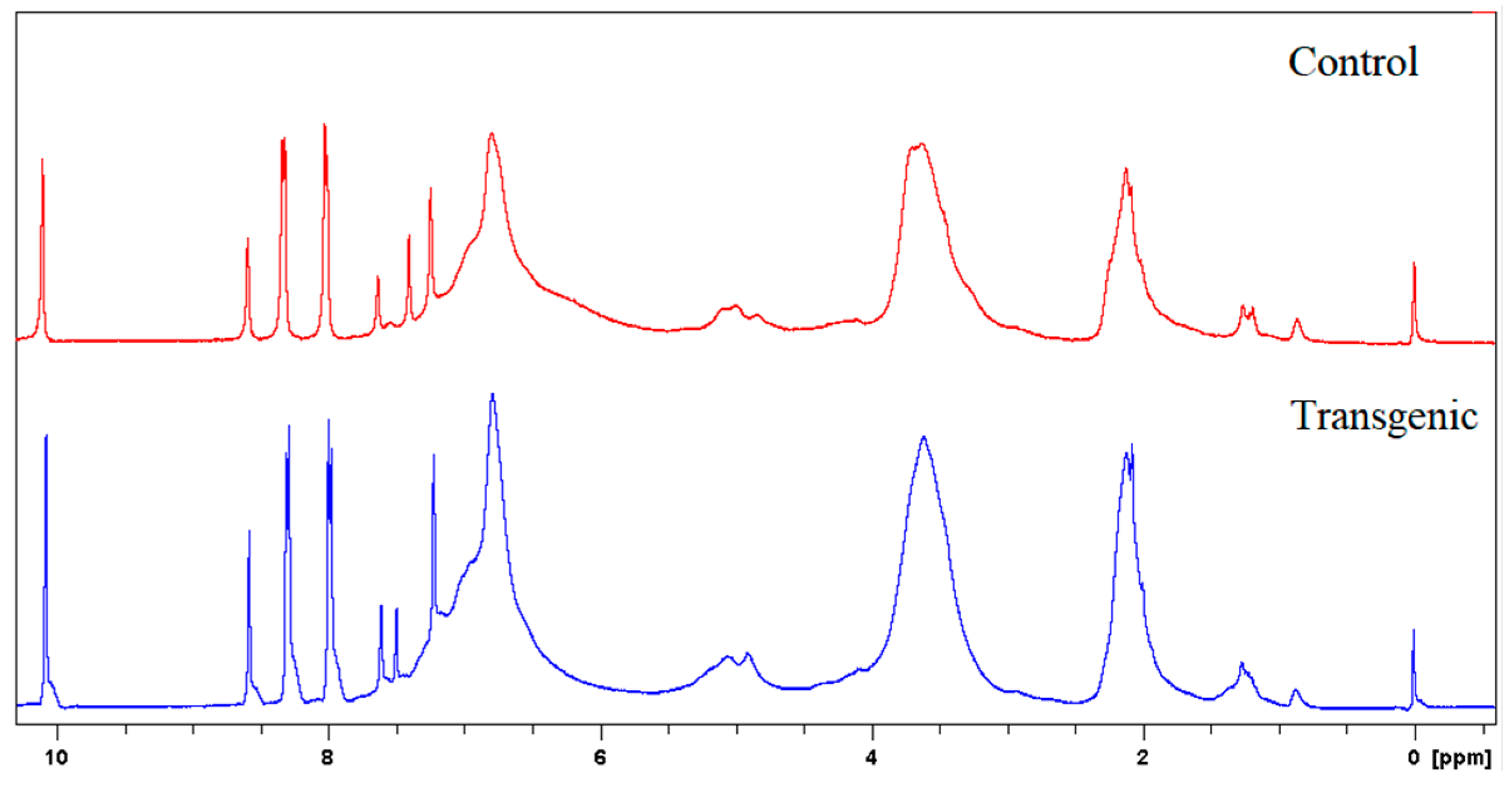
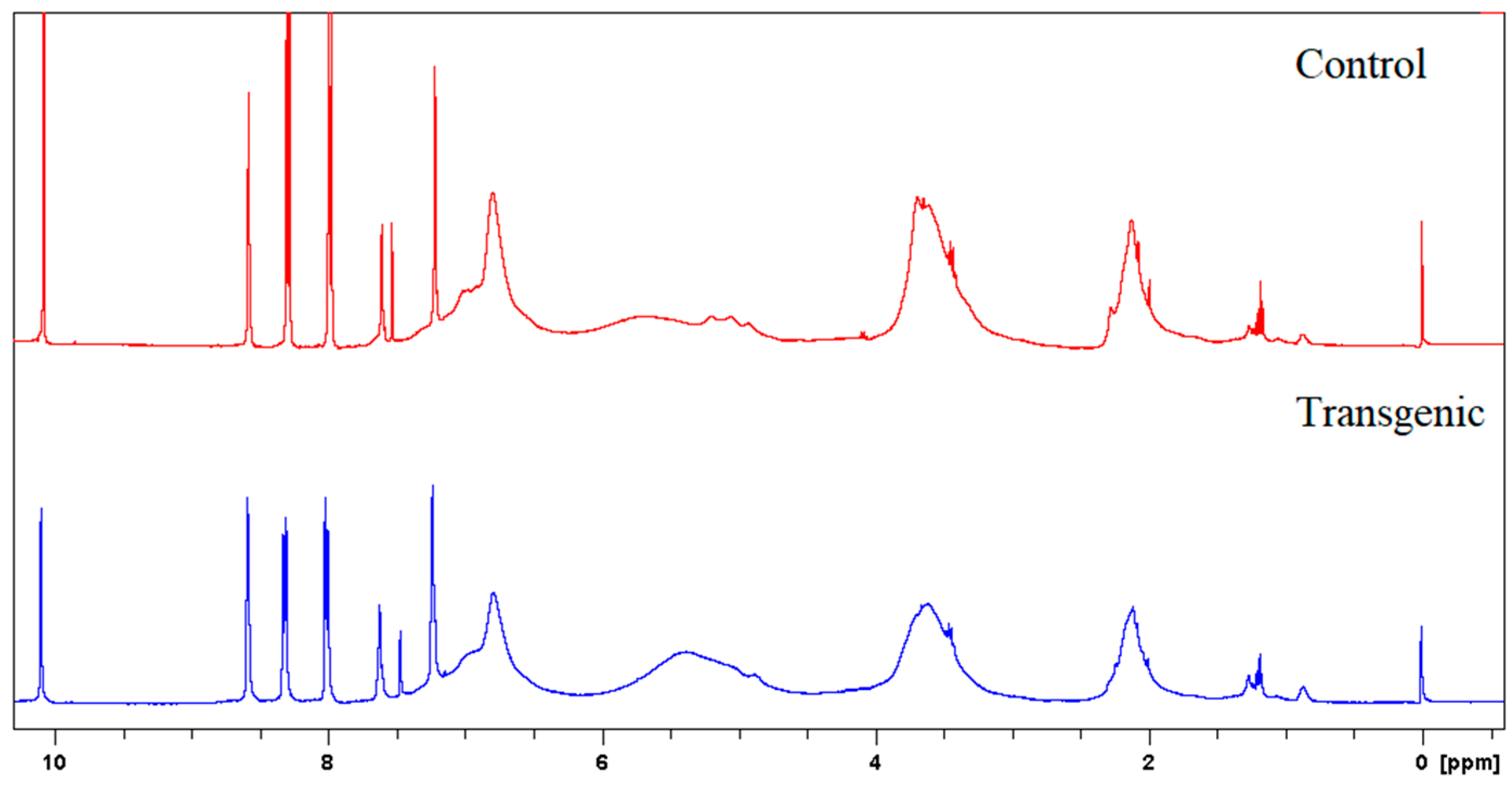
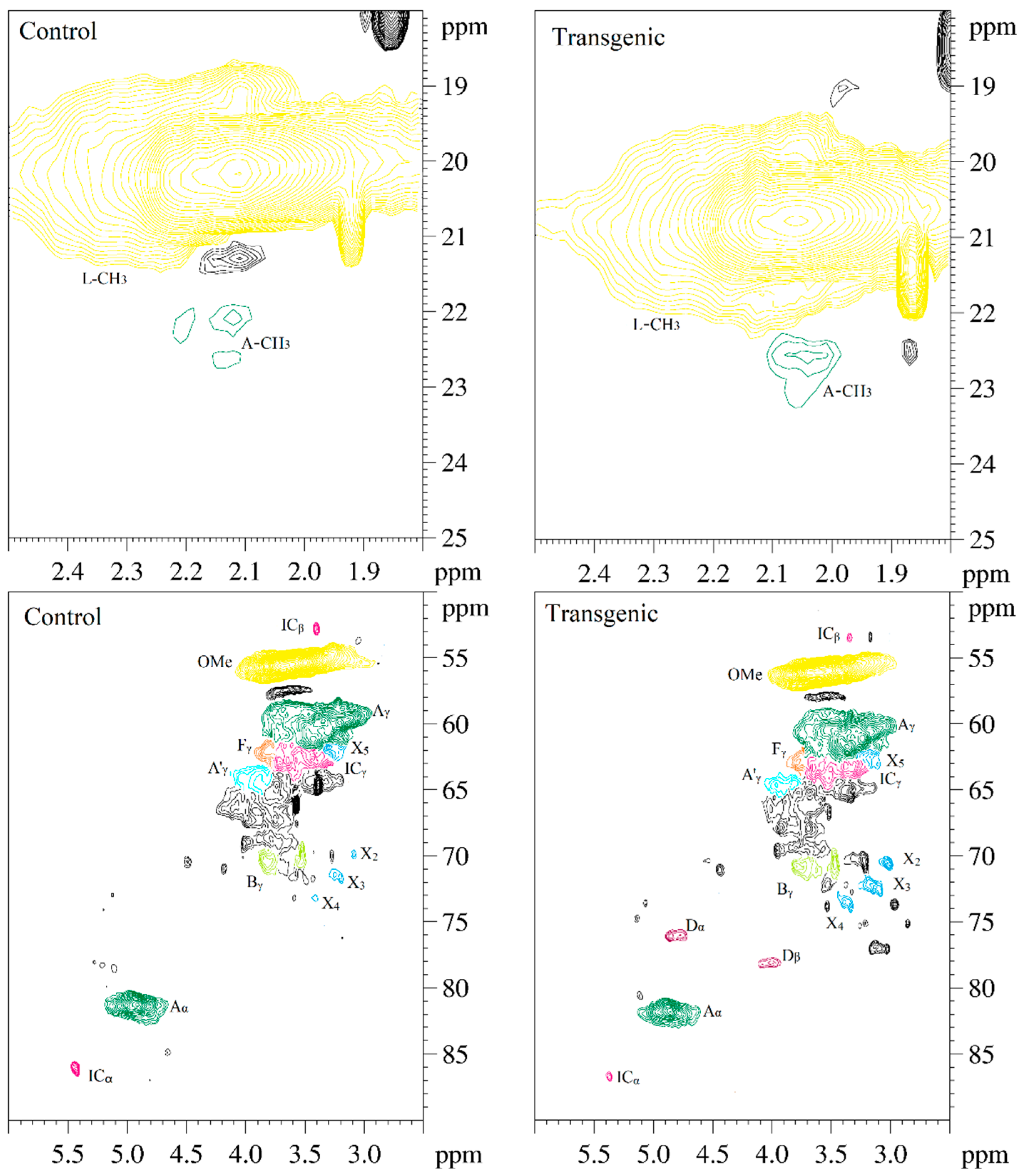
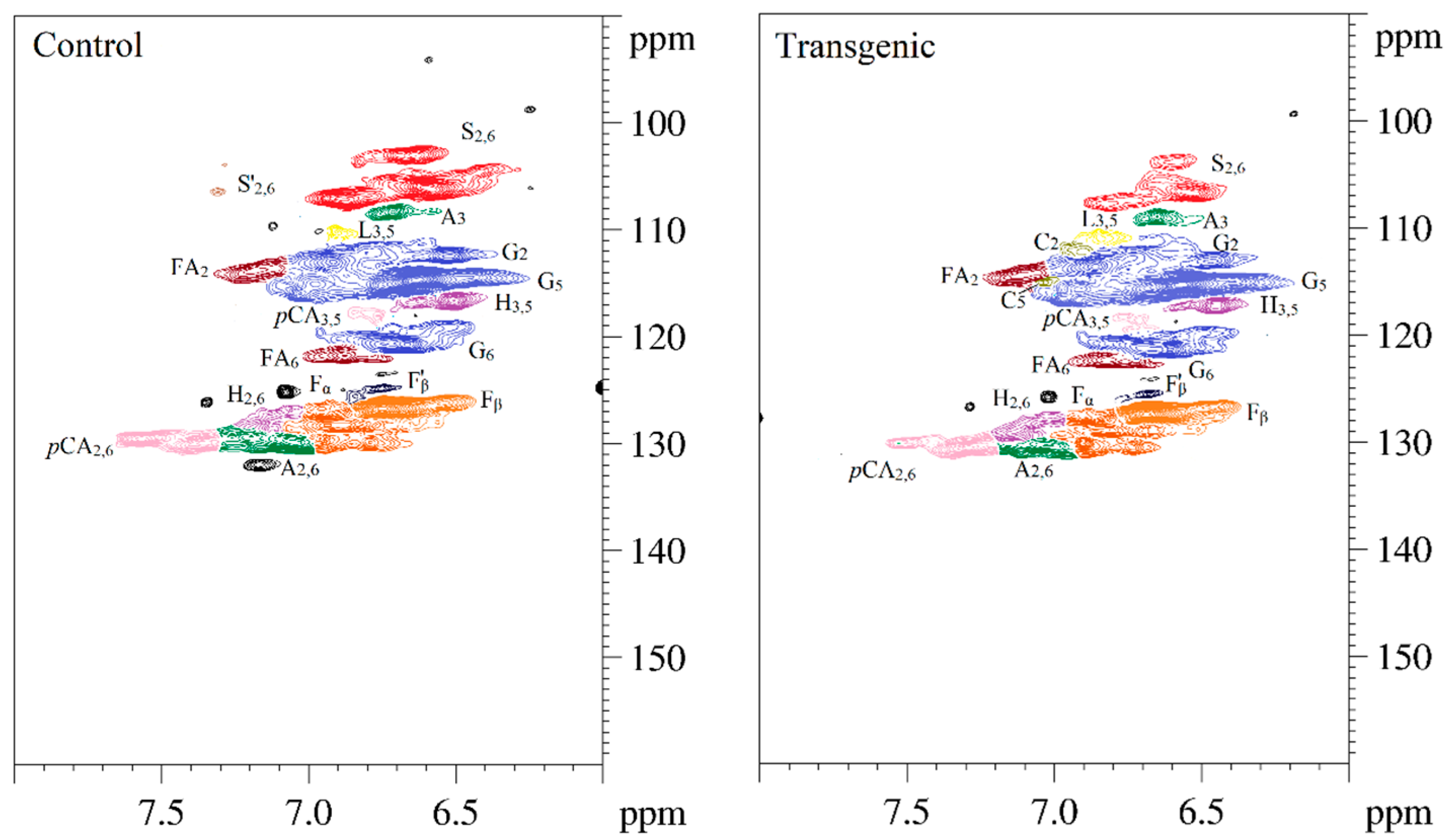
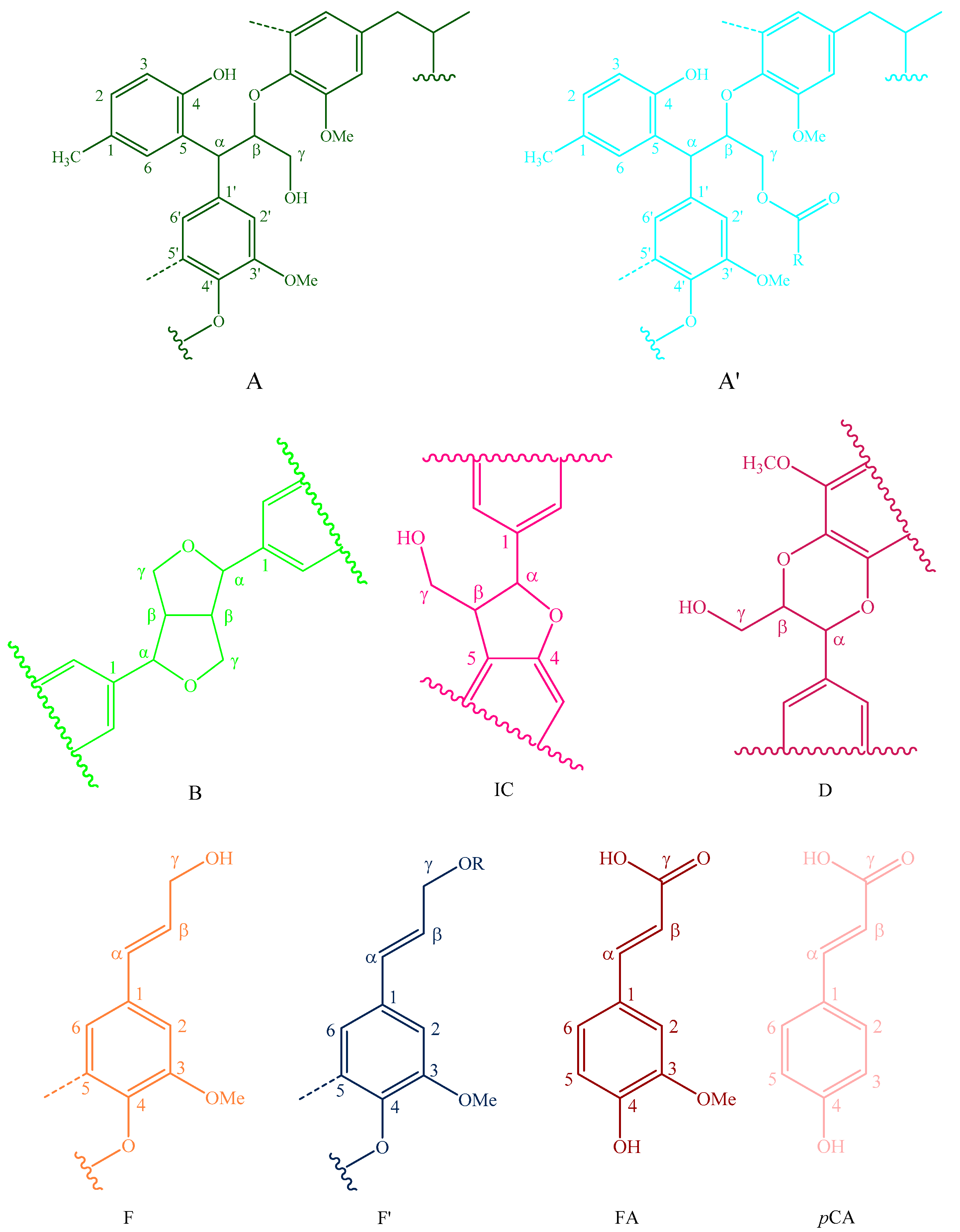
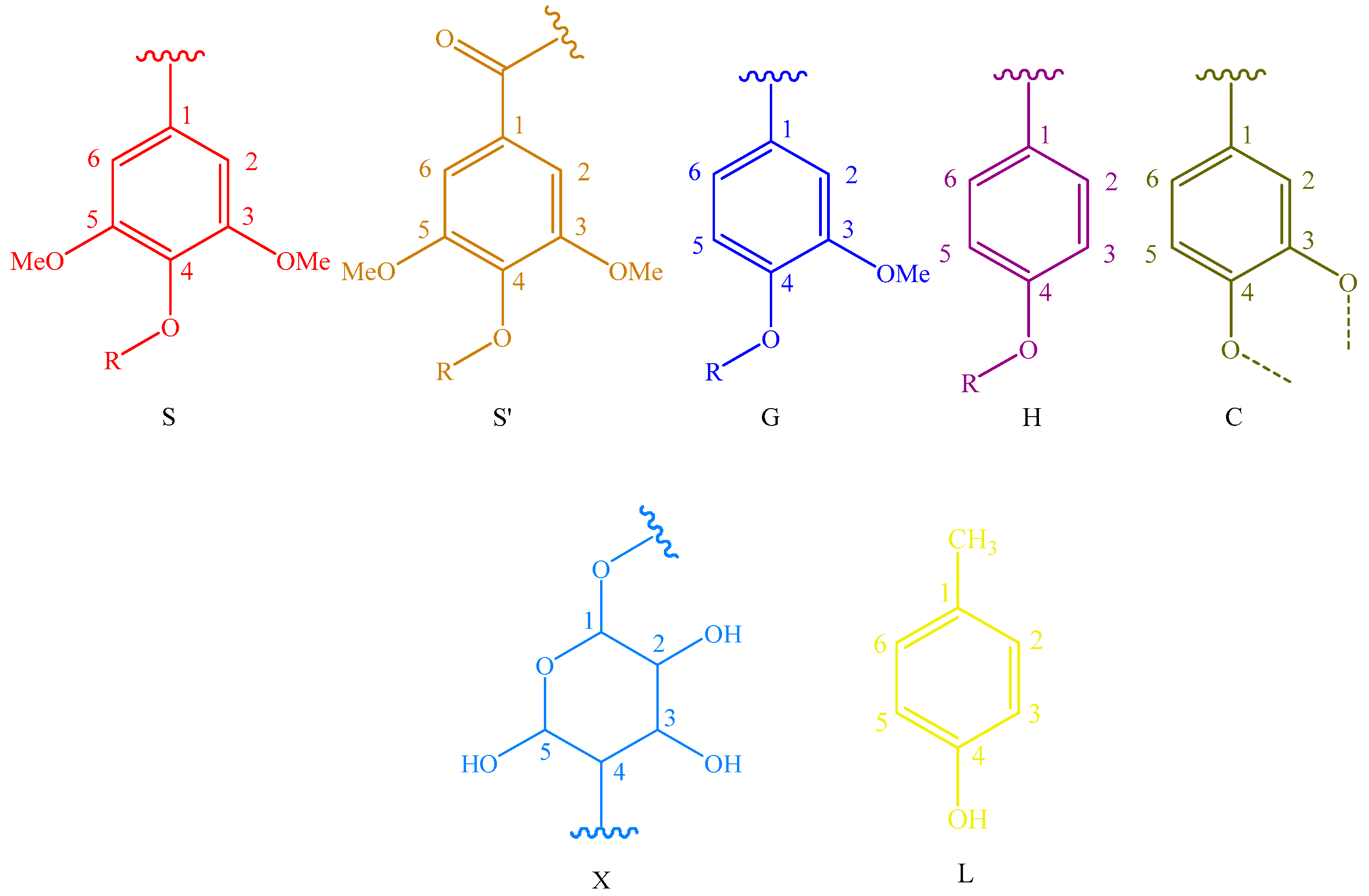
3.5. 2D-HSQC NMR Spectra of LCs from Transgenic and Control Switchgrass
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mcginnis, R.L.; Elimelech, M. Global challenges in energy and water supply: The promise of engineered osmosis. Environ. Sci. Technol. 2008, 42, 8625–8629. [Google Scholar] [CrossRef]
- Dorian, J.P.; Franssen, H.T.; Simbeck, D.R. Global challenges in energy. Energy Policy 2006, 34, 1984–1991. [Google Scholar] [CrossRef]
- Calvin, M. Solar energy by photosynthesis. Science 1974, 184, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Ragauskasa, J.; Williamsc, K.; Davison, B.H. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Mclaughlin, S.B.; Kszos, L.A. Development of switchgrass (Panicum virgatum) as a bioenergy feedstock in the United States. Biomass Bioenergy 2006, 28, 515–535. [Google Scholar] [CrossRef]
- Fu, C.X.; Mielenz, J.R.; Xiao, X.R. Genetix manipulation of lignin reduces recalcitrance and improves ethanol production from switchgrass. Proc. Natl. Acad. Sci. USA 2011, 108, 3803–3808. [Google Scholar] [CrossRef] [PubMed]
- Funaoka, M. A new type of phenolic lignin-based network polymer with the structure-variable function composed of 1,1-diarylpropane units. Polym. Int. 1998, 47, 277–290. [Google Scholar] [CrossRef]
- Mikame, K.; Funaoka, M. Polymer structure of lignophenol I—Structure and function of fractionated lignophenol—. Polymer 2006, 38, 585–591. [Google Scholar] [CrossRef]
- Funaoka, M.; Fukatsu, S. Characteristics of lignin structural conversion in a phase-separative reaction system composed of cresol and sulfuric acid. Holzforschung 1996, 50, 245–252. [Google Scholar] [CrossRef]
- Aberu, H.D.S.; Freire, M.D.F.I. Methoxyl content determination of lignins by 1H NMR. Anais da Academia Brasileira de Ciencias 1995, 67, 379–382. [Google Scholar]
- Mousavioun, P.; Doherty, W.O.S. Chemical and thermal properties of fractionated bagasse soda lignin. Ind. Crop. Prod. 2010, 31, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Ralph, J.; Akiyama, T. Solution-state 2D NMR of ball-milled plant cell wall gels. Bioenerg. Res. 2008, 1, 56–66. [Google Scholar] [CrossRef]
- Wen, J.L.; Sun, Z.J.; Sun, R.C. Structural characterization of alkali-extractable lignin fractions from bamboo. J. Biobased Mater. Bioenergy 2010, 4, 1–18. [Google Scholar] [CrossRef]
- Del Río, J.C.; Rencoret, J.; Prinsen, P.; Martínez, Á.T.; Ralph, J.; Gutiérrez, A. Structural characterization of wheat straw lignin as revealed by analytical pyrolysis, 2D-NMR, and reductive cleavage methods. J. Agric. Food Chem. 2012, 60, 5922–5935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funaoka, M. Sequential transformation and utilization of natural network polymer “LIGNIN”. React. Funct. Polym. 2013, 73, 396–404. [Google Scholar] [CrossRef]
- Ren, H.; Funaoka, M. Potential of herbaceous lignocellulosics as industrial raw materials. Trans. Mater. Res. Soc. Jpn. 2009, 34, 727–730. [Google Scholar] [CrossRef]
- Ren, H. Potential of Bamboo as Industrial Raw Materials. Ph.D. Thesis, Mie University, Mie, Japan, September 2008. [Google Scholar]
- Ralph, J.; Lapierre, C.; Lu, F.; Marita, J.M.; Pilate, G.; Van Doorsselaere, J.; Boerjan, W.; Jouanin, L. NMR evidence for benzodioxane structures resulting from incorporation of 5-hydroxyconiferyl alcohol into lignins of O-methyl-transferase-deficient poplars. J. Agric. Food Chem. 2001, 49, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Marita, J.M.; Lapierre, C.; Jouanin, L.; Morreel, K.; Boerjan, W.; Ralph, J. Sequencing around 5-hydroxyconiferyl alcohol-derived units in caffeic acid O-methyltransferase-deficient poplar lignins. Plant Physiol. 2010, 153, 569–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuel, R.; Pu, Y.Q.; Jiang, N.; Fu, C.X.; Wang, Z.Y.; Ragauskas, A. Structural characterization of lignin in wild-type versus COMT down-regulated switchgrass. Front. Energy Res. 2014, 1, 1–9. [Google Scholar] [CrossRef]
| Sample | Yield % (Based on Klason Lignin) | |||
|---|---|---|---|---|
| (Reaction Time) | 10 min | 20 min | 30 min | 60 min |
| Transgenic | 53.75 ± 0.12 | 56.89 ± 0.13 | 71.01 ± 0.12 | 54.85 ± 0.15 |
| Control | 44.46 ± 0.15 | 44.89 ± 0.10 | 57.39 ± 0.13 | 43.87 ± 0.14 |
| Sample | Mw | Mn | Mw/Mn | Combined Cresol (mol/C9) | PhOH (mol/C9) | AliOH (mol/C9) |
|---|---|---|---|---|---|---|
| Transgenic | 3547 ± 23 | 2087 ± 28 | 1.70 | 0.84 | 1.12 | 1.06 |
| Control | 2341 ± 36 | 1636 ± 53 | 1.43 | 0.85 | 1.20 | 1.13 |
| Acetylated-Transgenic | 3444 ± 42 | 2464 ± 37 | 1.40 | - | - | - |
| Acetylated-Control | 4354 ± 45 | 2822 ± 32 | 1.54 | - | - | - |
| Analytical Composition (%) | Empirical Formula | ||||||
|---|---|---|---|---|---|---|---|
| C | H | O | N | S | OCH3 | ||
| Transgenic | 66.76 | 6.29 | 28.83 | 2.75 | 0.37 | 18.10 | C9H8.22O2.2N0.35S0.02(OCH3)1.05 |
| Control | 62.92 | 5.97 | 28.45 | 2.27 | 1.39 | 19.58 | C9H7.97O2.1N0.32S0.08(OCH3)1.23 |
| Lable | δC/δH (ppm) a | δC/δH (ppm) b | Assignment |
|---|---|---|---|
| A-CH3 | 22.02/2.12 | 22.5/2.05 | C−H in lignocresol-CH3 (A) |
| ICβ | 52.75/3.40 | 53.45/3.34 | Cβ−Hβ in phenylcoumaran substructures (IC) |
| OMe | 55.54/3.67 | 56.07/3.59 | C–H in methoxyls (OMe) |
| Aγ | 61.07/3.42 | 61.73/3.36 | Cγ−Hγ in β–O–4 substructure of lignocresol (A) |
| A’γ | 64.24/4.03 | 64.63/3.96 | Cγ−Hγ in γ-hydroxylated β–O–4 substructures (A’) |
| Fγ | 62.05/3.81 | 62.91/3.78 | Cγ−Hγ in p-hydroxycinnamyl alcohol (F) |
| ICγ | 62.62/3.57 | 63.26/3.65 | Cγ−Hγ in phenylcoumaran substructures (IC) |
| Bγ | 70.29/3.83 | 70.82/3.79 | Cγ−Hγ in β−β (resinol) substructures (B) |
| Bγ | 69.68/3.52 | 70.55/3.46 | Cγ−Hγ in β−β (resinol) substructures (B) |
| X2 | 69.86/3.08 | 70.54/3.02 | C2−H2 in β–d–xylopyranoside (X) |
| X3 | 71.66/3.19 | 72.26/3.14 | C3−H3 in β–d–xylopyranoside (X) |
| X4 | 73.20/3.41 | 73.69/3.36 | C4−H4 in β–d–xylopyranoside (X) |
| X5 | 61.68/3.29 | 63.05/3.10 | C5−H5 in β–d–xylopyranoside (X) |
| Dα | ND | 76.2/4.84 | Cα−Hα in benzodioxane substructures (D) |
| Dβ | ND | 78.6/4.10 | Cβ−Hβ in benzodioxane substructures (D) |
| Aα | 81.17/4.91 | 81.71/4.86 | Cα−Hα in lignocresol (A) |
| ICα | 86.07/5.43 | 86.61/5.37 | Cα−Hα in phenylcoumaran substructures (IC) |
| S2,6 | 106.1/6.58 | 106.7/6.53 | C2, 6−H2, 6 in syringyl units (S) |
| S’2,6 | 106.2/7.30 | ND | C2,6−H2, 6, C(α)=O in syringyl units (S’) |
| G2 | 112.9/6.91 | 113.6/6.86 | C2−H2 in guaiacyl units (G) |
| G5 | 114.6/6.63 | 115.3/6.57 | C5−H5 in guaiacyl units (G) |
| G6 | 120.6/6.66 | 119.8/6.50 | C6−H6 in guaiacyl units (G) |
| H3,5 | 116.6/6.50 | 117.3/6.45 | C3, 5−H3,5 in p-hydroxyphenyl units (H) |
| H2,6 | 129.7/7.11 | 129.1/7.07 | C2, 6−H2,6 in p-hydroxyphenyl units (H) |
| C2 | ND | 112.2/6.90 | C2−H2 in catechyl units (C) |
| C5 | ND | 115.1/7.04 | C5−H5 in catechyl units (C) |
| A2,6 | 129.8/7.04 | 130.2/7.02 | C2,6−H2,6 in lignocresol (A) |
| A3 | 108.3/6.71 | 108.9/6.65 | C3−H3 in lignocresol (A) |
| L3,5 | 110.2/6.90 | 110.8/6.84 | C3,5−H3,5 in cresol (L) |
| pCA2,6 | 130.0/7.35 | 130.5/7.29 | C2, 6−H2,6 in p-coumarate (pCA) |
| pCA3,5 | 118.0/6.76 | 118.7/6.71 | C3, 5−H3,5 in p-coumarate (pCA) |
| FA2 | 113.6/7.12 | 114.4/7.11 | C2−H2 in ferulate (FA) |
| FA6 | 121.8/6.87 | 122.3/6.82 | C6−H6 in ferulate (FA) |
| Fα | 128.1/6.88 | 128.8/6.82 | Cα−Hα in p-hydroxycinnamyl alcohol (F) |
| Fβ | 126.6/6.74 | 127.3/6.69 | Cβ−Hβ in p-hydroxycinnamyl alcohol (F) |
| F’β | 124.8/6.74 | 125.4/6.68 | Cβ−Hβ in cinnamaldehyde end groups (F’) |
| Sample | S/% | G/% | S/G | H/% | β-O-4% | β-5/% | β-β/% | pCA/FA |
|---|---|---|---|---|---|---|---|---|
| Transgenic | 19.70 | 69.46 | 0.28 | 10.84 | 74.51 | 19.34 | 6.14 | 2.56 |
| Control | 41.22 | 48.42 | 0.85 | 10.36 | 80.72 | 14.56 | 4.68 | 3.25 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.; Tian, W.; Shu, F.; Xu, D.; Fu, C.; Zhai, H. Structural Characterization of Lignocresols from Transgenic and Wild-Type Switchgrass. Polymers 2018, 10, 727. https://doi.org/10.3390/polym10070727
Ren H, Tian W, Shu F, Xu D, Fu C, Zhai H. Structural Characterization of Lignocresols from Transgenic and Wild-Type Switchgrass. Polymers. 2018; 10(7):727. https://doi.org/10.3390/polym10070727
Chicago/Turabian StyleRen, Hao, Wenyuan Tian, Fan Shu, Dongliang Xu, Chunxiang Fu, and Huamin Zhai. 2018. "Structural Characterization of Lignocresols from Transgenic and Wild-Type Switchgrass" Polymers 10, no. 7: 727. https://doi.org/10.3390/polym10070727