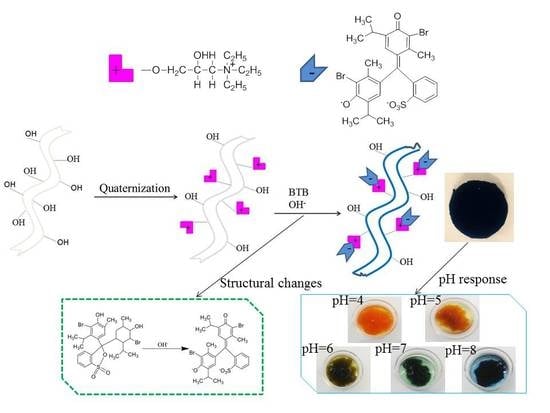
In-Situ pH-Sensitive Fibers via the Anchoring of Bromothymol Blue on Cellulose Grafted with Hydroxypropyltriethylamine Groups via Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Quaternized Cellulose Fiber (QCF)
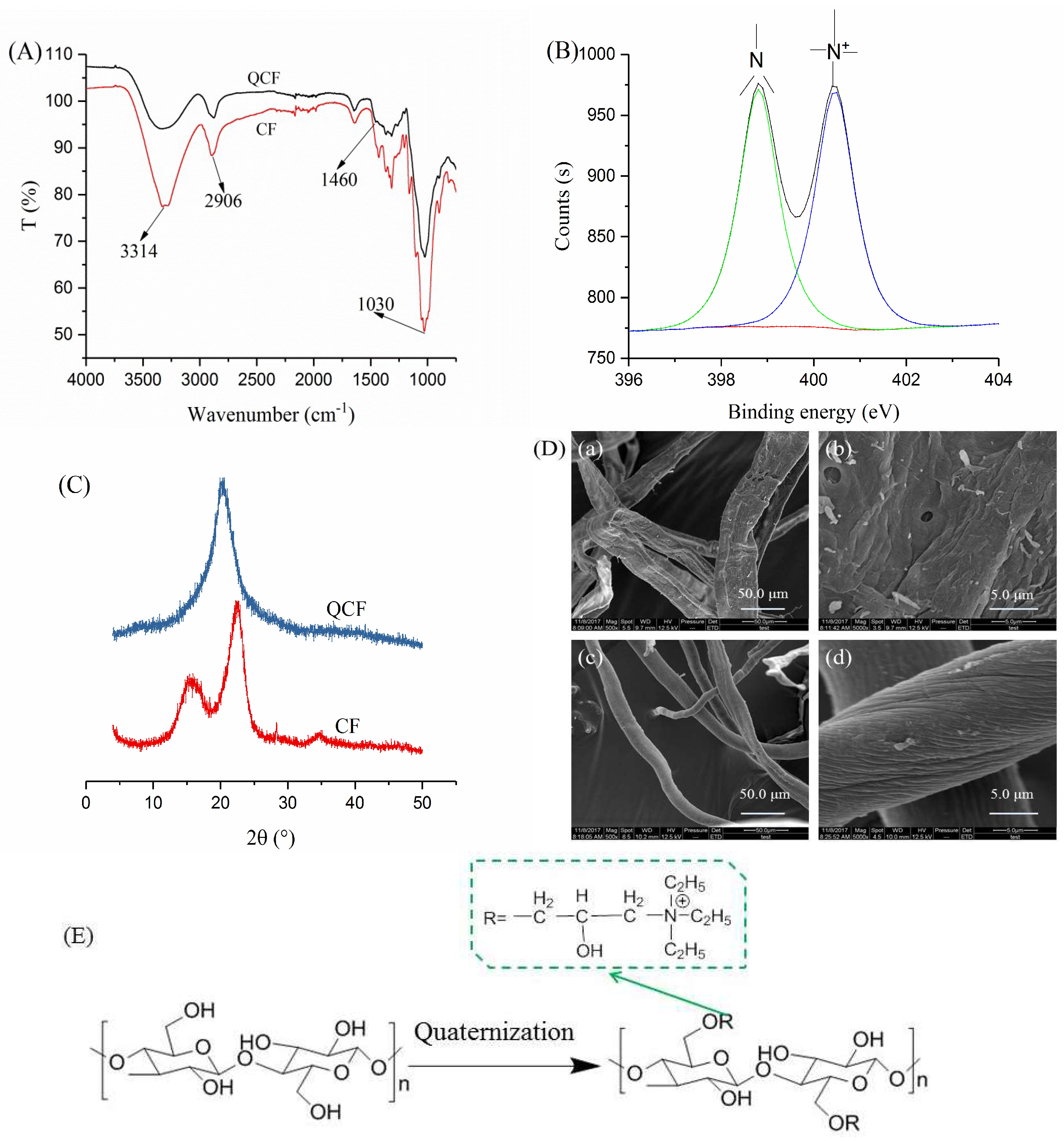
2.2.1. Preparation of the QCF
2.2.2. Characterization of the QCF
2.3. Adsorption Experiments
2.4. Adsorption Kinetics and Isotherms
2.4.1. Kinetic Models
2.4.2. Isotherm Models
2.5. Preparation and Characterization of In-Situ pH-Sensitive Cellulose Fibers (IS-pH-SCF)
2.5.1. Preparation of IS-pH-SCF
2.5.2. Characterization of IS-pH-SCF
2.6. pH Response of IS-pH-SCF
2.7. Release of BTB from IS-pH-SCF
3. Results and Discussion
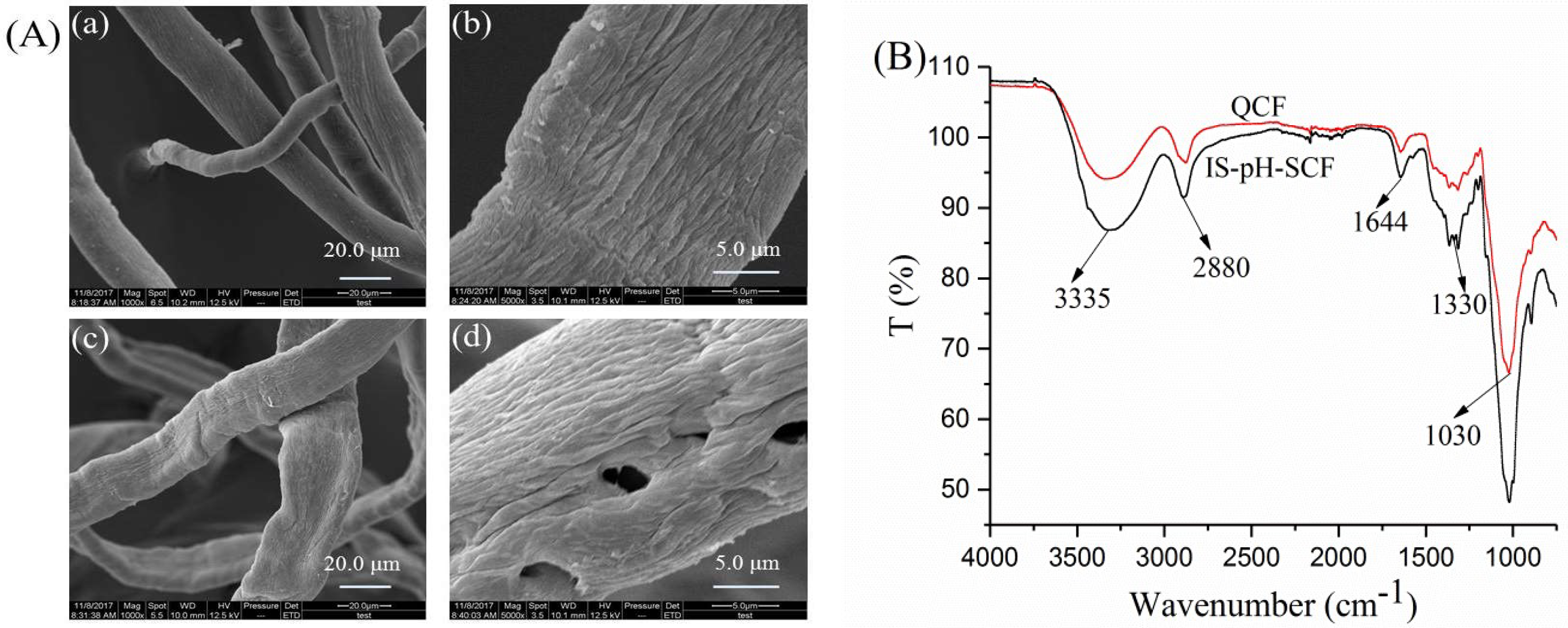
3.1. Analyses of the QCF
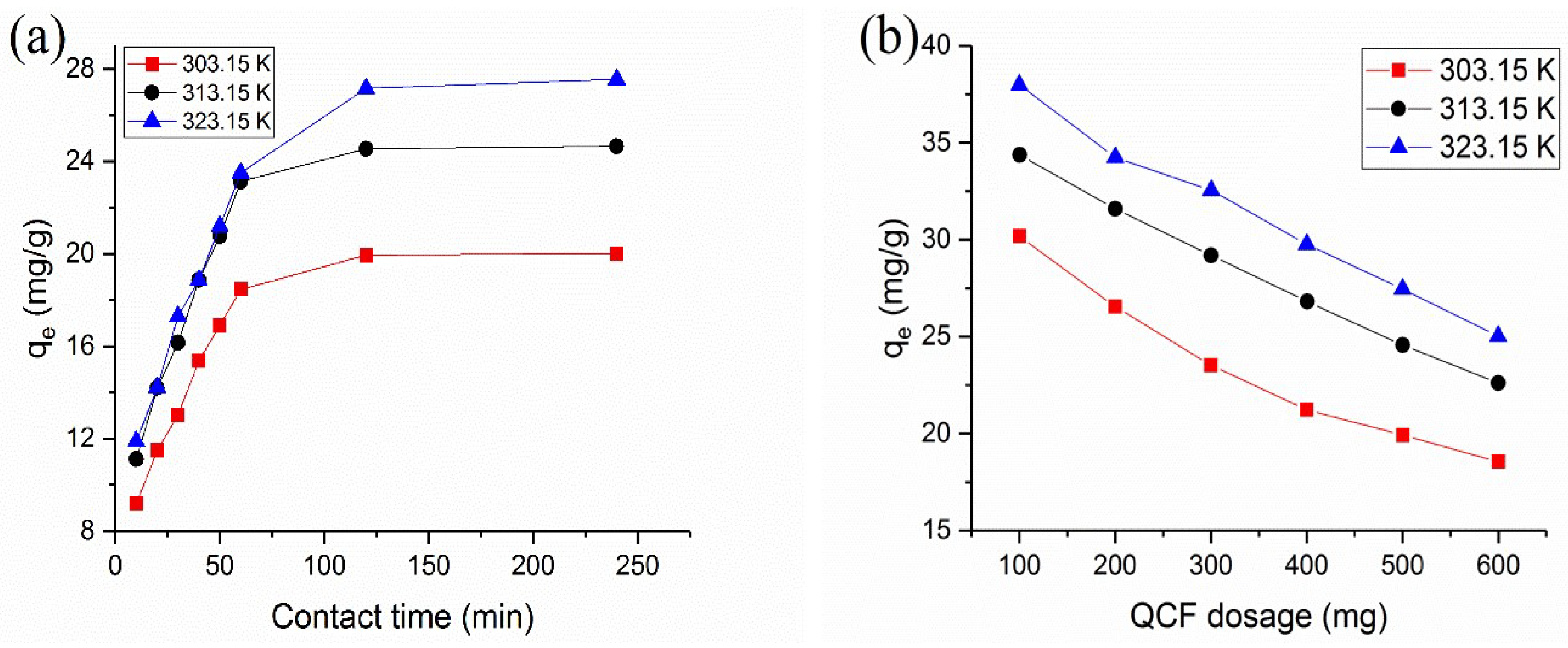
3.2. Effect of Contact Time
3.3. Effect of Adsorbent Dosage
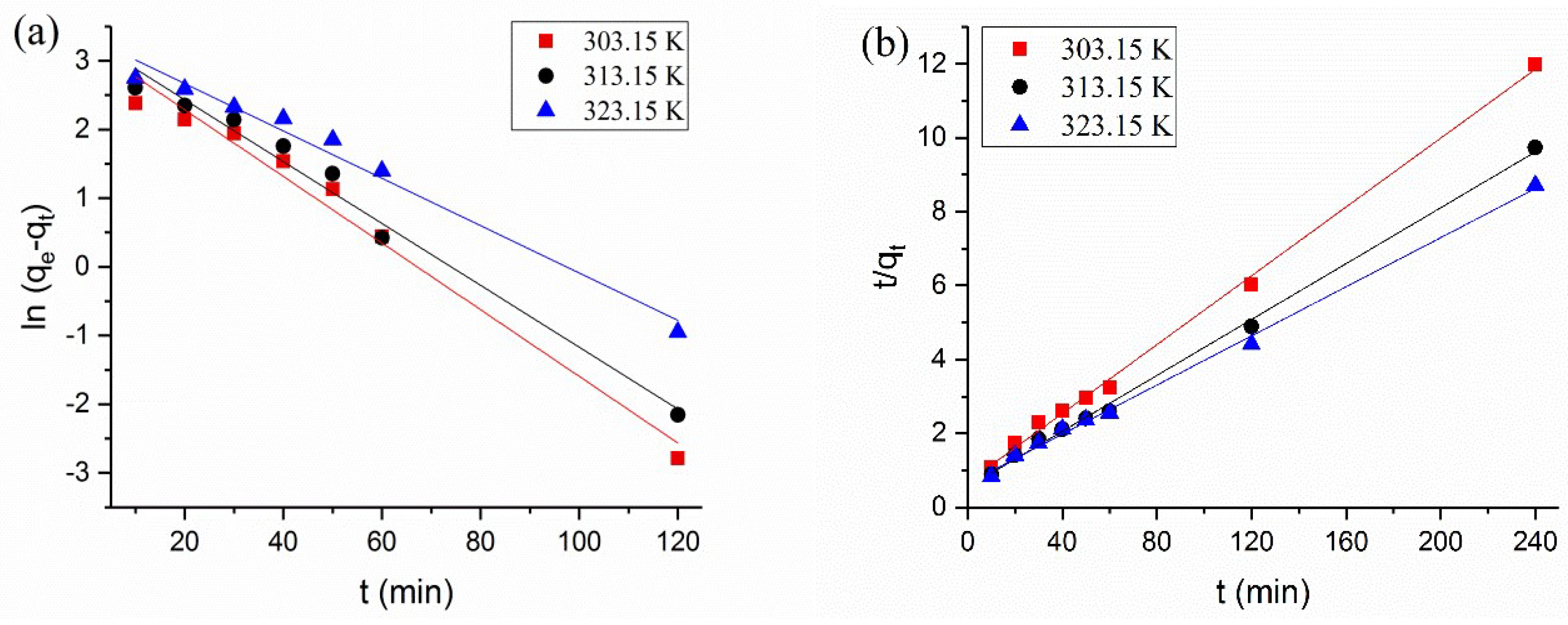
3.4. Adsorption Kinetics
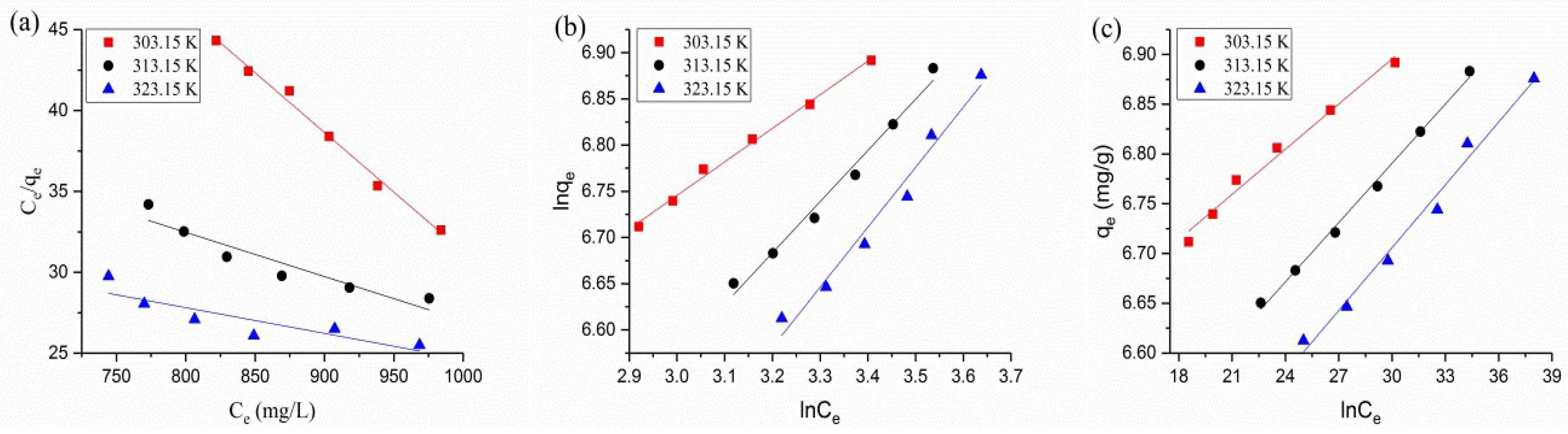
3.5. Adsorption Isotherms
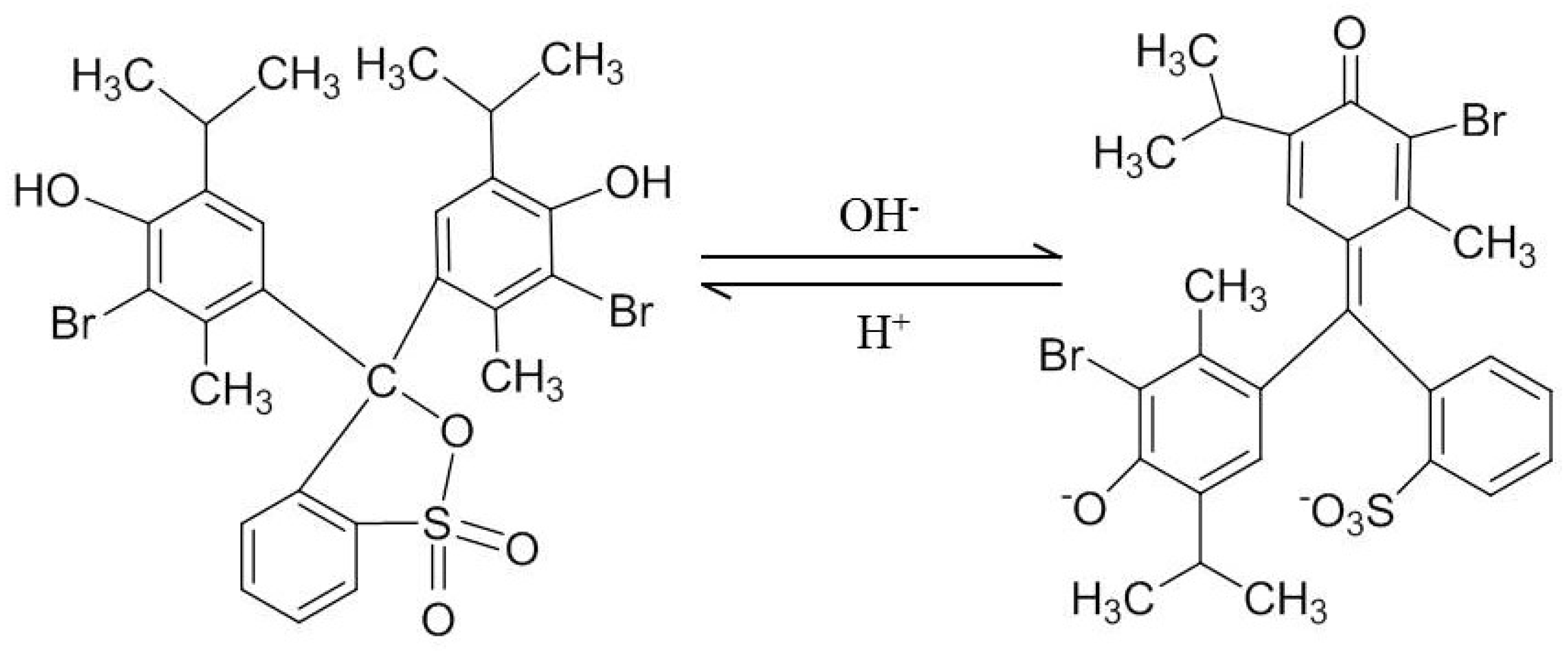
3.6. Analyses of IS-pH-SCF
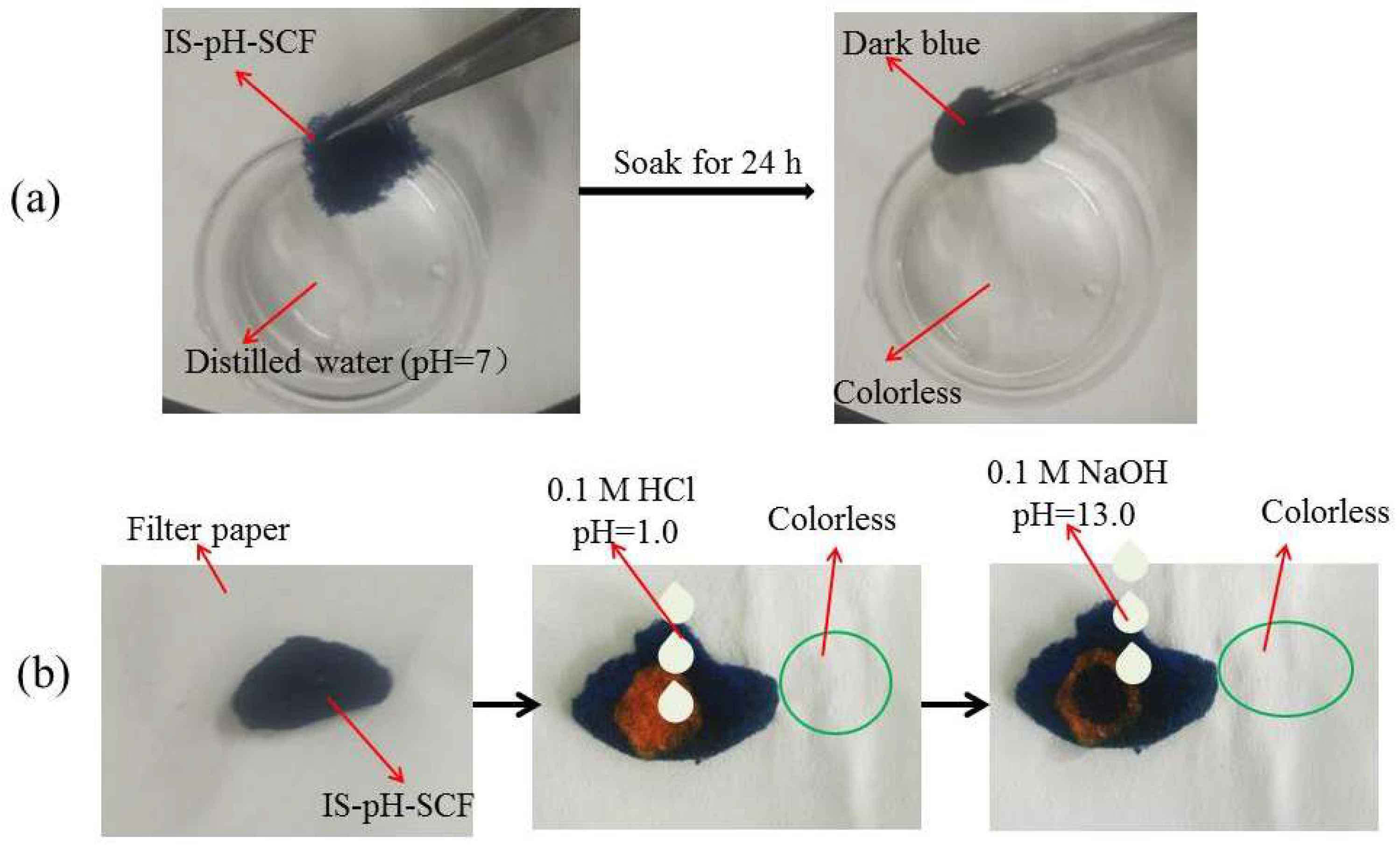
3.7. pH Response of IS-pH-SCF
3.8. Release of BTB from IS-pH-SCF
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hua, M.Z.; Feng, S.; Wang, S.; Lu, X. Rapid detection and quantification of 2,4-dichlorophenoxyacetic acid in milk using molecularly imprinted polymers-surface-enhanced raman spectroscopy. Food Chem. 2018, 258, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Poyatos-Racionero, E.; Ros-Lis, J.V.; Vivancos, J.-L.; Martínez-Máñez, R. Recent advances on intelligent packaging as tools to reduce food waste. J. Clean. Prod. 2018, 172, 3398–3409. [Google Scholar] [CrossRef]
- Lee, G.-Y.; Lee, S.; Shin, H.-S. Evaluation of gas freshness indicator for determination of skate (Raja kenojei) quality during storage. Food Sci. Biotechnol. 2016, 25, 1497–1500. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Y.; Cao, A. Strategy for sensor based on fluorescence emission red shift of conjugated polymers: Applications in pH response and enzyme activity detection. Anal. Chem. 2013, 85, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Del Ben, F.; Turetta, M.; Celetti, G.; Piruska, A.; Bulfoni, M.; Cesselli, D.; Huck, W.T.S.; Scoles, G. A method for detecting circulating tumor cells based on the measurement of single-cell metabolism in droplet-based microfluidics. Angew. Chem. 2016, 128, 8723–8726. [Google Scholar] [CrossRef]
- Peng, H.S.; Stolwijk, J.A.; Sun, L.N.; Wegener, J.; Wolfbeis, O.S. A nanogel for ratiometric fluorescent sensing of intracellular pH values. Angew. Chem. Int. Ed. Engl. 2010, 49, 4246–4249. [Google Scholar] [CrossRef] [PubMed]
- Swietach, P.; Vaughan-Jones, R.D.; Harris, A.L.; Hulikova, A. The chemistry, physiology and pathology of pH in cancer. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2014, 369, 20130099. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Takebayashi, N.; Qi, Y.; Hickerson, M.J. MTML-msBayes: Approximate Bayesian comparative phylogeographic inference from multiple taxa and multiple loci with rate heterogeneity. BMC Bioinform. 2011, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Wencel, D.; Abel, T.; McDonagh, C. Optical chemical pH sensors. Anal. Chem. 2014, 86, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Huang, F.; Cai, G.; Wang, M.; Lin, J. An optical biosensor using immunomagnetic separation, urease catalysis and pH indication for rapid and sensitive detection of Listeria monocytogenes. Sens. Actuators B Chem. 2018, 258, 447–453. [Google Scholar] [CrossRef]
- Kuswandi, B.; Wicaksono, Y.; Abdullah, A.; Heng, L.Y.; Ahmad, M. Smart packaging: Sensors for monitoring of food quality and safety. Sens. Instrum. Food Qual. Saf. 2011, 5, 137–146. [Google Scholar] [CrossRef]
- Yam, K.L. Intelligent packaging to enhance food safety and quality. Emerg. Food Packag. Technol. 2012, 137–152. [Google Scholar]
- O’Grady, M.N.; Kerry, J.P. Smart packaging technologies and their application in conventional meat packaging systems. In Meat Biotechnology; Springer: New York, NY, USA, 2008; pp. 425–451. [Google Scholar]
- Ma, Q.; Ren, Y.; Gu, Z.; Wang, L. Developing an intelligent film containing Vitis amurensis husk extracts: The effects of pH value of the film-forming solution. J. Clean. Prod. 2017, 166, 851–859. [Google Scholar] [CrossRef]
- Nopwinyuwong, A.; Trevanich, S.; Suppakul, P. Development of a novel colorimetric indicator label for monitoring freshness of intermediate-moisture dessert spoilage. Talanta 2010, 81, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, S.; Chen, X. A visual pH sensing film using natural dyes from Bauhinia blakeana Dunn. Sens. Actuators B Chem. 2014, 198, 268–273. [Google Scholar] [CrossRef]
- Prietto, L.; Mirapalhete, T.C.; Pinto, V.Z.; Hoffmann, J.F.; Vanier, N.L.; Lim, L.-T.; Guerra Dias, A.R.; da Rosa Zavareze, E. pH-sensitive films containing anthocyanins extracted from black bean seed coat and red cabbage. LWT Food Sci. Technol. 2017, 80, 492–500. [Google Scholar] [CrossRef]
- Yoshida, C.M.P.; Maciel, V.B.V.; Mendonça, M.E.D.; Franco, T.T. Chitosan biobased and intelligent films: Monitoring pH variations. LWT Food Sci. Technol. 2014, 55, 83–89. [Google Scholar] [CrossRef]
- Ma, Q.; Liang, T.; Cao, L.; Wang, L. Intelligent poly (vinyl alcohol)-chitosan nanoparticles-mulberry extracts films capable of monitoring pH variations. Int. J. Biol. Macromol. 2018, 108, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, T.J.; Guzman, R.; Medina Jaramillo, C.; Fama, L. Effect of beet flour on films made from biological macromolecules: Native and modified plantain flour. Int. J. Biol. Macromol. 2016, 82, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, T.J.; Ponce, A.G.; Alvarez, V.A. Nano-clays from natural and modified montmorillonite with and without added blueberry extract for active and intelligent food nanopackaging materials. Mater. Chem. Phys. 2017, 194, 283–292. [Google Scholar] [CrossRef]
- Chen, L.X. Discoloration property for pH-sensitivity of fabric dyed by turmeric. J. Text. Res. 2016, 38, 5. [Google Scholar]
- Liu, X.; Zhang, S.Q.; Wei, X.; Yang, T.; Chen, M.L.; Wang, J.H. A novel “modularized” optical sensor for pH monitoring in biological matrixes. Biosens. Bioelectron. 2018, 109, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Rukchon, C.; Nopwinyuwong, A.; Trevanich, S.; Jinkarn, T.; Suppakul, P. Development of a food spoilage indicator for monitoring freshness of skinless chicken breast. Talanta 2014, 130, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yang, X.; Shu, Y.; Chen, X.; Wang, J. Ionic liquid-based slab optical waveguide sensor for the detection of ammonia in human breath. J. Colloid Interface Sci. 2018, 512, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Chen, C.; Rosswurm, K.; Yao, T.; Janaswamy, S. A facile route to prepare cellulose-based films. Carbohydr. Polym. 2016, 149, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Yu, S.; Zhu, H.; Wang, S.; Chen, Y.; Liu, S. Dissolution and structure change of bagasse cellulose in zinc chloride solution. BioResources 2016, 11, 3813–3824. [Google Scholar] [CrossRef]
- Hu, D.; Wang, L. Adsorption of amoxicillin onto quaternized cellulose from flax noil: Kinetic, equilibrium and thermodynamic study. J. Taiwan Inst. Chem. E 2016, 64, 227–234. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Changmai, M.; Banerjee, P.; Nahar, K.; Purkait, M.K. A novel adsorbent from carrot, tomato and polyethylene terephthalate waste as a potential adsorbent for Co (II) from aqueous solution: Kinetic and equilibrium studies. J. Environ. Chem. Eng. 2018, 6, 246–257. [Google Scholar] [CrossRef]
- Cheah, W.; Hosseini, S.; Khan, M.A.; Chuah, T.G.; Choong, T.S.Y. Acid modified carbon coated monolith for methyl orange adsorption. Chem. Eng. J. 2013, 215, 747–754. [Google Scholar] [CrossRef]
- Namasivayam, C.; Kavitha, D. Removal of Congo Red from water by adsorption onto activated carbon prepared from coir pith, an agricultural solid waste. Dyes Pigment. 2002, 54, 47–58. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Kowalkowski, T.; Tutu, H.; Cukrowska, E.M.; Buszewski, B. Ionic liquid modified diatomite as a new effective adsorbent for uranium ions removal from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2015, 465, 159–167. [Google Scholar] [CrossRef]
- Zavareh, S.; Farrokhzad, Z.; Darvishi, F. Modification of zeolite 4A for use as an adsorbent for glyphosate and as an antibacterial agent for water. Ecotoxicol. Environ. Saf. 2018, 155, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Elwakeel, K.Z.; Elgarahy, A.M.; Mohammad, S.H. Use of beach bivalve shells located at port said coast (Egypt) as a green approach for methylene blue removal. J. Environ. Chem. Eng. 2017, 5, 578–587. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, J.; Wei, X.; Wang, L. Removal of remazol turquoise blue G-133 from aqueous solution using modified waste newspaper fiber. Carbohydr. Polym. 2013, 92, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Dang, Z.; Zhou, X.-Q.; Yi, X.-Y.; Wu, P.-X.; Zhu, N.-W.; Lu, G.-N. Removal of sulphate from aqueous solution using modified rice straw: Preparation, characterization and adsorption performance. Carbohydr. Polym. 2011, 85, 571–577. [Google Scholar] [CrossRef]
- Tastet, D.; Save, M.; Charrier, F.; Charrier, B.; Ledeuil, J.-B.; Dupin, J.-C.; Billon, L. Functional biohybrid materials synthesized via surface-initiated MADIX/RAFT polymerization from renewable natural wood fiber: Grafting of polymer as non leaching preservative. Polymer 2011, 52, 606–616. [Google Scholar] [CrossRef]
- Hu, D.; Wang, P.; Li, J.; Wang, L. Functionalization of microcrystalline cellulose with N,N-dimethyldodecylamine for the removal of Congo Red dye from an aqueous solution. BioResources 2014, 9, 5951–5962. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Kinetic models for the sorption of dye from aqueous solution by wood. Process Saf. Environ. Prot. 1998, 76, 183–191. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Konicki, W.; Sibera, D.; Mijowska, E.; Lendzion-Bielun, Z.; Narkiewicz, U. Equilibrium and kinetic studies on acid dye Acid Red 88 adsorption by magnetic ZnFe2O4 spinel ferrite nanoparticles. J. Colloid Interface Sci. 2013, 398, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Elwakeel, K.Z.; El-Bindary, A.A.; El-Sonbati, A.Z.; Hawas, A.R. Adsorption of toxic acidic dye from aqueous solution onto diethylenetriamine functionalized magnetic glycidyl methacrylate-N,N′-methylenebisacrylamide. RSC Adv. 2016, 6, 3350–3361. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; El-Bindary, A.A.; Ismail, A.; Morshidy, A.M. Sorptive removal of Remazol Brilliant Blue R from aqueous solution by diethylenetriamine functionalized magnetic macro-reticular hybrid material. RSC Adv. 2016, 6, 22395–22410. [Google Scholar] [CrossRef]
- Pourjavaher, S.; Almasi, H.; Meshkini, S.; Pirsa, S.; Parandi, E. Development of a colorimetric pH indicator based on bacterial cellulose nanofibers and red cabbage (Brassica oleraceae) extract. Carbohydr. Polym. 2017, 156, 193–201. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Temperature (K) | ||
|---|---|---|---|
| 303.15 | 313.15 | 323.15 | |
| qe,exp (mg/g) | 21.016 | 24.661 | 27.549 |
| Pseudo-first-order | |||
| K1 (min−1) | 0.034 | 0.045 | 0.049 |
| qe,cal (mg/g) | 28.703 | 28.022 | 25.972 |
| R2 | 0.975 | 0.979 | 0.975 |
| Pseudo-second-order | |||
| K2 (mg·g−1·min−1) | 0.0032 | 0.0026 | 0.0017 |
| qe,cal (mg/g) | 21.459 | 26.316 | 28.303 |
| R2 | 0.998 | 0.997 | 0.997 |
| Ea (kJ·mol−1) | 52.89 | ||
| Parameters | Temperature (K) | ||
|---|---|---|---|
| 303.15 | 313.15 | 323.15 | |
| Langmuir | |||
| KL (L·mg−1 × 10−4) | −7.039 | −0.162 | −3.922 |
| qm (mg/g) | −13.514 | −37.037 | −62.893 |
| R2 | 0.992 | 0.892 | 0.770 |
| Freundlich | |||
| K2 ([mg1 − (1/n)/(g·L−1/n]) | 286.00 | 135.64 | 90.56 |
| 1/n | 0.363 | 0.554 | 0.649 |
| R2 | 0.995 | 0.981 | 0.967 |
| Temkin | |||
| A | 6.442 | 6.195 | 6.075 |
| B | 0.015 | 0.020 | 0.021 |
| R2 | 0.980 | 0.995 | 0.982 |
| pH | L | a | b | ∆E | Photos |
|---|---|---|---|---|---|
| 4 | 42.228 ± 1.569 | 48.675 ± 2.211 | 72.510 ± 2.716 | 104.503 ± 2.026 |  |
| 5 | 28.148 ± 0.614 | 33.510 ± 0.514 | 47.930 ± 1.067 | 92.277 ± 0.264 |  |
| 6 | 0.390 ± 0.064 | 2.230 ± 0.486 | 0.398 ± 0.097 | 85.055 ± 0.052 |  |
| 7 | 0.142 ± 0.004 | 0.470 ± 0.041 | −0.128 ± 0.074 | 74.276 ± 0.004 | 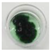 |
| 8 | 0.110 ± 0.001 | 0.240 ± 0.012 | −0.152 ± 0.041 | 68.307 ± 0.001 |  |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, L.; Liang, T.; Zhang, X.; Liu, W.; Li, J.; Zhan, X.; Wang, L. In-Situ pH-Sensitive Fibers via the Anchoring of Bromothymol Blue on Cellulose Grafted with Hydroxypropyltriethylamine Groups via Adsorption. Polymers 2018, 10, 709. https://doi.org/10.3390/polym10070709
Cao L, Liang T, Zhang X, Liu W, Li J, Zhan X, Wang L. In-Situ pH-Sensitive Fibers via the Anchoring of Bromothymol Blue on Cellulose Grafted with Hydroxypropyltriethylamine Groups via Adsorption. Polymers. 2018; 10(7):709. https://doi.org/10.3390/polym10070709
Chicago/Turabian StyleCao, Lele, Tieqiang Liang, Xipeng Zhang, Wenbo Liu, Jian Li, Xianxu Zhan, and Lijuan Wang. 2018. "In-Situ pH-Sensitive Fibers via the Anchoring of Bromothymol Blue on Cellulose Grafted with Hydroxypropyltriethylamine Groups via Adsorption" Polymers 10, no. 7: 709. https://doi.org/10.3390/polym10070709