Hyaluronic Acid in the Third Millennium
Abstract
:1. Introduction and Historical Background of HA
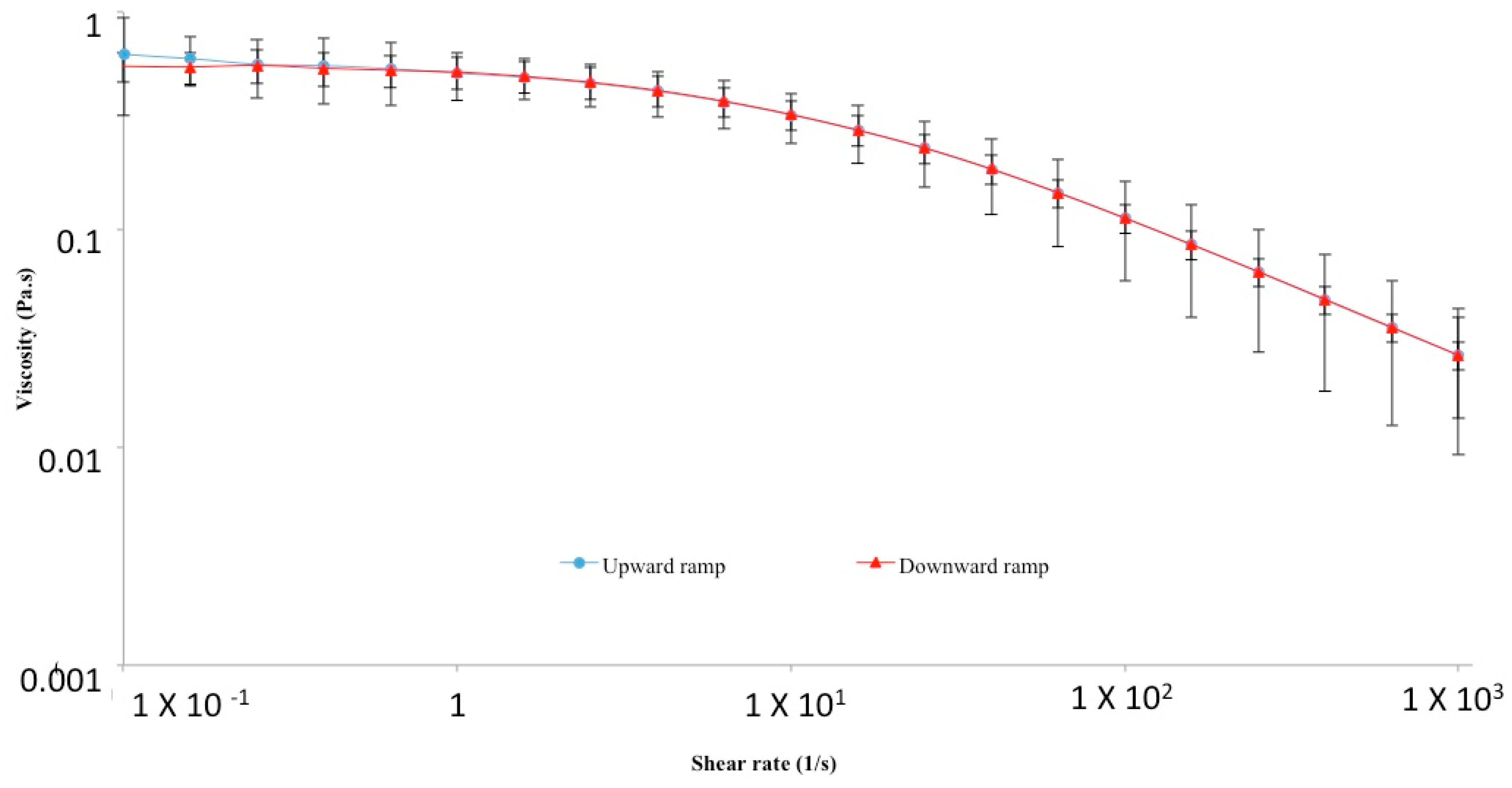
2. Physico-Chemical, Structural and Hydrodynamic Properties of HA
3. Biology of HA
3.1. HA Occurrence in Living Organism and Diffusion in the Human Body
3.2. HA Synthesis in the Human Body
3.3. HA Degradation in the Human Body
3.4. Biological Roles of HA in Relation to Its MW
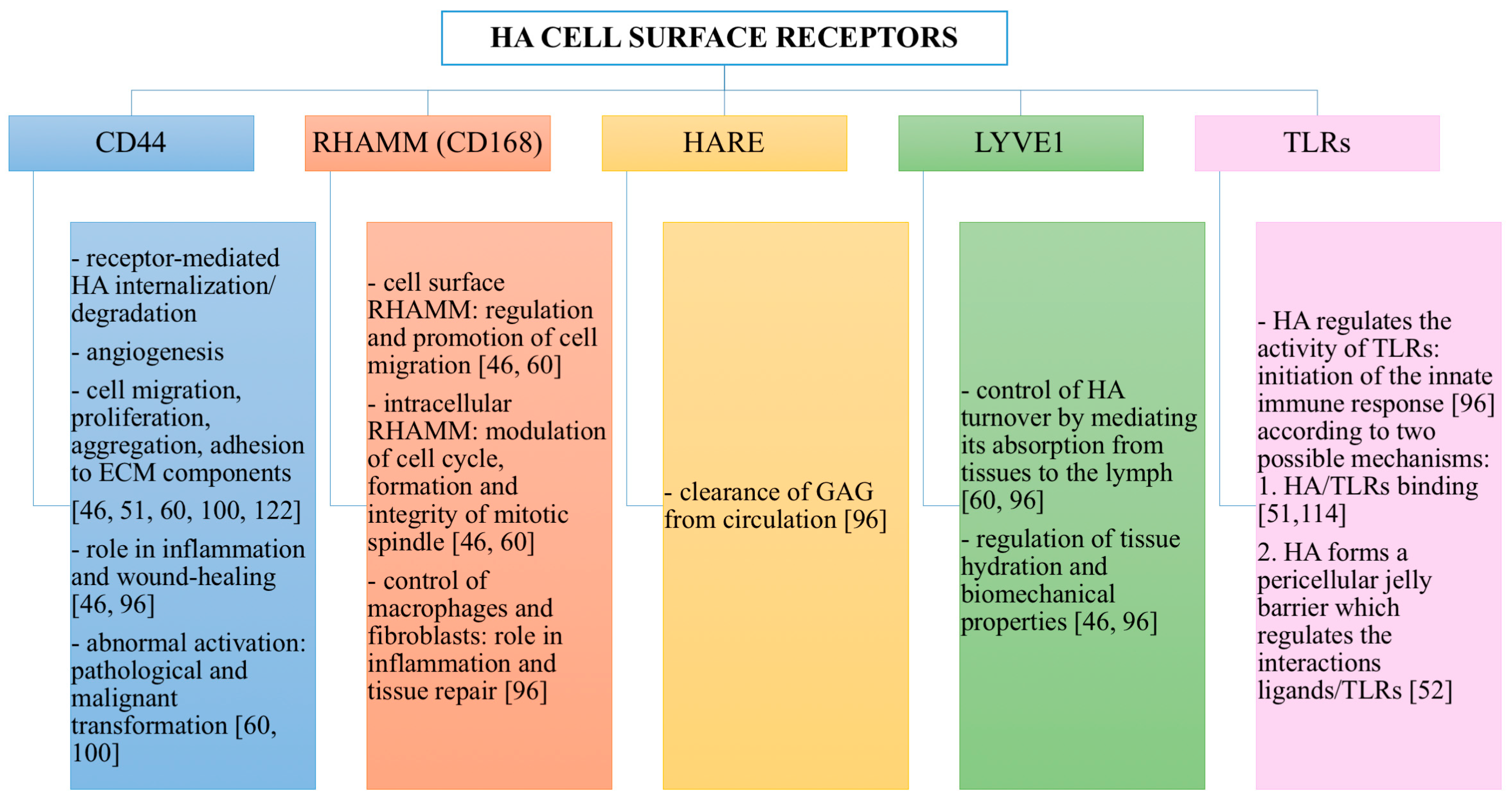
3.5. Mechanisms of Action of HA
HA Cell Surface Receptors
4. Industrial Production of HA
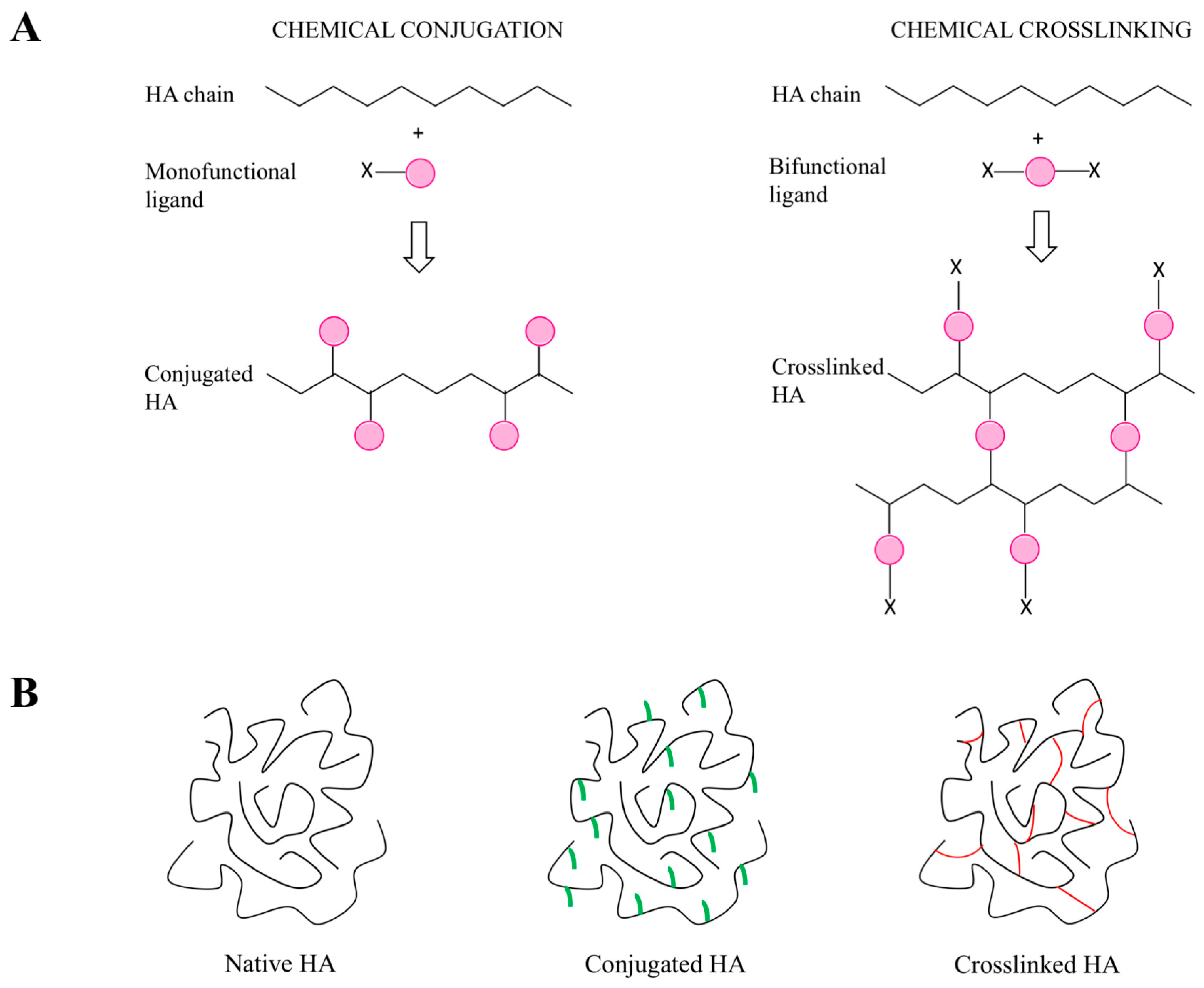
5. Synthetic Modifications of HA
5.1. General Introduction of the Chemical Approaches to Modify HA
5.2. Modification of HA Hydroxyl Groups
5.3. Modification of HA Carboxyl Groups
5.4. Modification of HA N-Acetyl Groups
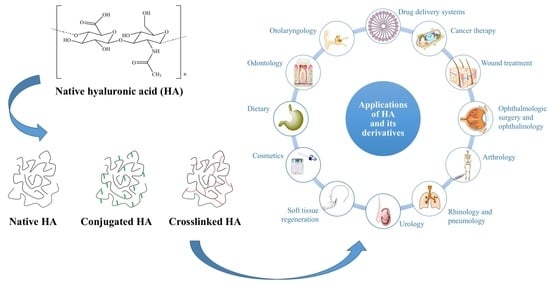
6. Applications of HA and Its Derivatives
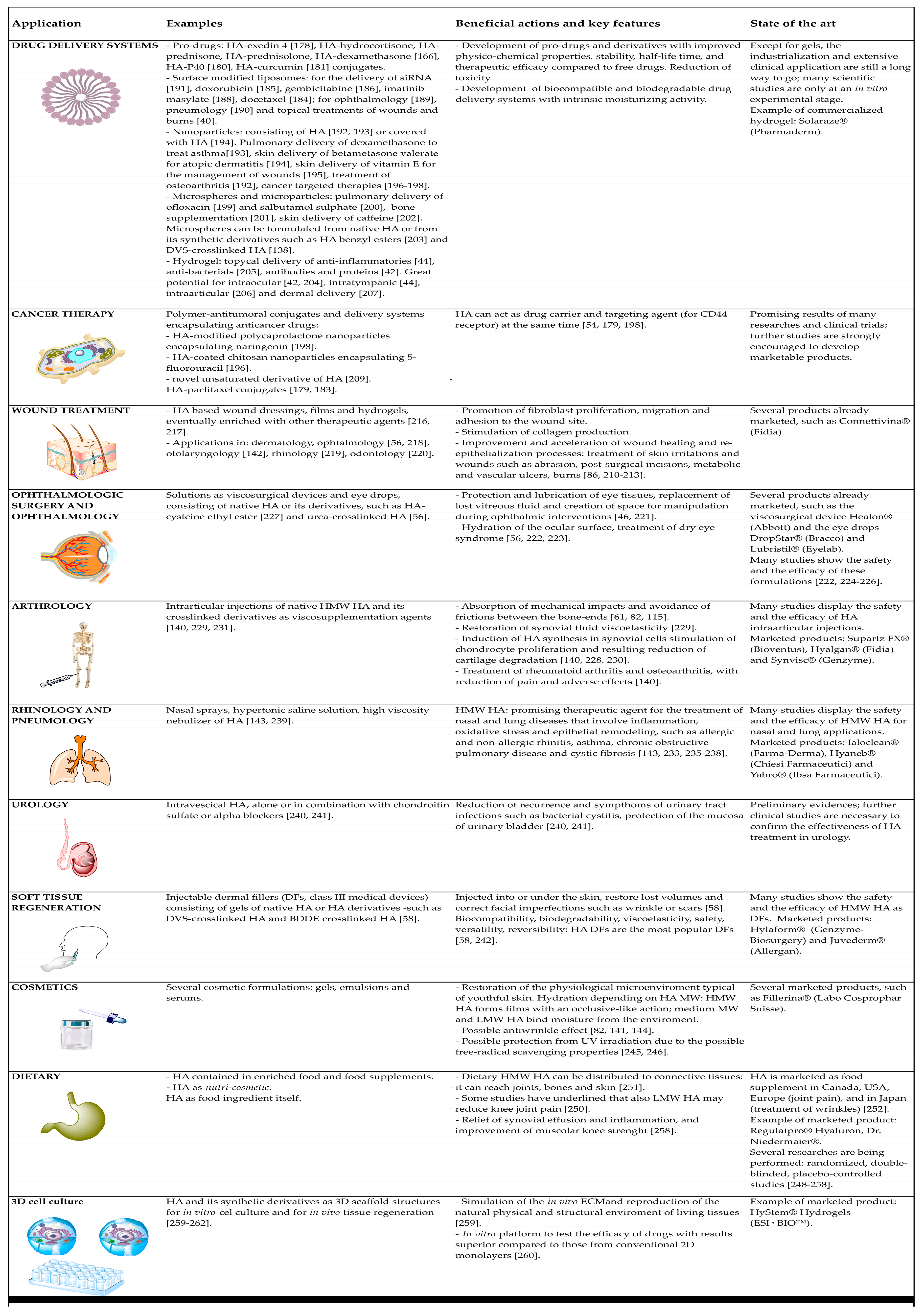
6.1. Drug Delivery Systems
6.2. Cancer Therapy
6.3. Wound Treatment
6.4. Ophthalmologic Surgery and Ophthalmology
6.5. Arthrology
6.6. Rhinology and Pneumology
6.7. Urology
6.8. Soft Tissue Regeneration
6.9. Cosmetics
6.10. Dietary
6.11. 3D Cell Culture Models
7. Conclusions, Future Trends and Perspectives
Acknowledgments
Conflicts of Interest
Abbreviations
| BDDE | butanediol-diglycidyl ether |
| CD44 | cluster of differentiation-44 |
| CDMT | 2-chloro-dimethoxy-1,3,5-triazine |
| DFs | dermal fillers |
| DMF | dimethylformamide |
| DMTMM | 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium |
| DMSO | dimethylsulfoxide |
| DVS | divinyl sulfone |
| ECM | extracellular matrix |
| EDC | N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride |
| HA | hyaluronic acid; hyaluronate; hyaluronan |
| HA-CL | urea-crosslinked hyaluronic acid |
| HARE | hyaluronan receptor for endocytosis |
| HAS | hyaluronan synthases |
| H-bonds | hydrogen bonds |
| HYAL | hyaluronidases |
| HMW | high molecular weight |
| GAGs | glycosaminoglycans |
| GRAS | generally regarded as safe |
| LYVE1 | lymphatic vessel endothelial hyaluronan receptor 1 |
| LMW | low molecular weight |
| MW | molecular weight |
| NHS | N-hydroxysuccinimide |
| oHA | oligosaccharides of hyaluronic acid |
| RHAMM | receptor for HA-mediated cell motility |
| ROS | reactive oxygen species |
| TBA | tetrabutylammonium |
| TLRs | Toll-like receptors |
References
- Boeriu, C.G.; Springer, J.; Kooy, F.K.; van den Broek, L.A.M.; Eggink, G. Production methods for hyaluronan. Int. J. Carbohydr. Chem. 2013, 2013, 14. [Google Scholar] [CrossRef]
- Meyer, K.; Palmer, J.W. The polysaccharide of the vitrous humor. J. Biol. Chem. 1934, 107, 629–634. [Google Scholar]
- Kendall, F.E.; Heidelberger, M.; Dawson, M.H. A serologically inactive polysaccharide elaborated by mucoid strains of group a hemolytic streptococcus. J. Biol. Chem. 1937, 118, 61–69. [Google Scholar]
- Boas, N.F. Isolation of hyaluronic acid from the cock’s comb. J. Biol. Chem. 1949, 181, 573–575. [Google Scholar] [PubMed]
- Kaye, M.A.; Stacey, M. Observations on the chemistry of hyaluronic acid. Biochem. J. 1950, 2, 13. [Google Scholar] [PubMed]
- Meyer, K. Highly viscous sodium hyaluronate. J. Biol. Chem. 1948, 176, 993–994. [Google Scholar] [PubMed]
- Varga, L. Studies on hyaluronic acid prepared from the vitreous body. J. Biol. Chem. 1955, 217, 651–658. [Google Scholar] [PubMed]
- Fletcher, E.; Jacobs, J.H.; Markham, R.L. Viscosity studies on hyaluronic acid of synovial fluid in rheumatoid arthritis and osteoarthritis. Clin. Sci. 1955, 14, 653–660. [Google Scholar] [PubMed]
- Blumberg, B.S.; Ogston, A.G.; Lowther, D.A.; Rogers, H.J. Physicochemical properties of hyaluronic acid formed by Streptococcus haemolyticus. Biochem. J. 1958, 70, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weissmann, B.; Meyer, K. The structure of hyalobiuronic acid and of hyaluronic acid from umbilical cord. J. Am. Chem. Soc. 1954, 76, 1753–1757. [Google Scholar] [CrossRef]
- Meyer, K. The biological significance of hyaluronic acid and hyaluronidase. Physiol. Rev. 1947, 27, 335–359. [Google Scholar] [CrossRef] [PubMed]
- Ogston, A.G.; Stanier, J.E. The physiological function of hyaluronic acid in synovial fluid; viscous, elastic and lubricant properties. J. Physiol. 1953, 119, 244–252. [Google Scholar] [CrossRef] [Green Version]
- Pinkus, H.; Perry, E.T. The influence of hyaluronic acid and other substances on tensile strength of healing wounds. J. Investig. Dermatol. 1953, 21, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Balazs, E.A. Ultrapure Hyaluronic Acid and the Use Thereof. U.S. Patent 4,141,973, 27 February 1979. [Google Scholar]
- Miller, D.; Stegmann, R. Use of Na-hyaluronate in anterior segment eye surgery. J. Am. Intraocul. Implant Soc. 1980, 6, 13–15. [Google Scholar] [CrossRef]
- Binkhorst, C.D. Advantages and disadvantages of intracamerular Na-hyaluronate (Healon) in intraocular lens surgery. Doc. Ophthalmol. 1981, 50, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Binkhorst, C.D. Inflammation and intraocular pressure after the use of Healon in intraocular lens surgery. J. Am. Intraocul. Implant Soc. 1980, 6, 340–341. [Google Scholar] [CrossRef]
- Percival, P. Results of a clinical trial of sodium hyaluronate in lens implantation surgery. J. Am. Intraocul. Implant Soc. 1985, 11, 257–259. [Google Scholar] [CrossRef]
- Graue, E.L.; Polack, F.M.; Balazs, E.A. The protective effect of Na-hyaluronate to corneal endothelium. Exp. Eye Res. 1980, 31, 119–127. [Google Scholar] [CrossRef]
- Percival, P. Protective role of Healon during lens implantation. Trans. Ophthalmol. Soc. UK 1981, 101, 77–78. [Google Scholar] [PubMed]
- Regnault, F.; Bregeat, P. Treatment of severe cases of retinal detachment with highly viscous hyaluronic acid. Mod. Probl. Ophthalmol. 1974, 12, 378–383. [Google Scholar] [PubMed]
- Kanski, J.J. Intravitreal hyaluronic acid injection. A long-term clinical evaluation. Br. J. Ophthalmol. 1975, 59, 255–256. [Google Scholar] [CrossRef] [PubMed]
- Auer, J.A.; Fackelman, G.E.; Gingerich, D.A.; Fetter, A.W. Effect of hyaluronic acid in naturally occurring and experimentally induced osteoarthritis. Am. J. Vet. Res. 1980, 41, 568–574. [Google Scholar] [PubMed]
- Namiki, O.; Toyoshima, H.; Morisaki, N. Therapeutic effect of intra-articular injection of high molecular weight hyaluronic acid on osteoarthritis of the knee. Int. J. Clin. Pharmacol. Ther. Toxicol. 1982, 20, 501–507. [Google Scholar] [PubMed]
- Leardini, G.; Perbellini, A.; Franceschini, M.; Mattara, L. Intra-articular injections of hyaluronic acid in the treatment of painful shoulder. Clin. Ther. 1988, 10, 521–526. [Google Scholar] [PubMed]
- Dougados, M.; Nguyen, M.; Listrat, V.; Amor, B. High molecular weight sodium hyaluronate (hyalectin) in osteoarthritis of the knee: A 1year placebo-controlled trial. Osteoarthr. Cartil. 1993, 1, 97–103. [Google Scholar] [CrossRef]
- Jones, A.C.; Pattrick, M.; Doherty, S.; Doherty, M. Intra-articular hyaluronic acid compared to intra-articular triamcinolone hexacetonide in inflammatory knee osteoarthritis. Osteoarthr. Cartil. 1995, 3, 269–273. [Google Scholar] [CrossRef]
- Juhlin, L. Hyaluronan in skin. J. Intern. Med. 1997, 242, 61–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavicic, T.; Gauglitz, G.G.; Lersch, P.; Schwach-Abdellaoui, K.; Malle, B.; Korting, H.C.; Farwick, M. Efficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatment. J. Drugs Dermatol. 2011, 10, 990–1000. [Google Scholar] [PubMed]
- Abatangelo, G.; Martelli, M.; Vecchia, P. Healing of hyaluronic acid-enriched wounds: Histological observations. J. Surg. Res. 1983, 35, 410–416. [Google Scholar] [CrossRef]
- Doillon, C.J.; Silver, F.H. Collagen-based wound dressing: Effects of hyaluronic acid and fibronectin on wound healing. Biomaterials 1986, 7, 3–8. [Google Scholar] [CrossRef]
- Hellström, S.; Laurent, C. Hyaluronan and healing of tympanic membrane perforations. An experimental study. Acta Otolaryngol. Suppl. 1987, 442, 54–61. [Google Scholar] [CrossRef] [PubMed]
- King, S.R.; Hickerson, W.L.; Proctor, K.G. Beneficial actions of exogenous hyaluronic acid on wound healing. Surgery 1991, 109, 76–84. [Google Scholar] [PubMed]
- Duranti, F.; Salti, G.; Bovani, B.; Calandra, M.; Rosati, M.L. Injectable hyaluronic acid gel for soft tissue augmentation. A clinical and histological study. Dermatol. Surg. 1998, 24, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Bartlett, S.P.; Matsuo, K.; LiVolsi, V.A.; Parry, C.; Hass, B.; Whitaker, L.A. Hyaluronic acid-filled mammary implants: An experimental study. Plast. Reconstr. Surg. 1994, 94, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, L.M.; Topp, E.M.; Stella, V.J. Microspheres of hyaluronic acid esters—Fabrication methods and in vitro hydrocortisone release. J. Control. Release 1990, 13, 33–41. [Google Scholar] [CrossRef]
- Lim, S.T.; Martin, G.P.; Berry, D.J.; Brown, M.B. Preparation and evaluation of the in vitro drug release properties and mucoadhesion of novel microspheres of hyaluronic acid and chitosan. J. Control. Release 2000, 66, 281–292. [Google Scholar] [CrossRef]
- Moreira, C.A.J.; Armstrong, D.K.; Jelliffe, R.W.; Moreira, A.T.; Woodford, C.C.; Liggett, P.E.; Trousdale, M.D. Sodium hyaluronate as a carrier for intravitreal gentamicin. An experimental study. Acta Ophthalmol. 1991, 69, 45–49. [Google Scholar] [CrossRef]
- Morimoto, K.; Yamaguchi, H.; Iwakura, Y.; Morisaka, K.; Ohashi, Y.; Nakai, Y. Effects of viscous hyaluronate-sodium solutions on the nasal absorption of vasopressin and an analogue. Pharm. Res. 1991, 8, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Yerushalmi, N.; Arad, A.; Margalit, R. Molecular and cellular studies of hyaluronic acid-modified liposomes as bioadhesive carriers for topical drug delivery in wound healing. Arch. Biochem. Biophys. 1994, 313, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Camber, O.; Edman, P. Sodium hyaluronate as an ophthalmic vehicle: Some factors governing its effect on the ocular absorption of pilocarpine. Curr. Eye Res. 1989, 8, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Egbu, R.; Brocchini, S.; Khaw, P.T.; Awwad, S. Antibody loaded collapsible hyaluronic acid hydrogels for intraocular delivery. Eur. J. Pharm. Biopharm. 2018, 124, 95–103. [Google Scholar] [CrossRef] [PubMed]
- El Kechai, N.; Geiger, S.; Fallacara, A.; Cañero Infante, I.; Nicolas, V.; Ferrary, E.; Huang, N.; Bochot, A.; Agnely, F. Mixtures of hyaluronic acid and liposomes for drug delivery: Phase behavior, microstructure and mobility of liposomes. Int. J. Pharm. 2017, 523, 246–259. [Google Scholar] [CrossRef] [PubMed]
- El Kechai, N.; Mamelle, E.; Nguyen, Y.; Huang, N.; Nicolas, V.; Chaminade, P.; Yen-Nicolaÿ, S.; Gueutin, C.; Granger, B.; Ferrary, E.; et al. Hyaluronic acid liposomal gel sustains delivery of a corticoid to the inner ear. J. Control. Release 2016, 226, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Upton, Z.; Richards, S.; Rizzi, S.C.; Leavesley, D.I. Hyaluronic acid: Evaluation as a potential delivery vehicle for vitronectin: Growth factor complexes in wound healing applications. J. Control. Release 2011, 153, 225–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knopf-Marques, H.; Pravda, M.; Wolfova, L.; Velebny, V.; Schaaf, P.; Vrana, N.E.; Lavalle, P. Hyaluronic Acid and Its Derivatives in Coating and Delivery Systems: Applications in Tissue Engineering, Regenerative Medicine and Immunomodulation. Adv. Healthc. Mater. 2016, 5, 2841–2855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamia, S.; Pilarski, P.M.; Belch, A.R.; Pilarski, L.M. Aberrant splicing, hyaluronan synthases and intracellular hyaluronan as drivers of oncogenesis and potential drug targets. Curr. Cancer Drug Targets 2013, 13, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Adamia, S.; Maxwell, C.A.; Pilarski, L.M. Hyaluronan and hyaluronan synthases: Potential therapeutic targets in cancer. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2005, 5, 3–14. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.D.; Carvalho, L.S.; Gomes, A.M.; Queiroz, L.R.; Magalhães, B.S.; Parachin, N.S. Genetic basis for hyper production of hyaluronic acid in natural and engineered microorganisms. Microb. Cell Fact. 2016, 15, 119. [Google Scholar] [CrossRef] [PubMed]
- Heldin, P.; Lin, C.Y.; Kolliopoulos, K.; Chen, Y.H.; Skandalis, S.S. Regulation of hyaluronan biosynthesis and clinical impact of excessive hyaluronan production. Matrix Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Cyphert, J.M.; Trempus, C.S.; Garantziotis, S. Size matters: Molecular weight specificity of hyaluronan effects in cell biology. Int. J. Cell Biol. 2015, 2015, 563818. [Google Scholar] [CrossRef] [PubMed]
- Ebid, R.; Lichtnekert, J.; Anders, H.J. Hyaluronan is not a ligand but a regulator of toll-like receptor signaling in mesangial cells: Role of extracellular matrix in innate immunity. ISRN Nephrol. 2014, 2014, 714081. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yang, C.X.; Mo, W.; Liu, Y.W.; He, Y.Q. Hyaluronan oligosaccharides are potential stimulators to angiogenesis via RHAMM mediated signal pathway in wound healing. Clin. Investig. Med. 2008, 31, E106–E116. [Google Scholar] [CrossRef]
- Mattheolabakis, G.; Milane, L.; Singh, A.; Amiji, M.M. Hyaluronic acid targeting of CD44 for cancer therapy: From receptor biology to nanomedicine. J. Drug Target. 2015, 23, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Supp, D.M.; Hahn, J.M.; McFarland, K.L.; Glaser, K. Inhibition of hyaluronan synthase 2 reduces the abnormal migration rate of keloid keratinocytes. J. Burn Care Res. 2014, 35, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Fallacara, A.; Vertuani, S.; Panozzo, G.; Pecorelli, A.; Valacchi, G.; Manfredini, S. Novel Artificial Tears Containing Cross-Linked Hyaluronic Acid: An In Vitro Re-Epithelialization Study. Molecules 2017, 22, 2104. [Google Scholar] [CrossRef] [PubMed]
- Larrañeta, E.; Henry, M.; Irwin, N.J.; Trotter, J.; Perminova, A.A.; Donnelly, R.F. Synthesis and characterization of hyaluronic acid hydrogels crosslinked using a solvent-free process for potential biomedical applications. Carbohydr. Polym. 2018, 181, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Fallacara, A.; Manfredini, S.; Durini, E.; Vertuani, S. Hyaluronic acid fillers in soft tissue regeneration. Facial Plast. Surg. 2017, 33, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.R.; Laurent, T.C.; Laurent, U.B. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Girish, K.S.; Kemparaju, K. The magic glue hyaluronan and its eraser hyaluronidase: A biological overview. Life Sci. 2007, 80, 1921–1943. [Google Scholar] [CrossRef] [PubMed]
- Laurent, T.C.; Fraser, J.R. Hyaluronan. FASEB J. 1992, 6, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Hascall, V.C.; Laurent, T.C. Hyaluronan: Structure and Physical Properties. GlycoForum—Hyaluronan Today Website. 1997. Available online: http://glycoforum.gr.jp/science/hyaluronan/HA01/HA01E.html (accessed on 22 February 2018).
- Scott, J.E. Secondary structures in hyaluronan solutions: Chemical and biological implications. Ciba Found. Symp. 1989, 143, 6–15; Discussion 15–20, 281–285. [Google Scholar] [PubMed]
- Scott, J.E.; Cummings, C.; Brass, A.; Chen, Y. Secondary and tertiary structures of hyaluronan in aqueous solution, investigated by rotary shadowing-electron microscopy and computer simulation. Hyaluronan is a very efficient network-forming polymer. Biochem. J. 1991, 274, 699–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, J.E.; Heatley, F. Hyaluronan forms specific stable tertiary structures in aqueous solution: A 13C NMR study. Proc. Natl. Acad. Sci. USA 1999, 96, 4850–4855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurent, T. The biology of hyaluronan. Introduction. Ciba Found. Symp. 1989, 143, 1–20. [Google Scholar] [PubMed]
- Balazs, E.A.; Laurent, T.C.; Jeanloz, R.W. Nomenclature of hyaluronic acid. Biochem. J. 1986, 235, 903. [Google Scholar] [CrossRef] [PubMed]
- Heatley, F.; Scott, J.E. A water molecule participates in the secondary structure of hyaluronan. Biochem. J. 1988, 254, 489–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, J.E. Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. FASEB J. 1992, 6, 2639–2645. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Okamoto, A.; Nishinari, K. Viscoelasticity of hyaluronic acid with different molecular weights. Biorheology 1994, 31, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Cleland, R.L. Ionic polysaccharides. II. Comparison of polyelectrolyte behavior of hyaluronate with that of carboxymethyl cellulose. Biopolymers 1968, 6, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Balazs, E.A. The physical properties of synovial fluid and the special role of hyaluronic acid. In Disorders of the Knee; Helfet, A.J., Ed.; J.B. Lippincott Company: Philadelphia, PA, USA, 1974; pp. 63–75. [Google Scholar]
- Rwei, S.P.; Chen, S.W.; Mao, C.F.; Fang, H.W. Viscoelasticity and wearability of hyaluronate solutions. Biochem. Eng. J. 2008, 40, 211–217. [Google Scholar] [CrossRef]
- Lapcík, L.J.; Lapcík, L.; De Smedt, S.; Demeester, J.; Chabrecek, P. Hyaluronan: Preparation, Structure, Properties, and Applications. Chem. Rev. 1998, 98, 2663–2684. [Google Scholar] [CrossRef]
- Maleki, A.; Kjøniksen, A.L.; Nystrom, B. Effect of pH on the behavior of hyaluronic acid in dilute and semidilute aqueous solutions. Macromol. Symp. 2008, 274, 131–140. [Google Scholar] [CrossRef]
- Ghosh, S.; Kobal, I.; Zanette, D.; Reed, W.F. Conformational contraction and hydrolysis of hyaluronate in sodium hydroxide solutions. Macromolecules 1993, 26, 4685–4693. [Google Scholar] [CrossRef]
- Morris, E.R.; Rees, D.A.; Welsh, E.J. Conformation and dynamic interactions in hyaluronate solutions. J. Mol. Biol. 1980, 138, 383–400. [Google Scholar] [CrossRef]
- Pisárčik, M.; Bakoš, D.; Čeppan, M. Non-Newtonian properties of hyaluronic acid aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 1995, 97, 197–202. [Google Scholar] [CrossRef]
- Gura, E.; Hückel, M.; Müller, P.J. Specific degradation of hyaluronic acid and its rheological properties. Polym. Degrad. Stab. 1998, 59, 297–302. [Google Scholar] [CrossRef]
- DeAngelis, P.L.; Jing, W.; Drake, R.R.; Achyuthan, A.M. Identification and molecular cloning of a unique hyaluronan synthase from Pasteurella multocida. J. Biol. Chem. 1998, 273, 8454–8458. [Google Scholar] [CrossRef] [PubMed]
- Balazs, E.A.; Leshchiner, E.; Larsen, N.E.; Band, P. Applications of hyaluronan and its derivatives. In Biotechnological Polymers; Gebelein, C.G., Ed.; Technomic: Lancaster, UK, 1993; pp. 41–65. [Google Scholar]
- Schiraldi, C.; La Gatta, A.; De Rosa, M. Biotechnological Production and Application of Hyaluronan. In Biopolymers; Elnashar, M., Ed.; IntechOpen: London, UK, 2010; Available online: https://www.intechopen.com/books/biopolymers/biotechnological-production-characterization-and-application-of-hyaluronan (accessed on 20 June 2018).[Green Version]
- DeAngelis, P.L. Hyaluronan synthases: Fascinating glycosyltransferases from vertebrates, bacterial pathogens, and algal viruses. Cell. Mol. Life Sci. 1999, 56, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Volpi, N.; Maccari, F. Purification and characterization of hyaluronic acid from the mollusc bivalve Mytilus galloprovincialis. Biochimie 2003, 85, 619–625. [Google Scholar] [CrossRef]
- Kogan, G.; Soltés, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007, 29, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Volpi, N.; Schiller, J.; Stern, R.; Soltés, L. Role, metabolism, chemical modifications and applications of hyaluronan. Curr. Med. Chem. 2009, 16, 1718–1745. [Google Scholar] [CrossRef] [PubMed]
- Sobolewski, K.; Bańkowski, E.; Chyczewski, L.; Jaworski, S. Collagen and glycosaminoglycans of Wharton’s jelly. Biol. Neonate 1997, 71, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Robert, L.; Robert, A.M.; Renard, G. Biological effects of hyaluronan in connective tissues, eye, skin, venous wall. Role in aging. Pathol. Biol. 2010, 58, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Weigel, P.H.; Hascall, V.C.; Tammi, M. Hyaluronan synthases. J. Biol. Chem. 1997, 272, 13997–14000. [Google Scholar] [CrossRef] [PubMed]
- Itano, N.; Kimata, K. Mammalian hyaluronan synthases. IUBMB Life 2002, 54, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Spicer, A.P.; McDonald, J.A. Characterization and molecular evolution of a vertebrate hyaluronan synthase gene family. J. Biol. Chem. 1998, 273, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Itano, N.; Sawai, T.; Yoshida, M.; Lenas, P.; Yamada, Y.; Imagawa, M.; Shinomura, T.; Hamaguchi, M.; Yoshida, Y.; Ohnuki, Y.; et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J. Biol. Chem. 1999, 274, 25085–25092. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan in tissue injury and repair. Annu. Rev. Cell Dev. Biol. 2007, 23, 435–461. [Google Scholar] [CrossRef] [PubMed]
- Vigetti, D.; Viola, M.; Karousou, E.; De Luca, G.; Passi, A. Metabolic control of hyaluronan synthases. Matrix Biol. 2014, 35, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuhlmeier, K.M.; Pollaschek, C. Differential effect of transforming growth factor beta (TGF-beta) on the genes encoding hyaluronan synthases and utilization of the p38 MAPK pathway in TGF-beta-induced hyaluronan synthase 1 activation. J. Biol. Chem. 2004, 279, 8753–8760. [Google Scholar] [CrossRef] [PubMed]
- Vigetti, D.; Karousou, E.; Viola, M.; Deleonibus, S.; De Luca, G.; Passi, A. Hyaluronan: Biosynthesis and signaling. Biochim. Biophys. Acta 2014, 1840, 2452–2459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Tao, D.; Zhang, P.; Liu, X.; Zhang, Y.; Cheng, J.; Yuan, H.; Liu, L.; Jiang, H. Hyaluronan synthase 2 expressed by cancer-associated fibroblasts promotes oral cancer invasion. J. Exp. Clin. Cancer Res. 2016, 35, 181. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, J.; Yang, T.; Monterrosa Mena, J.; Huan, C.; Xie, T.; Kurkciyan, A.; Liu, N.; Jiang, D.; Noble, P.W. Hyaluronan synthase 2 regulates fibroblast senescence in pulmonary fibrosis. Matrix Biol. 2016, 55, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsang, J.Y.; Ni, Y.B.; Chan, S.K.; Chan, K.F.; Cheung, S.Y.; Tse, G.M. Hyaluronan synthase 2 is an adverse prognostic marker in androgen receptor-negative breast cancer. J. Clin. Pathol. 2016, 69, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Toole, B.P. Hyaluronan: From extracellular glue to pericellular cue. Nat. Rev. Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.; Jedrzejas, M.J. Hyaluronidases: Their genomics, structures, and mechanisms of action. Chem. Rev. 2006, 106, 818–839. [Google Scholar] [CrossRef] [PubMed]
- Csoka, A.B.; Frost, G.I.; Stern, R. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 2001, 20, 499–508. [Google Scholar] [CrossRef]
- Lokeshwar, V.B.; Rubinowicz, D.; Schroeder, G.L.; Forgacs, E.; Minna, J.D.; Block, N.L.; Nadji, M.; Lokeshwar, B.L. Stromal and epithelial expression of tumor markers hyaluronic acid and HYAL1 hyaluronidase in prostate cancer. J. Biol. Chem. 2001, 276, 11922–11932. [Google Scholar] [CrossRef] [PubMed]
- Lepperdinger, G.; Strobl, B.; Kreil, G. HYAL2, a human gene expressed in many cells, encodes a lysosomal hyaluronidase with a novel type of specificity. J. Biol. Chem. 1998, 273, 22466–22470. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.I.; Csóka, A.B.; Wong, T.; Stern, R. Purification, cloning, and expression of human plasma hyaluronidase. Biochem. Biophys. Res. Commun. 1997, 236, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Csoka, A.B.; Frost, G.I.; Wong, T.; Stern, R. Purification and microsequencing of hyaluronidase isozymes from human urine. FEBS Lett. 1997, 417, 307–310. [Google Scholar] [CrossRef] [Green Version]
- Stern, R. Hyaluronidases in cancer biology. Semin. Cancer Biol. 2008, 18, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Cherr, G.N.; Yudin, A.I.; Overstreet, J.W. The dual functions of GPI-anchored PH-20: Hyaluronidase and intracellular signaling. Matrix Biol. 2001, 20, 515–525. [Google Scholar] [CrossRef]
- Soltés, L.; Mendichi, R.; Kogan, G.; Schiller, J.; Stankovska, M.; Arnhold, J. Degradative action of reactive oxygen species on hyaluronan. Biomacromolecules 2006, 7, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.; Kogan, G.; Jedrzejas, M.J.; Soltés, L. The many ways to cleave hyaluronan. Biotechnol. Adv. 2007, 25, 537–557. [Google Scholar] [CrossRef] [PubMed]
- Monzon, M.E.; Fregien, N.; Schmid, N.; Falcon, N.S.; Campos, M.; Casalino-Matsuda, S.M.; Forteza, R.M. Reactive oxygen species and hyaluronidase 2 regulate airway epithelial hyaluronan fragmentation. J. Biol. Chem. 2010, 285, 26126–26134. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.; Arnhold, J.; Arnold, K. Contribution of reactive oxygen species to cartilage degradation in rheumatic diseases: Molecular pathways, diagnosis and potential therapeutic strategies. Curr. Med. Chem. 2003, 10, 2123–2145. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.; Arnhold, J.; Arnold, K. Action of hypochlorous acid on polymeric components of cartilage. Use of 13C NMR spectroscopy. Z. Naturforsch. C 1995, 50, 721–728. [Google Scholar] [PubMed]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef] [PubMed]
- Tamer, T.M. Hyaluronan and synovial joint: Function, distribution and healing. Interdiscip. Toxicol. 2013, 6, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Cao, M.; He, Y.; Liu, Y.; Yang, C.; Du, Y.; Wang, W.; Gao, F. A novel role of low molecular weight hyaluronan in breast cancer metastasis. FASEB J. 2015, 29, 1290–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.Y.; Muto, J.; Gallo, R.L. Hyaluronic acid oligosaccharides suppress TLR3-dependent cytokine expression in a TLR4-dependent manner. PLoS ONE 2013, 8, e72421. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Toole, B.P.; Ghatak, S. Hyaluronan constitutively regulates activation of multiple receptor tyrosine kinases in epithelial and carcinoma cells. J. Biol. Chem. 2006, 281, 34936–34941. [Google Scholar] [CrossRef] [PubMed]
- Toole, B.P.; Ghatak, S.; Misra, S. Hyaluronan oligosaccharides as a potential anticancer therapeutic. Curr. Pharm. Biotechnol. 2008, 9, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Campo, G.M.; Avenoso, A.; D’Ascola, A.; Prestipino, V.; Scuruchi, M.; Nastasi, G.; Calatroni, A.; Campo, S. 4-mer hyaluronan oligosaccharides stimulate in ammation response in synovial broblasts in part via TAK-1 and in part via p38-MAPK. Curr. Med. Chem. 2013, 20, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Cao, M.; Liu, H.; He, Y.; Xu, J.; Du, Y.; Liu, Y.; Wang, W.; Cui, L.; Hu, J.; et al. The high and low molecular weight forms of hyaluronan have distinct effects on CD44 clustering. J. Biol. Chem. 2012, 287, 43094–43107. [Google Scholar] [CrossRef] [PubMed]
- Turley, E.A.; Noble, P.W.; Bourguignon, LY. Signaling properties of hyaluronan receptors. J. Biol. Chem. 2002, 277, 4589–4592. [Google Scholar] [CrossRef] [PubMed]
- Research, G.V. Hyaluronic Acid Market Size Worth USD 15.4 Billion by 2025|CAGR: 8.8%. Available online: https://www.grandviewresearch.com/press-release/global-hyaluronic-acid-market (accessed on 8 March 2018).
- Shiedlin, A.; Bigelow, R.; Christopher, W.; Arbabi, S.; Yang, L.; Maier, R.V.; Wainwright, N.; Childs, A.; Miller, R.J. Evaluation of hyaluronan from different sources: Streptococcus zooepidemicus, rooster comb, bovine vitreous, and human umbilical cord. Biomacromolecules 2004, 5, 2122–2127. [Google Scholar] [CrossRef] [PubMed]
- Rangaswamy, V.; Jain, D. An efficient process for production and purification of hyaluronic acid from Streptococcus equi subsp. zooepidemicus. Biotechnol. Lett. 2008, 30, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yoo, S.J.; Oh, D.K.; Kweon, Y.G.; Park, D.W.; Lee, C.H.; Gil, G.H. Selection of a Streptococcus equi mutant and optimization of culture conditions for the production of high molecular weight hyaluronic acid. Enzyme Microb. Technol. 1996, 19, 440–445. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Li, J.; Du, G.; Chen, J. Microbial production of hyaluronic acid: Current state, challenges, and perspectives. Microb. Cell Fact. 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Jayaraman, G. Hyaluronan production and molecular weight is enhanced in pathway-engineered strains of lactate dehydrogenase-deficient Lactococcus lactis. Metab. Eng. Commun. 2016, 3, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Chien, L.J.; Lee, C.K. Enhanced hyaluronic acid production in Bacillus subtilis by coexpressing bacterial hemoglobin. Biotechnol. Prog. 2007, 23, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Stephanopoulos, G. Metabolic engineering of Escherichia coli for biosynthesis of hyaluronic acid. Metab. Eng. 2008, 10, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Gong, Q.; Yu, H.; Stephanopoulos, G. High-titer biosynthesis of hyaluronic acid by recombinant Corynebacterium glutamicum. Biotechnol. J. 2016, 11, 574–584. [Google Scholar] [CrossRef] [PubMed]
- DeAngelis, P.L.; Achyuthan, A.M. Yeast-derived recombinant DG42 protein of Xenopus can synthesize hyaluronan in vitro. J. Biol. Chem. 1996, 271, 23657–23660. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.; Shim, W.Y.; Kim, J.H. Metabolic engineering of Pichia pastoris for production of hyaluronic acid with high molecular weight. J. Biotechnol. 2014, 185, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Rakkhumkaew, N.; Shibatani, S.; Kawasaki, T.; Fujie, M.; Yamada, T. Hyaluronan synthesis in cultured tobacco cells (BY-2) expressing a chlorovirus enzyme: Cytological studies. Biotechnol. Bioeng. 2013, 110, 1174–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeAngelis, P.L. Monodisperse hyaluronan polymers: Synthesis and potential applications. Curr. Pharm. Biotechnol. 2008, 9, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Malson, T.; Lindqvist, B. Gels of Crosslinked Hyaluronic Acid for Use as a Vitreous Humor Substitute. International Publication No. WO1986000079 A1, 3 January 1986. [Google Scholar]
- Shimojo, A.A.; Pires, A.M.; Lichy, R.; Rodrigues, A.A.; Santana, M.H. The crosslinking degree controls the mechanical, rheological, and swelling properties of hyaluronic acid microparticles. J. Biomed. Mater. Res. A 2015, 103, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Birkinshaw, C. Physical properties of crosslinked hyaluronic acid hydrogels. J. Mater. Sci. Mater. Med. 2008, 19, 3335–3343. [Google Scholar] [CrossRef] [PubMed]
- Bowman, S.; Awad, M.E.; Hamrick, M.W.; Hunter, M.; Fulzele, S. Recent advances in hyaluronic acid based therapy for osteoarthritis. Clin. Transl. Med. 2018, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Nobile, V.; Buonocore, D.; Michelotti, A.; Marzatico, F. Anti-aging and filling efficacy of six types hyaluronic acid based dermo-cosmetic treatment: Double blind, randomized clinical trial of efficacy and safety. J. Cosmet. Dermatol. 2014, 13, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Singh, H.; Singh, M. Repair of tympanic membrane perforation by topical application of 1% sodium hyaluronate. Indian J. Otolaryngol. Head Neck Surg. 2006, 58, 241–244. [Google Scholar] [PubMed]
- Gelardi, M.; Iannuzzi, L.; Quaranta, N. Intranasal sodium hyaluronate on the nasal cytology of patients with allergic and nonallergic rhinitis. Int. Forum Allergy Rhinol. 2013, 3, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Gelardi, M.; Guglielmi, A.V.; De Candia, N.; Maffezzoni, E.; Berardi, P.; Quaranta, N. Effect of sodium hyaluronate on mucociliary clearance after functional endoscopic sinus surgery. Eur. Ann. Allergy Clin. Immunol. 2013, 45, 103–108. [Google Scholar] [PubMed]
- Maleki, A.; Kjøniksen, A.L.; Nyström, B. Characterization of the chemical degradation of hyaluronic acid during chemical gelation in the presence of different cross-linker agents. Carbohydr. Res. 2007, 342, 2776–2792. [Google Scholar] [CrossRef] [PubMed]
- Bulpitt, P.; Aeschlimann, D. New strategy for chemical modification of hyaluronic acid: Preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J. Biomed. Mater. Res. 1999, 47, 152–169. [Google Scholar] [CrossRef]
- Lim, D.G.; Prim, R.E.; Kang, E.; Jeong, S.H. One-pot synthesis of dopamine-conjugated hyaluronic acid/polydopamine nanocomplexes to control protein drug release. Int. J. Pharm. 2018, 542, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Magnani, A.; Rappuoli, R.; Lamponi, S.; Barbucci, R. Novel polysaccharide hydrogels: Characterization and properties. Polym. Adv. Technol. 2000, 11, 488–495. [Google Scholar] [CrossRef]
- Sigen, A.; Xu, Q.; McMichael, P.; Gao, Y.; Li, X.; Wang, X.; Greiser, U.; Zhou, D.; Wang, W. A facile one-pot synthesis of acrylated hyaluronic acid. Chem. Commun. 2018, 54, 1081–1084. [Google Scholar]
- Felgueiras, H.P.; Wang, L.M.; Ren, K.F.; Querido, M.M.; Jin, Q.; Barbosa, M.A.; Ji, J.; Martins, M.C. Octadecyl chains immobilized onto hyaluronic acid coatings by thiol-ene “click chemistry” increase the surface antimicrobial properties and prevent platelet adhesion and activation to polyurethane. ACS Appl. Mater. Interfaces 2017, 9, 7979–7989. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.J.; Taimoory, S.M.; Tam, R.Y.; Baker, A.E.G.; Binth Mohammad, N.; Trant, J.F.; Shoichet, M.S. Diels-Alder Click-Cross-Linked Hydrogels with Increased Reactivity Enable 3D Cell Encapsulation. Biomacromolecules 2018, 19, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Dong, H.; Deng, X.; Zhuo, R.; Zhong, Z. Injectable hyaluronic acid/poly(ethylene glycol) hydrogels crosslinked via strain-promoted azide-alkyne cycloaddition click reaction. Carbohydr. Polym. 2017, 169, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.Z.; Liu, Y.; Luo, Y.; Roberts, M.C.; Prestwich, G.D. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules 2002, 3, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Bencherif, S.A.; Srinivasan, A.; Horkay, F.; Hollinger, J.O.; Matyjaszewski, K.; Washburn, N.R. Influence of the degree of methacrylation on hyaluronic acid hydrogels properties. Biomaterials 2008, 29, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, P.E.; Chen, T.; Finch, A.; Brial, C.; Maher, S.A.; Torzilli, P.A. Photocrosslinked tyramine-substituted hyaluronate hydrogels with tunable mechanical properties improve immediate tissue-hydrogel interfacial strength in articular cartilage. J. Biomater. Sci. Polym. Ed. 2017, 28, 582–600. [Google Scholar] [CrossRef] [PubMed]
- Yui, N.; Okano, T.; Sakurai, Y. Inflammation responsive degradation of crosslinked hyaluronic acid gels. J. Control. Release 1992, 22, 105–116. [Google Scholar]
- Zhao, X. Process for the Production of Multiple Cross-Linked Hyaluronic Acid Derivatives. International Publication No. WO/2000/046253, 10 August 2000. [Google Scholar]
- Collins, M.; Birkinshaw, C. Comparison of the effectiveness of four different crosslinking agents with hyaluronic acid hydrogel films for tissue-culture applications. J. Appl. Polym. Sci. 2007, 104, 3183–3191. [Google Scholar] [CrossRef]
- Serban, M.; Yang, G.; Prestwich, G. Synthesis, characterization and chondroprotective properties of a hyaluronan thioethyl ether derivative. Biomaterials 2008, 29, 1388–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crescenzi, V.; Francescangeli, A.; Taglienti, A.; Capitani, D.; Mannina, L. Synthesis and partial characterization of hydrogels obtained via glutaraldehyde crosslinking of acetylated chitosan and of hyaluronan derivatives. Biomacromolecules 2003, 4, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Tomihata, K.; Ikada, Y. Crosslinking of hyaluronic acid with glutaraldehyde. J. Polym. Sci. Part A Polym. Chem. 1997, 35, 3553–3559. [Google Scholar] [CrossRef]
- Toemmeraas, K.; Eenschooten, C. Aryl/Alkyl Succinic Anhydride Hyaluronan Derivatives. International Publication No. WO/2007/033677, 29 March 2007. [Google Scholar]
- Seidlits, S.K.; Khaing, Z.Z.; Petersen, R.R.; Nickels, J.D.; Vanscoy, J.E.; Shear, J.B.; Schmidt, C.E. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials 2010, 31, 3930–3940. [Google Scholar] [CrossRef] [PubMed]
- Pravata, L.; Braud, C.; Boustta, M.; El Ghzaoui, A.; Tømmeraas, K.; Guillaumie, F.; Schwach-Abdellaoui, K.; Vert, M. New amphiphilic lactic acid oligomer–hyaluronan conjugates: Synthesis and physicochemical characterization. Biomacromolecules 2008, 9, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Mlcochová, P.; Bystrický, S.; Steiner, B.; Machová, E.; Koós, M.; Velebný, V.; Krcmár, M. Synthesis and characterization of new biodegradable hyaluronan alkyl derivatives. Biopolymers 2006, 82, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Della Valle, F.; Romeo, A. Esters of Hyaluronic Acid. U.S. Patent 4,851,521, 25 July 1989. [Google Scholar]
- Huin-Amargier, C.; Marchal, P.; Payan, E.; Netter, P.; Dellacherie, E. New physically and chemically crosslinked hyaluronate (HA)-based hydrogels for cartilage repair. J. Biomed. Mater. Res. A 2006, 76, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Sakai, S.; Ishikawa, T.; Avci, F.Y.; Linhardt, R.J.; Toida, T. Preparation of the methyl ester of hyaluronan and its enzymatic degradation. Carbohydr. Res. 2005, 340, 2297–2304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prata, J.E.; Barth, T.A.; Bencherif, S.A.; Washburn, N.R. Complex fluids based on methacrylated hyaluronic acid. Biomacromolecules 2010, 11, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, L.; Cortivo, R.; Berti, T.; Berti, A.; Pea, F.; Mazzo, M.; Moras, M.; Abatangelo, G. Biocompatibility and biodegradation of different hyaluronan derivatives (Hyaff) implanted in rats. Biomaterials 1993, 14, 1154–1160. [Google Scholar] [CrossRef]
- Bellini, D.; Topai, A. Amides of Hyaluronic Acid and the Derivatives Thereof and a Process for Their Preparation. International Application No. PCT/IB1999/001254, 13 January 2000. [Google Scholar]
- Kaczmarek, B.; Sionkowska, A.; Kozlowska, J.; Osyczka, A.M. New composite materials prepared by calcium phosphate precipitation in chitosan/collagen/hyaluronic acid sponge cross-linked by EDC/NHS. Int. J. Biol. Macromol. 2018, 107, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Kirk, J.F.; Ritter, G.; Finger, I.; Sankar, D.; Reddy, J.D.; Talton, J.D.; Nataraj, C.; Narisawa, S.; Millán, J.L.; Cobb, R.R. Mechanical and biocompatible characterization of a cross-linked collagen-hyaluronic acid wound dressing. Biomatter 2013, 3, pii:E25633. [Google Scholar] [CrossRef] [PubMed]
- Bergman, K.; Elvingson, C.; Hilborn, J.; Svensk, G.; Bowden, T. Hyaluronic acid derivatives prepared in aqueous media by triazine-activated amidation. Biomacromolecules 2007, 8, 2190–2195. [Google Scholar] [CrossRef] [PubMed]
- D’Este, M.; Eglin, D.; Alini, M. A systematic analysis of DMTMM vs EDC/NHS for ligation of amines to hyaluronan in water. Carbohydr. Polym. 2014, 108, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, V.; Francescangeli, A.; Segre, A.; Capitani, D.; Mannina, L.; Renier, D.; Bellini, D. NMR structural study of hydrogels based on partially deacetylated hyaluronan. Macromol. Biosci. 2002, 2, 272–279. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Svechkarev, D.; Souchek, J.J.; Hill, T.K.; Taylor, M.A.; Natarajan, A.; Mohs, A.M. Impact of structurally modifying hyaluronic acid on CD44 interaction. J. Mater. Chem. B. 2017, 5, 8183–8192. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.H.; Oh, E.J.; Chae, S.Y.; Lee, K.C.; Hahn, S.K. Long acting hyaluronate—Exendin 4 conjugate for the treatment of type 2 diabetes. Biomaterials 2010, 31, 4121–4128. [Google Scholar] [CrossRef] [PubMed]
- Mero, A.; Campisi, M. Hyaluronic acid bioconjugates for the delivery of bioactive molecules. Polymers 2014, 6, 346–369. [Google Scholar] [CrossRef]
- Mangano, K.; Vergalito, F.; Mammana, S.; Mariano, A.; De Pasquale, R.; Meloscia, A.; Bartollino, S.; Guerra, G.; Nicoletti, F.; Di Marco, R. Evaluation of hyaluronic acid-P40 conjugated cream in a mouse model of dermatitis induced by oxazolone. Exp. Ther. Med. 2017, 14, 2439–2444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manju, S.; Sreenivasan, K. Conjugation of curcumin onto hyaluronic acid enhances its aqueous solubility and stability. J. Colloid Interface Sci. 2011, 359, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Sahu, K.; Singh, S.P.; Jain, B. Wound healing activity of curcumin conjugated to hyaluronic acid: In vitro and in vivo evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Peng, F.; Song, X.; Wu, J.; Yao, W.; Gao, X. Conjugation of paclitaxel to C-6 hexanediamine-modified hyaluronic acid for targeted drug delivery to enhance antitumor efficacy. Carbohydr. Polym. 2018, 181, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.D.; Zheng, S.; Han, J.; Le, V.H.; Park, J.O.; Park, S. Nanohybrid magnetic liposome functionalized with hyaluronic acid for enhanced cellular uptake and near-infrared-triggered drug release. Colloids Surf. B. Biointerfaces 2017, 154, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Hayward, S.L.; Wilson, C.L.; Kidambi, S. Hyaluronic acid-conjugated liposome nanoparticles for targeted delivery to CD44 overexpressing glioblastoma cells. Oncotarget 2016, 7, 34158–34171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, N.K.; Shin, D.H.; Kim, J.S.; Weon, K.Y.; Jang, C.Y.; Kim, J.S. Hyaluronan-conjugated liposomes encapsulating gemcitabine for breast cancer stem cells. Int. J. Nanomed. 2016, 11, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Yin, X.; Sun, K.; Feng, S.; Liu, J.; Chen, D.; Guo, C.; Wu, Z. Redox-sensitive and hyaluronic acid functionalized liposomes for cytoplasmic drug delivery to osteosarcoma in animal models. J. Control. Release 2017, 261, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Negi, L.M.; Jaggi, M.; Joshi, V.; Ronodip, K.; Talegaonkar, S. Hyaluronan coated liposomes as the intravenous platform for delivery of imatinib mesylate in MDR colon cancer. Int. J. Biol. Macromol. 2015, 73, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, M.A.; Elnaggar, Y.S.R.; El-Refaie, W.M.; Abdallah, O.Y. Hyalugel-integrated liposomes as a novel ocular nanosized delivery system of fluconazole with promising prolonged effect. Int. J. Pharm. 2017, 534, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Manconi, M.; Manca, M.L.; Valenti, D.; Escribano, E.; Hillaireau, H.; Fadda, A.M.; Fattal, E. Chitosan and hyaluronan coated liposomes for pulmonary administration of curcumin. Int. J. Pharm. 2017, 525, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Leite Nascimento, T.; Hillaireau, H.; Vergnaud, J.; Rivano, M.; Deloménie, C.; Courilleau, D.; Arpicco, S.; Suk, J.S.; Hanes, J.; Fattal, E. Hyaluronic acid-conjugated lipoplexes for targeted delivery of siRNA in a murine metastatic lung cancer model. Int. J. Pharm. 2016, 514, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Maudens, P.; Meyer, S.; Seemayer, C.A.; Jordan, O.; Allémann, E. Self-assembled thermoresponsive nanostructures of hyaluronic acid conjugates for osteoarthritis therapy. Nanoscale 2018, 10, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, H.T.; Tae, Y.M.; Kong, W.H.; Sung, D.K.; Hwang, B.W.; Kim, K.S.; Kim, Y.K.; Hahn, S.K. Bioimaging and pulmonary applications of self-assembled Flt1 peptide-hyaluronic acid conjugate nanoparticles. Biomaterials 2013, 34, 8478–8490. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Choudhury, H.; Gunasegaran, T.A.P.; Nathan, S.S.; Md, S.; Gorain, B.; Tripathy, M.; Hussain, Z. Hyaluronic acid-modified betamethasone encapsulated polymeric nanoparticles: Fabrication, characterisation, in vitro release kinetics, and dermal targeting. Drug Deliv. Transl. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.G.; Detoni, C.B.; Balducci, A.G.; Rondelli, V.; Colombo, P.; Guterres, S.S.; Sonvico, F. Hyaluronate nanoparticles included in polymer films for the prolonged release of vitamin E for the management of skin wounds. Eur. J. Pharm. Sci. 2016, 83, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hou, J.; Su, C.; Zhao, L.; Shi, Y. Hyaluronic acid-coated chitosan nanoparticles induce ROS-mediated tumor cell apoptosis and enhance antitumor efficiency by targeted drug delivery via CD44. J. Nanobiotechnol. 2017, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Yoon, H.Y.; Koo, H.; Ko, S.H.; Shim, J.S.; Lee, J.H.; Kim, K.; Kwon, I.C.; Kim, D.D. Self-assembled nanoparticles based on hyaluronic acid-ceramide (HA-CE) and Pluronic® for tumor-targeted delivery of docetaxel. Biomaterials 2011, 32, 7181–7190. [Google Scholar] [CrossRef] [PubMed]
- Parashar, P.; Rathor, M.; Dwivedi, M.; Saraf, S.A. Hyaluronic acid decorated naringenin nanoparticles: Appraisal of chemopreventive and curative potential for lung cancer. Pharmaceutics 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.M.; Kim, D.D.; Chung, S.J.; Shim, C.K. Delivery of ofloxacin to the lung and alveolar macrophages via hyaluronan microspheres for the treatment of tuberculosis. J. Control. Release 2008, 129, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, M.; Liu, T.; Cun, D.; Fang, L.; Yang, M. Inhaled hyaluronic acid microparticles extended pulmonary retention and suppressed systemic exposure of a short-acting bronchodilator. Carbohydr. Polym. 2017, 172, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Fatnassi, M.; Jacquart, S.; Brouillet, F.; Rey, C.; Combes, C.; Girod Fullana, S. Optimization of spray-dried hyaluronic acid microspheres to formulate drug-loaded bone substitute materials. Powder Technol. 2014, 255, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Simsolo, E.E.; Eroğlu, İ.; Tanrıverdi, S.T.; Özer, Ö. Formulation and evaluation of organogels containing hyaluronan microparticles for topical delivery of caffeine. AAPS PharmSciTech 2018, 19, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Menegatti, E.; Cortesi, R. Hyaluronan-based microspheres as tools for drug delivery: A comparative study. Int. J. Pharm. 2005, 288, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Koivusalo, L.; Karvinen, J.; Sorsa, E.; Jönkkäri, I.; Väliaho, J.; Kallio, P.; Ilmarinen, T.; Miettinen, S.; Skottman, H.; Kellomäki, M. Hydrazone crosslinked hyaluronan-based hydrogels for therapeutic delivery of adipose stem cells to treat corneal defects. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 85, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Kirker, K.R.; Prestwich, G.D. Cross-linked hyaluronic acid hydrogel films: New biomaterials for drug delivery. J. Control. Release 2000, 69, 169–184. [Google Scholar] [CrossRef]
- Kroin, J.S.; Kc, R.; Li, X.; Hamilton, J.L.; Das, V.; van Wijnen, A.J.; Dall, O.M.; Shelly, D.A.; Kenworth, T.; Im, H.J. Intraarticular slow-release triamcinolone acetate reduces allodynia in an experimental mouse knee osteoarthritis model. Gene 2016, 591, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirard, D.; Vereecken, P.; Mélot, C.; Heenen, M. Three percent diclofenac in 2.5% hyaluronan gel in the treatment of actinic keratoses: A meta-analysis of the recent studies. Arch. Dermatol. Res. 2005, 297, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Foster, R.; Yang, X.; Feng, Y.; Shen, J.K.; Mankin, H.J.; Hornicek, F.J.; Amiji, M.M.; Duan, Z. Up-regulation of CD44 in the development of metastasis, recurrence and drug resistance of ovarian cancer. Oncotarget 2015, 6, 9313–9326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buffa, R.; Šedová, P.; Basarabová, I.; Bobula, T.; Procházková, P.; Vágnerová, H.; Dolečková, I.; Moravčíková, S.; Hejlová, L.; Velebný, V. A new unsaturated derivative of hyaluronic acid—Synthesis, analysis and applications. Carbohydr. Polym. 2017, 163, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Aya, K.L.; Stern, R. Hyaluronan in wound healing: Rediscovering a major player. Wound Repair Regen. 2014, 22, 579–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagliagambe, M.; Elstrom, T.A.; Ward, D.B. Hyaluronic Acid Sodium Salt 0.2% Gel in the Treatment of a Recalcitrant Distal Leg Ulcer: A Case Report. J. Clin. Aesthet. Dermatol. 2017, 10, 49–51. [Google Scholar] [PubMed]
- Neuman, M.G.; Nanau, R.M.; Oruña-Sanchez, L.; Coto, G. Hyaluronic acid and wound healing. J. Pharm. Pharm. Sci. 2015, 18, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Rueda Lópex, J.; Segovia Gómez, T.; Guerrero Palmero, A.; Bermejo Martínez, M.; Muñoz Bueno, A.M. Hyaluronic acid: A new trend to cure skin injuries an observational study. Rev. Enferm. 2005, 28, 53–57. [Google Scholar] [PubMed]
- Shi, L.; Zhao, Y.; Xie, Q.; Fan, C.; Hilborn, J.; Dai, J.; Ossipov, D.A. Moldable Hyaluronan Hydrogel Enabled by Dynamic Metal-Bisphosphonate Coordination Chemistry for Wound Healing. Adv. Healthc. Mater. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xue, Y.; Jia, B.; Bai, Y.; Zuo, Y.; Wang, S.; Zhao, Y.; Yang, W.; Tang, H. The preparation of hyaluronic acid grafted pullulan polymers and their use in the formation of novel biocompatible wound healing film. Carbohydr. Polym. 2018, 188, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Berce, C.; Muresan, M.S.; Soritau, O.; Petrushev, B.; Tefas, L.; Rigo, I.; Ungureanu, G.; Catoi, C.; Irimie, A.; Tomuleasa, C. Cutaneous wound healing using polymeric surgical dressings based on chitosan, sodium hyaluronate and resveratrol. A preclinical experimental study. Colloids Surf. B Biointerfaces 2018, 163, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Sanad, R.A.; Abdel-Bar, H.M. Chitosan-hyaluronic acid composite sponge scaffold enriched with Andrographolide-loaded lipid nanoparticles for enhanced wound healing. Carbohydr. Polym. 2017, 173, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Wirostko, B.; Mann, B.K.; Williams, D.L.; Prestwich, G.D. Ophthalmic Uses of a Thiol-Modified Hyaluronan-Based Hydrogel. Adv. Wound Care 2014, 3, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Cassano, M.; Russo, G.M.; Granieri, C.; Cassano, P. Cytofunctional changes in nasal ciliated cells in patients treated with hyaluronate after nasal surgery. Am. J. Rhinol. Allergy 2016, 30, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, P.; Kamal, R. Hyaluronic acid: A boon in periodontal therapy. N. Am. J. Med. Sci. 2013, 5, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Neumayer, T.; Prinz, A.; Findl, O. Effect of a new cohesive ophthalmic viscosurgical device on corneal protection and intraocular pressure in small-incision cataract surgery. J. Cataract Refract. Surg. 2008, 34, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Vandermeer, G.; Chamy, Y.; Pisella, P.J. Comparison of objective optical quality measured by double-pass aberrometry in patients with moderate dry eye: Normal saline vs. artificial tears: A pilot study. J. Fr. Ophtalmol. 2018, 41, e51–e57. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, G.; Villa-Collar, C.; Martin-Gil, A.; Serramito, M.; Santamaría, L. Comparison Between Viscous Teardrops and Saline Solution to Fill Orthokeratology Contact Lenses Before Overnight Wear. Eye Contact Lens. 2017. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.E.; Murphy, P.J.; Boulton, M. Effectiveness of sodium hyaluronate eyedrops in the treatment of dry eye. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006, 244, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Aragona, P.; Papa, V.; Micali, A.; Santocono, M.; Milazzo, G. Long term treatment with sodium hyaluronate–containing artificial tears reduces ocular surface damage in patients with dry eye. Br. J. Ophthalmol. 2002, 86, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.T.; Chiang, T.H.; Chang, S.W.; Chen, Y.H.; Hu, F.R.; Wang, I.J. Enhanced corneal wound healing with hyaluronic acid and high-potassium artificial tears. Clin. Exp. Optom. 2013, 96, 536–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laffleur, F.; Dachs, S. Development of novel mucoadhesive hyaluronic acid derivate as lubricant for the treatment of dry eye syndrome. Ther. Deliv. 2015, 6, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Guidolin, D. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: Are the effects molecular weight dependent? Semin. Arthritis Rheum. 2002, 32, 10–37. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.J.; Laurent, U.B.; Fraser, J.R. Turnover of hyaluronan in synovial joints: Elimination of labelled hyaluronan from the knee joint of the rabbit. Exp. Physiol. 1991, 76, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, D.D.; Stoker, A.; Kane, S.; Cockrell, M.; Cook, J.L. Biochemical effects of two different hyaluronic acid products in a co-culture model of osteoarthritis. Osteoarthr. Cartil. 2006, 14, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.F.; Hsu, C.W.; Lin, H.S.; Liou, I.H.; Chen, Y.H.; Hung, C.L. Comparison of single intra-articular injection of novel hyaluronan (HYA-JOINT Plus) with synvisc-one for knee osteoarthritis: A randomized, controlled, double-blind trial of efficacy and safety. J. Bone Joint Surg. Am. 2017, 99, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Monzon, M.E.; Casalino-Matsuda, S.M.; Forteza, R.M. Identification of glycosaminoglycans in human airway secretions. Am. J. Respir. Cell Mol. Biol. 2006, 34, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Garantziotis, S.; Brezina, M.; Castelnuovo, P.; Drago, L. The role of hyaluronan in the pathobiology and treatment of respiratory disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L785–L795. [Google Scholar] [CrossRef] [PubMed]
- Gerdin, B.; Hällgren, R. Dynamic role of hyaluronan (HYA) in connective tissue activation and inflammation. J. Intern. Med. 1997, 242, 49–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furnari, M.L.; Termini, L.; Traverso, G.; Barrale, S.; Bonaccorso, M.R.; Damiani, G.; Piparo, C.L.; Collura, M. Nebulized hypertonic saline containing hyaluronic acid improves tolerability in patients with cystic fibrosis and lung disease compared with nebulized hypertonic saline alone: A prospective, randomized, double-blind, controlled study. Ther. Adv. Respir. Dis. 2012, 6, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Gavina, M.; Luciani, A.; Villella, V.R.; Esposito, S.; Ferrari, E.; Bressani, I.; Casale, A.; Bruscia, E.M.; Maiuri, L.; Raia, V. Nebulized hyaluronan ameliorates lung inflammation in cystic fibrosis mice. Pediatr. Pulmonol. 2013, 48, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Petrigni, G.; Allegra, L. Aerosolised hyaluronic acid prevents exercise-induced bronchoconstriction, suggesting novel hypotheses on the correction of matrix defects in asthma. Pulm. Pharmacol. Ther. 2006, 19, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Turino, G.M.; Ma, S.; Lin, Y.Y.; Cantor, J.O. The therapeutic potential of hyaluronan in COPD. Chest 2017. [Google Scholar] [CrossRef] [PubMed]
- Nenna, R.; Papasso, S.; Battaglia, M.; De Angelis, D.; Petrarca, L.; Felder, D.; Salvadei, S.; Berardi, R.; Roberti, M.; Papoff, P.; et al. 7% hypertonic saline and hyaluronic acid and in the treatment of infants mild-moderate bronchiolitis. Eur. Respir. J. 2011, 38, 1717. [Google Scholar]
- Goddard, J.C.; Janssen, D.A.W. Intravesical hyaluronic acid and chondroitin sulfate for recurrent urinary tract infections: Systematic review and meta-analysis. Int. Urogynecol. J. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ząbkowski, T.; Jurkiewicz, B.; Saracyn, M. Treatment of recurrent bacterial cystitis by intravesical instillations of hyaluronic acid. Urol. J. 2015, 12, 2192–2195. [Google Scholar] [PubMed]
- American Society of Plastic Surgeons. 2017 Plastic Surgery Statistics Report. Available online: https://www.plasticsurgery.org/documents/News/Statistics/2017/plastic-surgery-statistics-report-2017.pdf (accessed on 8 March 2018).
- Muhn, C.; Rosen, N.; Solish, N.; Bertucci, V.; Lupin, M.; Dansereau, A.; Weksberg, F.; Remington, B.K.; Swift, A. The evolving role of hyaluronic acid fillers for facial volume restoration and contouring: A Canadian overview. Clin. Cosmet. Investig. Dermatol. 2012, 5, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Janiš, R.; Pata, V.; Egner, P.; Pavlačková, J.; Zapletalová, A.; Kejlová, K. Comparison of metrological techniques for evaluation of the impact of a cosmetic product containing hyaluronic acid on the properties of skin surface. Biointerphases 2017, 12, 021006. [Google Scholar] [CrossRef] [PubMed]
- Trommer, H.; Wartewig, S.; Böttcher, R.; Pöppl, A.; Hoentsch, J.; Ozegowski, J.H.; Neubert, R.H. The effects of hyaluronan and its fragments on lipid models exposed to UV irradiation. Int. J. Pharm. 2003, 254, 223–234. [Google Scholar] [CrossRef]
- Hašová, M.; Crhák, T.; Safránková, B.; Dvořáková, J.; Muthný, T.; Velebný, V.; Kubala, L. Hyaluronan minimizes effects of UV irradiation on human keratinocytes. Arch. Dermatol. Res. 2011, 303, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Drealos, Z.D. Nutrition and enhancing youthful-appearing skin. Clin. Dermatol. 2010, 28, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Maeshima, T.; Kubota, T.; Kurihara, H.; Masuda, Y.; Nomura, Y. Adsorption of orally administerd hyaluronan. J. Med. Food 2016, 19, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Zgheib, C.; Xu, J.; Liechty, K.W. Targeting Inflammatory Cytokines and Extracellular Matrix Composition to Promote Wound Regeneration. Adv. Wound Care 2014, 3, 344–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, T.; Iwaso, H. An effectiveness study of hyaluronic acid [Hyabest® (J)] in the treatment of osteoarthritis of the knee on the patients in the United States. J. New Rem. Clin. 2009, 58, 260–269. [Google Scholar]
- Balogh, L.; Polyak, A.; Mathe, D.; Kiraly, R.; Thuroczy, J.; Terez, M.; Janoki, G.; Ting, Y.; Bucci, L.R.; Schauss, A.G. An effectiveness study of hyaluronic acid [Hyabest® (J)] in the treatment of osteoarthritis of the knee on the patients in the United States. J. Agric. Food Chem. 2008, 56, 10582–10593. [Google Scholar] [CrossRef] [PubMed]
- Oe, M.; Mitsugi, K.; Odanaka, W.; Yoshida, H.; Matsuoka, R.; Seino, S.; Kanemitsu, T.; Masuda, Y. Dietary hyaluronic acid migrates into the skin of rats. Sci. World J. 2014, 2014, 378024. [Google Scholar] [CrossRef] [PubMed]
- Solà, R.; Valls, R.M.; Martorell, I.; Giralt, M.; Pedret, A.; Taltavull, N.; Romeu, M.; Rodríguez, À.; Moriña, D.; Lopez de Frutos, V.; Montero, M.; et al. A low-fat yoghurt supplemented with a rooster comb extract on muscle joint function in adults with mild knee pain: A rondomized, double blind, parallel, placebo-controlled, clinical trial of efficacy. Food Funct. 2015, 6, 3531–3539. [Google Scholar] [CrossRef] [PubMed]
- Göllner, I.; Voss, W.; von Hehn, U.; Kammerer, S. Ingestion of an Oral Hyaluronan Solution Improves Skin Hydration, Wrinkle Reduction, Elasticity, and Skin Roughness: Results of a Clinical Study. J. Evid. Based Complement. Altern. Med. 2017, 22, 816–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawada, C.; Yoshida, T.; Yoshida, H.; Matsuoka, R.; Sakamoto, W.; Odanaka, W.; Sato, T.; Yamasaki, T.; Kanemitsu, T.; Masuda, Y.; et al. Ingested hyaluronan moisturizes dry skin. Nutr. J. 2014, 13, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawada, C.; Yoshida, T.; Yoshida, H.; Sakamoto, W.; Odanaka, W.; Sato, T.; Yamasaki, T.; Kanemitsu, T.; Masuda, Y.; Urushibata, O. Ingestion of hyaluronans (molecular weights 800 k and 300 k) improves dry skin conditions: A randomized, double blind, controlled study. J. Clin. Biochem. Nutr. 2015, 56, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Kawada, C.; Kimura, M.; Masuda, Y.; Nomura, Y. Oral administration of hyaluronan prevents skin dryness and epidermal thickening in ultraviolet irradiated hairless mice. J. Photochem. Photobiol. B 2015, 153, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Oe, M.; Tashiro, T.; Yoshida, H.; Nishiyama, H.; Masuda, Y.; Maruyama, K.; Koikeda, T.; Maruya, R.; Fukui, N. Oral hyaluronan relieves knee pain: A review. Nutr. J. 2016, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Larson, B. 3D Cell Culture: A Review of Current Techniques. 2015. Available online: http://mktg.biotek.com/ news/2015/Fall/featured-application.html (accessed on 14 June 2018).
- Gurski, L.A.; Jha, A.K.; Zhang, C.; Jia, X.; Farach-Carson, M.C. Hyaluronic acid-based hydrogels as 3D matrices for in vitro evaluation of chemotherapeutic drugs using poorly adherent prostate cancer cells. Biomaterials 2009, 30, 6076–6085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffery, A.F.; Churchward, M.A.; Mushahwar, V.K.; Todd, K.G.; Elias, A.L. Hyaluronic acid-based 3D culture model for in vitro testing of electrode biocompatibility. Biomacromolecules 2014, 15, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Highley, C.B.; Rodell, C.B.; Sun, W.; Burdick, J.A. 3D Printing of Shear-Thinning Hyaluronic Acid Hydrogels with Secondary Cross-Linking. ACS Biomater. Sci. Eng. 2016, 2, 1743–1751. [Google Scholar] [CrossRef]
- Fallacara, A.; Busato, L.; Pozzoli, M.; Ghadiri, M.; Ong, H.X.; Young, P.M.; Manfredini, S.; Traini, D. Combination of urea-crosslinked Hyaluronic acid and sodium ascorbyl phosphate for the treatment of inflammatory lung diseases: An in vitro study. Eur. J. Pharm. Sci. 2018, 120, 96–106. [Google Scholar] [CrossRef] [PubMed]



| Total | 13,684 |
|---|---|
| Alive | 8749 |
| Dead | 4935 |
| 1st application year | unknown |
| After 2015 | 2717 |
| 2011–2015 | 4568 |
| 2006–2010 | 2647 |
| 2001–2005 | 1694 |
| Before 2001 | 2058 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. https://doi.org/10.3390/polym10070701
Fallacara A, Baldini E, Manfredini S, Vertuani S. Hyaluronic Acid in the Third Millennium. Polymers. 2018; 10(7):701. https://doi.org/10.3390/polym10070701
Chicago/Turabian StyleFallacara, Arianna, Erika Baldini, Stefano Manfredini, and Silvia Vertuani. 2018. "Hyaluronic Acid in the Third Millennium" Polymers 10, no. 7: 701. https://doi.org/10.3390/polym10070701