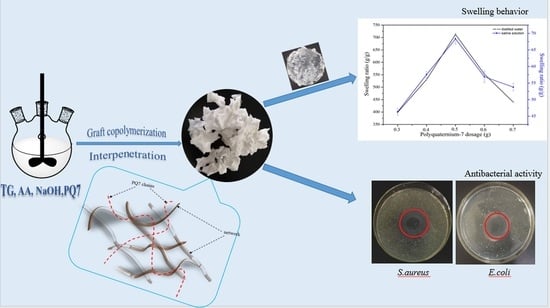
A One Pot Method for Preparing an Antibacterial Superabsorbent Hydrogel with a Semi-IPN Structure Based on Tara Gum and Polyquaternium-7
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TG-g-PAA/PQ7 Superabsorbent Polymers
2.3. Characterization
2.3.1. Swelling Behavior
2.3.2. FTIR Spectroscopy
2.3.3. X-ray Diffraction
2.3.4. Scanning Electronic Microscopy
2.3.5. Thermogravimetric Analysis
2.3.6. Antibacterial Activity
2.3.7. Mechanical Property Test
3. Results and Discussion
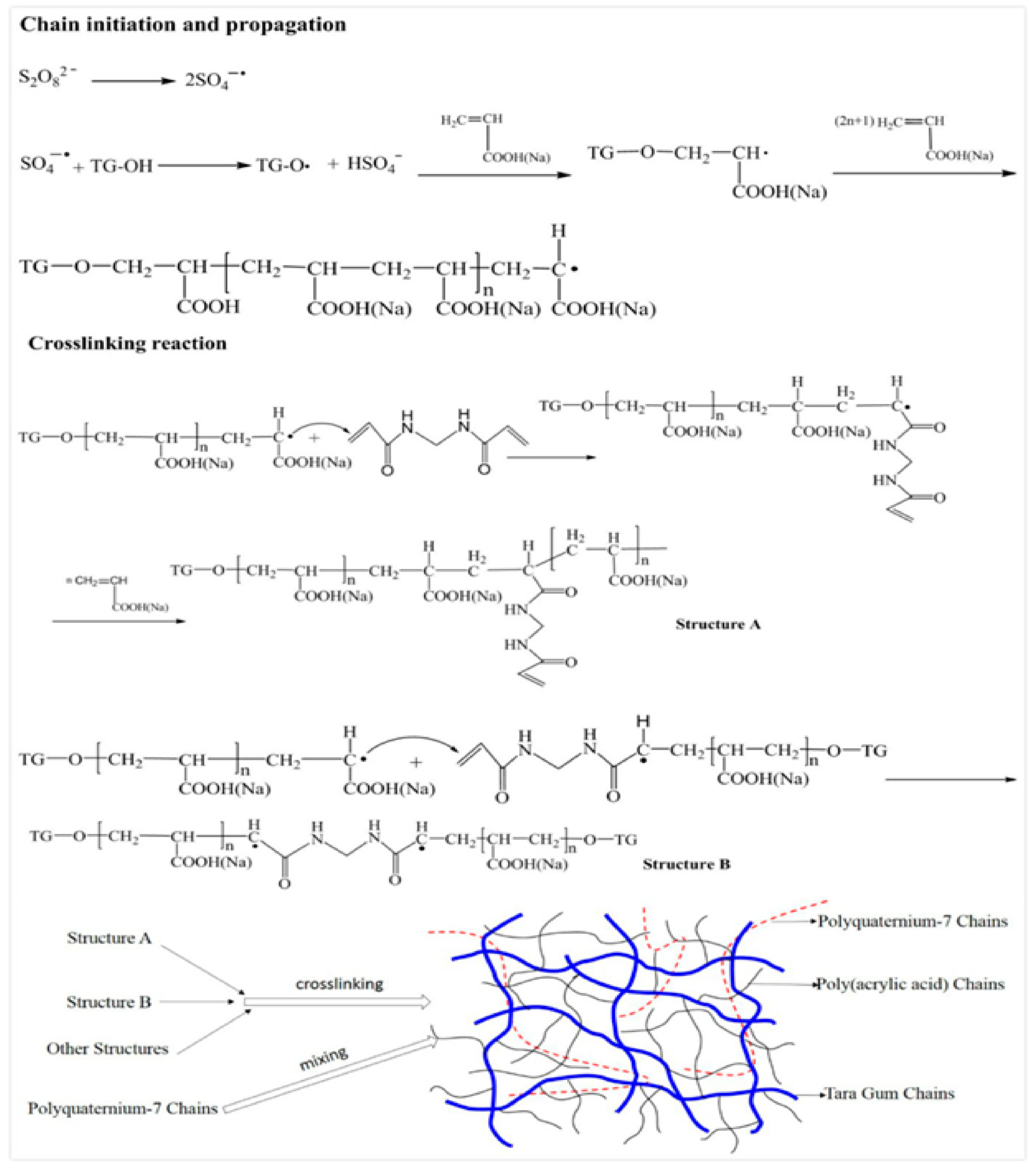
3.1. Mechanism of the Superabsorbent Polymer
3.2. Swelling Behavior
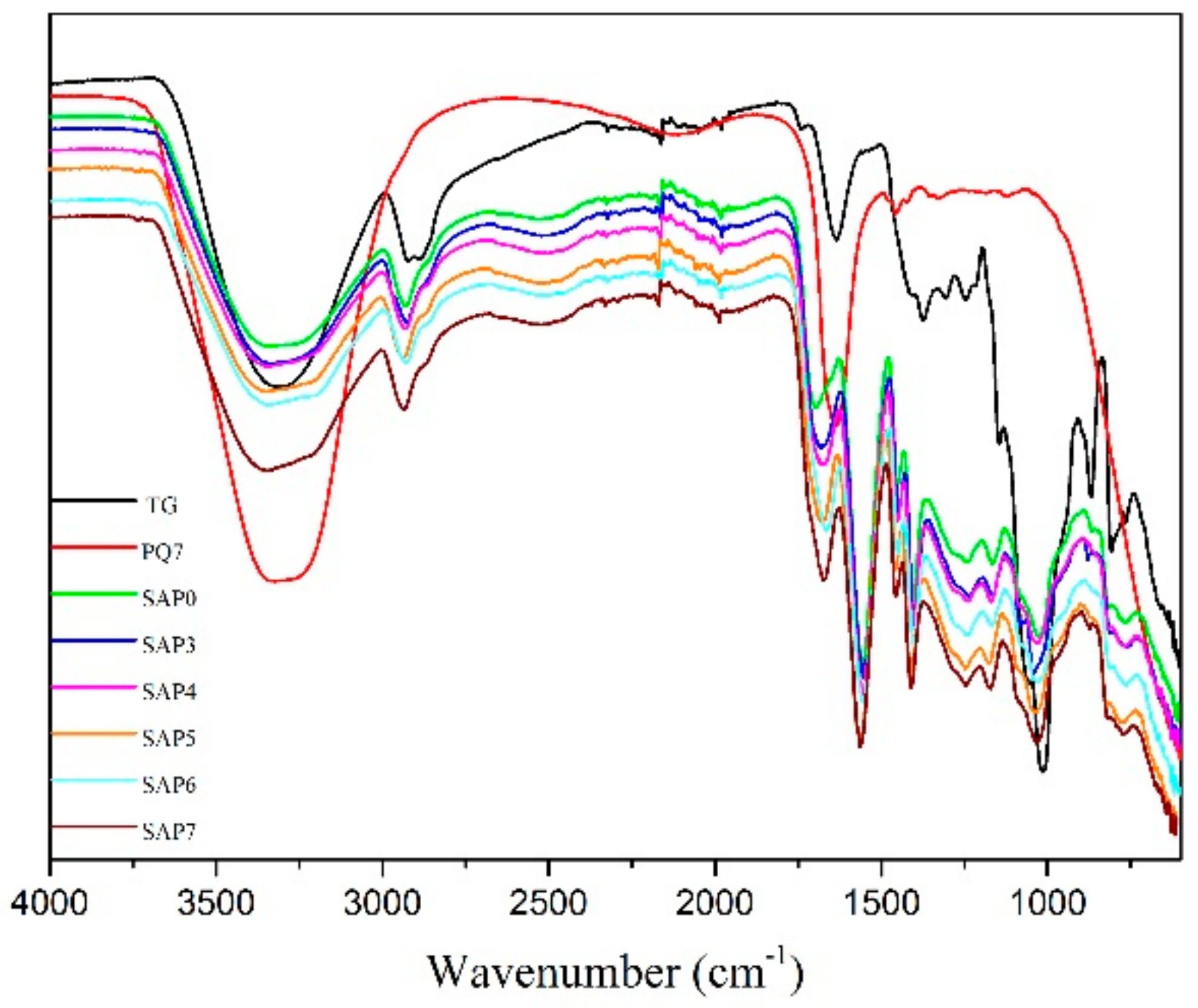
3.3. FTIR Analysis
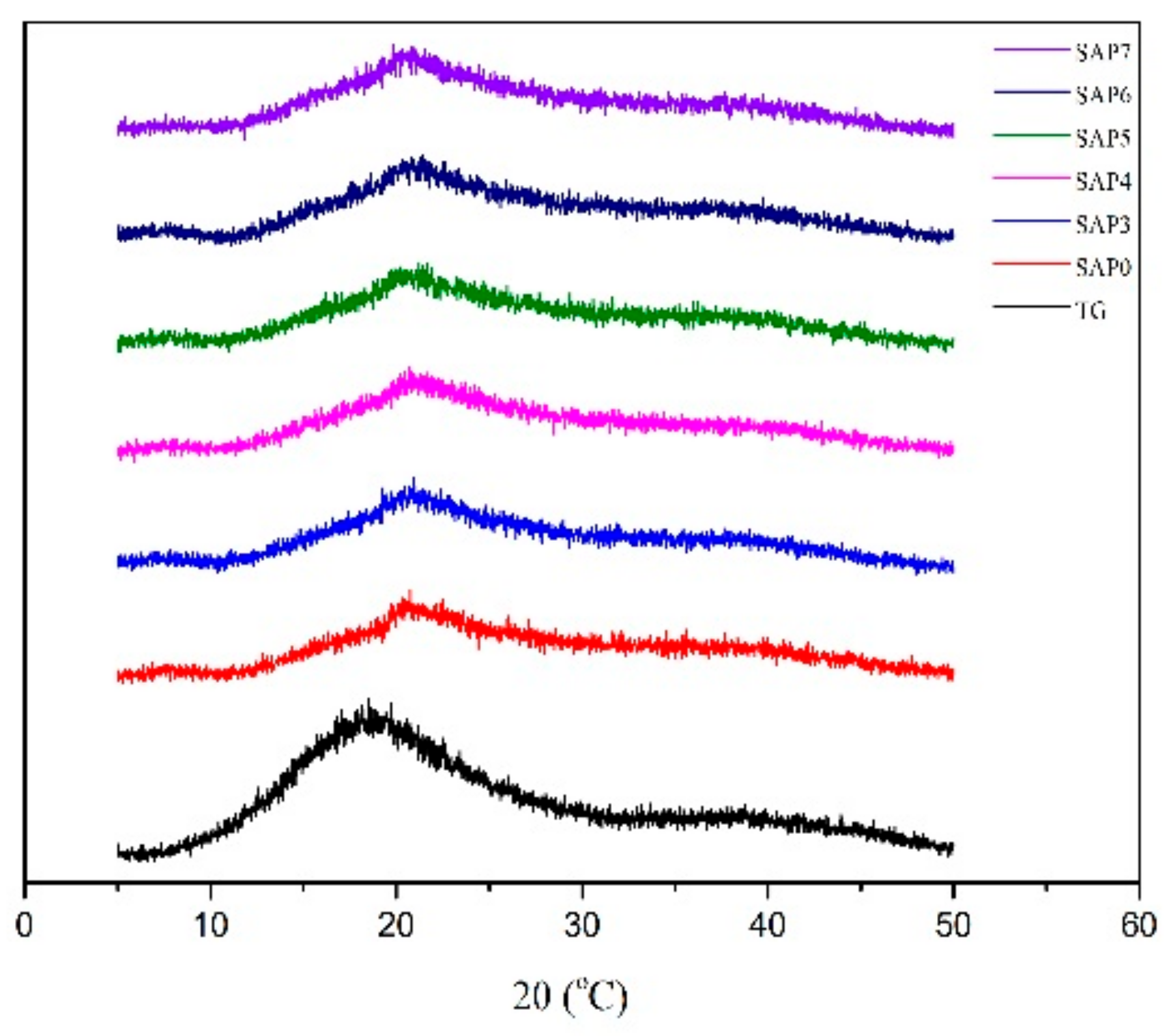
3.4. XRD Analysis
3.5. Morphological Analysis
3.6. Thermal Stability
3.7. Antibacterial Test
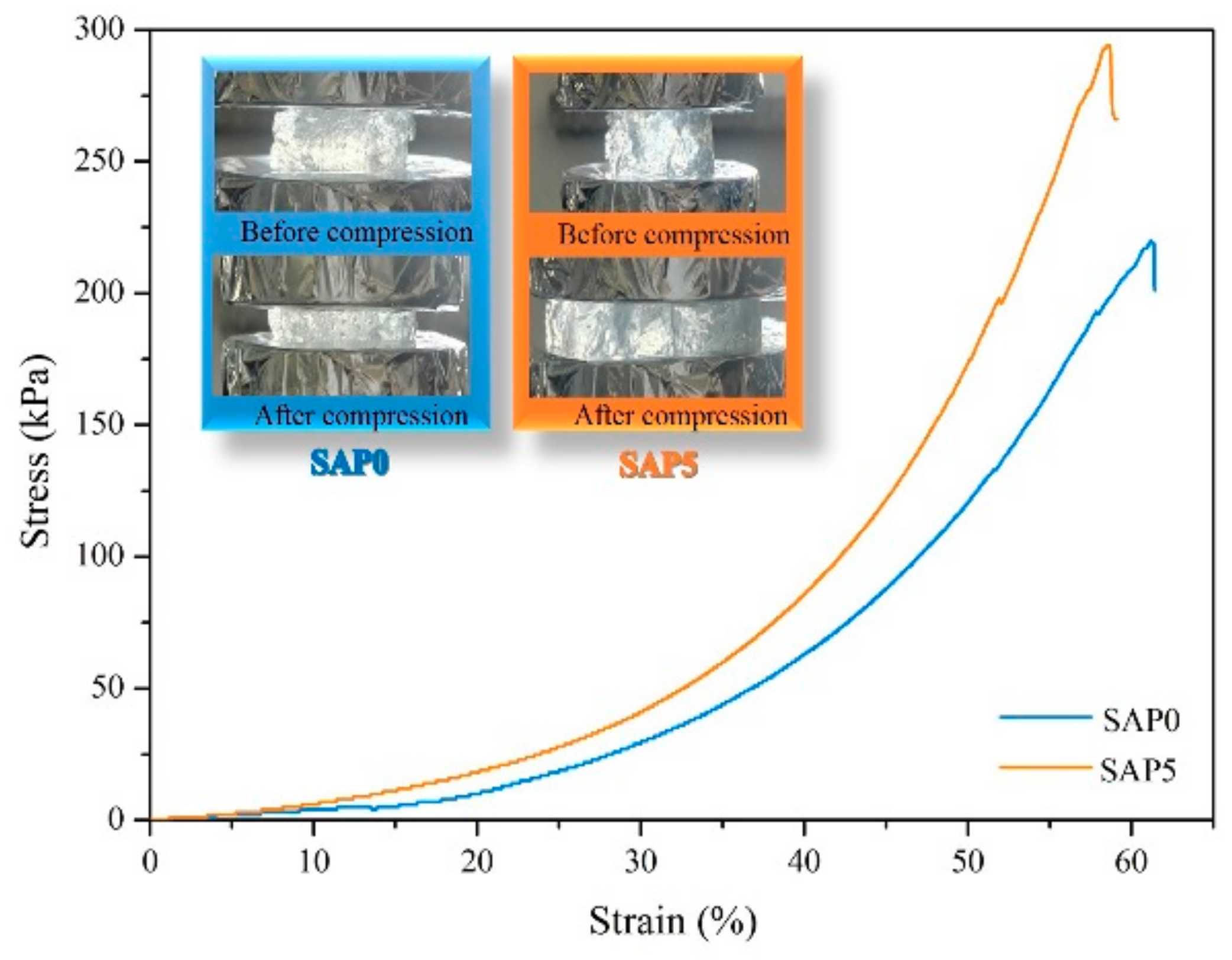
3.8. Mechnical Property
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bao, Y.; Ma, J.; Li, N. Synthesis and swelling behaviors of sodium carboxymethyl cellulose-g-poly(aa-co-am-co-amps)/mmt superabsorbent hydrogel. Carbohydr. Polym. 2011, 84, 76–82. [Google Scholar] [CrossRef]
- Kuang, J.; Yuk, K.Y.; Huh, K.M. Polysaccharide-based superporous hydrogels with fast swelling and superabsorbent properties. Carbohydr Polym 2011, 83, 284–290. [Google Scholar] [CrossRef]
- Zheng, Y.; Hua, S.; Wang, A. Adsorption behavior of Cu2+ from aqueous solutions onto starch-g-poly(acrylic acid)/sodium humate hydrogels. Desalination 2010, 263, 170–175. [Google Scholar] [CrossRef]
- Chang, C.; Duan, B.; Cai, J.; Zhang, L. Superabsorbent hydrogels based on cellulose for smart swelling and controllable delivery. Eur. Polym. J. 2010, 46, 92–100. [Google Scholar] [CrossRef]
- Kosemund, K.; Schlatter, H.; Ochsenhirt, J.L.; Krause, E.L.; Marsman, D.S.; Erasala, G.N. Safety evaluation of superabsorbent baby diapers. Regul. Toxicol. Pharmacol. 2009, 53, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Paulino, A.T.; Belfiore, L.A.; Kubota, L.T.; Muniz, E.C.; Almeida, V.C.; Tambourgi, E.B. Effect of magnetite on the adsorption behavior of Pb(II), Cd(II), and Cu(II) in chitosan-based hydrogels. Desalination 2011, 275, 187–196. [Google Scholar] [CrossRef]
- Puoci, F.; Iemma, F.; Spizzirri, U.G.; Cirillo, G.; Curcio, M.; Picci, N. Polymer in agriculture: A review. Am. J. Agric. Biol. Sci. 2008, 3, 299–314. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Wang, A. Preparation and characterization of a novel ph-sensitive chitosan-g-poly (acrylic acid)/attapulgite/sodium alginate composite hydrogel bead for controlled release of diclofenac sodium. Carbohydr. Polym. 2009, 78, 731–737. [Google Scholar] [CrossRef]
- Masoumi, A.; Ghaemy, M. Removal of metal ions from water using nanohydrogel tragacanth gum-g-polyamidoxime: Isotherm and kinetic study. Carbohydr. Polym. 2014, 108, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.; Kumar, M.; Varshney, L. Radiation synthesis of superabsorbent poly(acrylic acid)–carrageenan hydrogels. Radiat. Phys. Chem. 2004, 69, 481–486. [Google Scholar] [CrossRef]
- Kaith, B.S.; Sharma, R.; Kalia, S. Guar gum based biodegradable, antibacterial and electrically conductive hydrogels. Int. J. Biol. Macromol. 2015, 75, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, H.; Gao, Q.; Liu, X.; Tong, Z. Alginate–calcium carbonate porous microparticle hybrid hydrogels with versatile drug loading capabilities and variable mechanical strengths. Carbohydr. Polym. 2008, 71, 476–480. [Google Scholar] [CrossRef]
- Wang, W.; Wang, A. Preparation, characterization and properties of superabsorbent nanocomposites based on natural guar gum and modified rectorite. Carbohydr. Polym. 2009, 77, 891–897. [Google Scholar] [CrossRef]
- Yadav, M.; Mishra, D.K.; Behari, K. Synthesis of partially hydrolyzed graft copolymer (H-partially carboxymethylated guar gum-g-methacrylic acid): A superabsorbing material. Carbohydr. Polym. 2011, 85, 29–36. [Google Scholar] [CrossRef]
- Hemmati, K.; Masoumi, A.; Ghaemy, M. Tragacanth gum-based nanogel as a superparamagnetic molecularly imprinted polymer for quercetin recognition and controlled release. Carbohydr. Polym. 2016, 136, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Matsuo, K.; Fujioka, R. Novel biodegradable superabsorbent hydrogels derived from cotton cellulose and succinic anhydride: Synthesis and characterization. J. Appl. Polym. Sci. 2006, 99, 3251–3256. [Google Scholar] [CrossRef]
- Berdy, J. Thoughts and facts about antibiotics: Where we are now and where we are heading. J. Antibiot. 2012, 65, 385–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Peng, J.; Li, J.; Wu, J. Radiation crosslinking of CMC-Na at low dose and its application as substitute for hydrogel. Radiat. Phys. Chem. 2005, 72, 635–638. [Google Scholar] [CrossRef]
- Li, H.; Yang, J.; Hu, X.; Liang, J.; Fan, Y.; Zhang, X. Superabsorbent polysaccharide hydrogels based on pullulan derivate as antibacterial release wound dressing. J. Biomed. Mater. Res. Part A 2011, 98, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-F.; Huang, Y.-C. Swelling and antibacterial properties for the superabsorbent hydrogels containing silver nanoparticles. J. Appl. Polym. Sci. 2007, 106, 1992–1999. [Google Scholar] [CrossRef]
- Ferfera-Harrar, H.; Aiouaz, N.; Dairi, N.; Hadj-Hamou, A.S. Preparation of chitosan-g-poly(acrylamide)/montmorillonite superabsorbent polymer composites: Studies on swelling, thermal, and antibacterial properties. J. Appl. Polym. Sci. 2014, 131, 39747. [Google Scholar] [CrossRef]
- Sharma, R.; Kalia, S.; Kaith, B.S.; Kumar, A.; Thakur, P.; Pathania, D.; Srivastava, M.K. Ggum-poly(itaconic acid) based superabsorbents via two-step free-radical aqueous polymerization for environmental and antibacterial applications. J. Polym. Environ. 2016, 25, 176–191. [Google Scholar] [CrossRef]
- Murali Mohan, Y.; Lee, K.; Premkumar, T.; Geckeler, K.E. Hydrogel networks as nanoreactors: A novel approach to silver nanoparticles for antibacterial applications. Polymer 2007, 48, 158–164. [Google Scholar] [CrossRef]
- Yadollahi, M.; Gholamali, I.; Namazi, H.; Aghazadeh, M. Synthesis and characterization of antibacterial carboxymethylcellulose/CuO bio-nanocomposite hydrogels. Int. J. Biol. Macromol. 2015, 73, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Lakes, A.L.; Peyyala, R.; Ebersole, J.L.; Puleo, D.A.; Hilt, J.Z.; Dziubla, T.D. Synthesis and characterization of an antibacterial hydrogel containing covalently bound vancomycin. Biomacromolecules 2014, 15, 3009–3018. [Google Scholar] [CrossRef] [PubMed]
- Pakzad, Y.; Ganji, F. Thermosensitive hydrogel for periodontal application: In vitro drug release, antibacterial activity and toxicity evaluation. J. Biomater. Appl. 2016, 30, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Shimojo, A.A.M.; Santana, M.H.A. Rheological properties of citrus pectin dispersions and its blends with polyquaternium-7 and colloidal particles. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Leng, M.T.; Hu, S.D.; Lu, A.J.; Cai, M.T.; Luo, X.L. The anti-bacterial poly(caprolactone)-poly(quaternary ammonium salt) as drug delivery carriers. Appl. Microbiol. Biotechnol. 2016, 100, 3049–3059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Liu, H.L.; Chen, X.; Zhan, X.L.; Chen, F.Q. Preparation, surface properties, and antibacterial activity of a poly(dimethyl siloxane) network containing a quaternary ammonium salt side chain. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Li, J.; Zhou, M.-L.; Lin, J.-Y.; Ye, W.-Y.; Xu, Y.-Q.; Shen, J.-N.; Gao, C.-J.; Bruggen, B.V.D. Mono-valent cation selective membranes for electrodialysis by introducing polyquaternium-7 in a commercial cation exchange membrane. J. Membr. Sci. 2015, 486, 89–96. [Google Scholar] [CrossRef]
- Abd Alla, S.G.; Sen, M.; El-Naggar, A.W. Swelling and mechanical properties of superabsorbent hydrogels based on tara gum/acrylic acid synthesized by gamma radiation. Carbohydr. Polym. 2012, 89, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Bedia, J.; Belver, C.; Ponce, S.; Rodriguez, J.; Rodriguez, J.J. Adsorption of antipyrine by activated carbons from fecl3-activation of tara gum. Chem. Eng. J. 2018, 333, 58–65. [Google Scholar] [CrossRef]
- Wang, W.B.; Kang, Y.R.; Wang, A.Q. Synthesis, characterization and swelling properties of guar gum-g-poly(sodium acrylate-co-styrene)/muscovite superabsorbent composites. Sci. Technol. Adv. Mater. 2010, 11. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Su, M.X.; Zhang, J.C.; Jin, F.Q.; Zha, C.H.; Li, M.; Hao, X.M. Biodiesel production from rubber seed oil using poly (sodium acrylate) supporting naoh as a water-resistant catalyst. Bioresour. Technol. 2011, 102, 2665–2671. [Google Scholar] [CrossRef] [PubMed]
- Yuen, S.-N.; Choi, S.-M.; Phillips, D.L.; Ma, C.-Y. Raman and ftir spectroscopic study of carboxymethylated non-starch polysaccharides. Food Chem. 2009, 114, 1091–1098. [Google Scholar] [CrossRef]
- Dabrzalska, M.; Benseny-Cases, N.; Barnadas-Rodriguez, R.; Mignani, S.; Zablocka, M.; Majoral, J.P.; Bryszewska, M.; Klajnert-Maculewicz, B.; Cladera, J. Fourier transform infrared spectroscopy (FTIR) characterization of the interaction of anti-cancer photosensitizers with dendrimers. Anal. Bioanal. Chem. 2016, 408, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Hebeish, A.; Hashem, M.; El-Hady, M.M.; Sharaf, S. Development of cmc hydrogels loaded with silver nano-particles for medical applications. Carbohydr. Polym. 2013, 92, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Li, J.; Liu, S.; Chen, Q.-y. Preparation of copper nanoparticles in a water/oleic acid mixed solvent via two-step reduction method. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 373, 29–35. [Google Scholar] [CrossRef]
- Huang, J.S.; Meng, F.; Fu, J.G.; Xu, X.Y.; Wu, E.W. Preparation and proprety of dimethyldiallyammonium chloride and acrylamide superabsorbent copolymer. New Chem. Mater. 2015, 43, 77–79. [Google Scholar]
- Qiao, J.L.; Fu, J.; Lsiu, L.L.; Liu, Y.Y.; Sheng, J.W. Highly stable hydroxyl anion conducting membranes poly(vinyl alcohol)/poly(acrylamide-co-diallyldimethylammonium chloride) (PVA/PAADDA) for alkaline fuel cells: Effect of cross-linking. Int. J. Hydrogen Energy 2012, 37, 4580–4589. [Google Scholar] [CrossRef]
- Codling, C.E.; Maillard, J.Y.; Russell, A.D. Aspects of the antimicrobial mechanisms of action of a polyquaternium and an amidoamine. J. Antimicrob. Chemother. 2003, 51, 1153–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Cui, R.Q.; Peng, L.C.; Cai, S.B.; Li, P.; Lan, T.Q. Preparation of antibacterial cellulose paper using layer-by-layer assembly for cooked beef preservation at ambient temperature. Polymers 2018, 10, 15. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Li, B.; Zhan, X.; Wang, L. A One Pot Method for Preparing an Antibacterial Superabsorbent Hydrogel with a Semi-IPN Structure Based on Tara Gum and Polyquaternium-7. Polymers 2018, 10, 696. https://doi.org/10.3390/polym10070696
Shen J, Li B, Zhan X, Wang L. A One Pot Method for Preparing an Antibacterial Superabsorbent Hydrogel with a Semi-IPN Structure Based on Tara Gum and Polyquaternium-7. Polymers. 2018; 10(7):696. https://doi.org/10.3390/polym10070696
Chicago/Turabian StyleShen, Jie, Bingjie Li, Xianxu Zhan, and Lijuan Wang. 2018. "A One Pot Method for Preparing an Antibacterial Superabsorbent Hydrogel with a Semi-IPN Structure Based on Tara Gum and Polyquaternium-7" Polymers 10, no. 7: 696. https://doi.org/10.3390/polym10070696