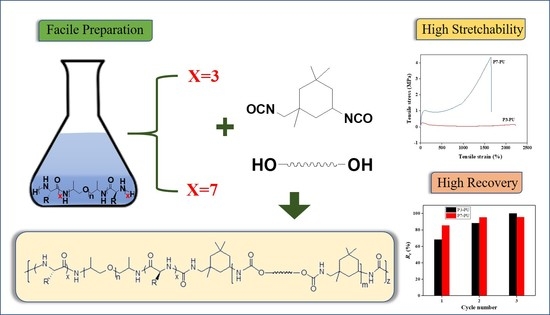
Facile Preparation of Highly Stretchable and Recovery Peptide-Polyurethane/Ureas
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
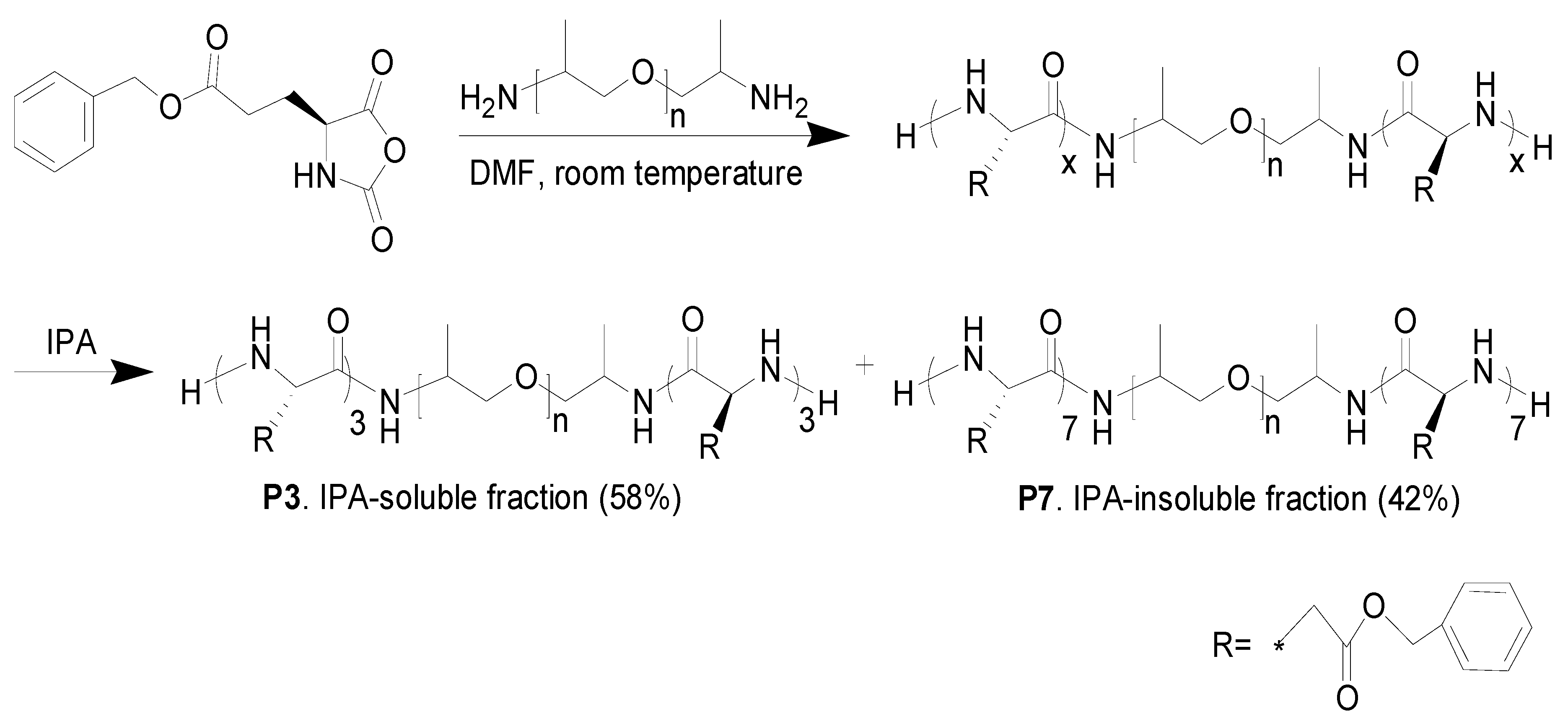
2.2. Synthesis of Poly(γ-benzyl-l-glutamate)-b-poly(propylene glycol)-b-poly(γ-benzyl-l-glutamate) (PBLG-b-PPG-b-PBLG) Triblock
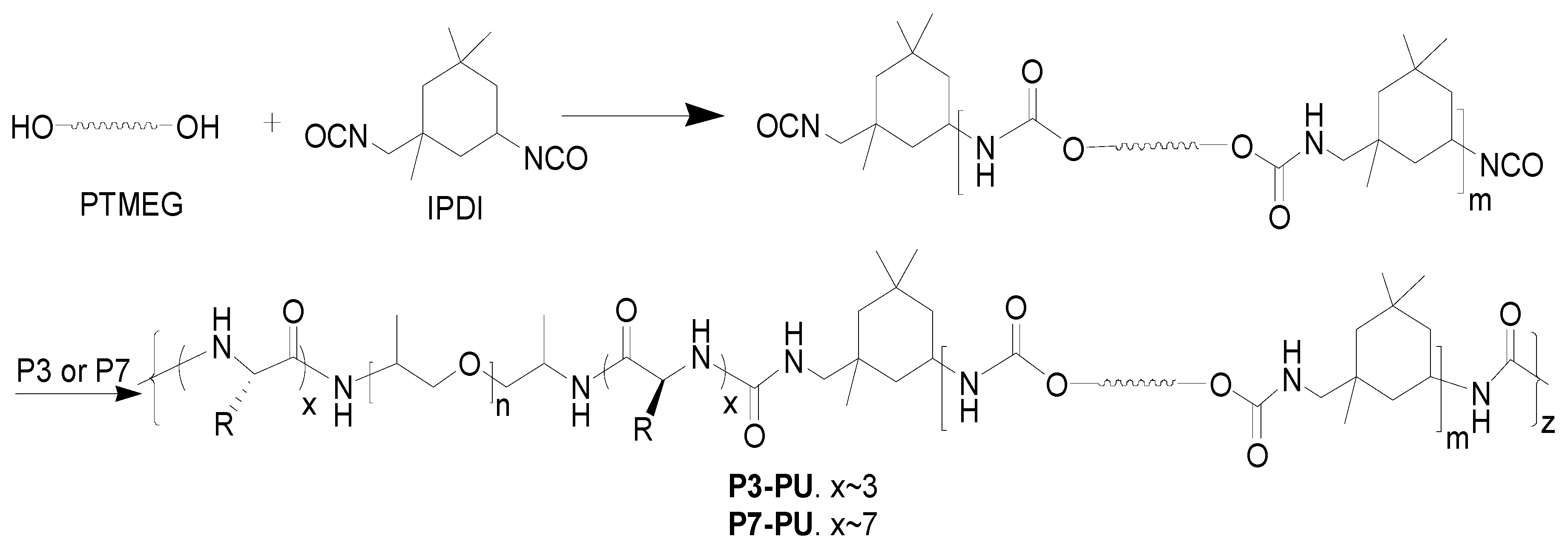
2.3. Preparation of Peptide-Polyurethane/Ureas and Films
2.4. Characterization
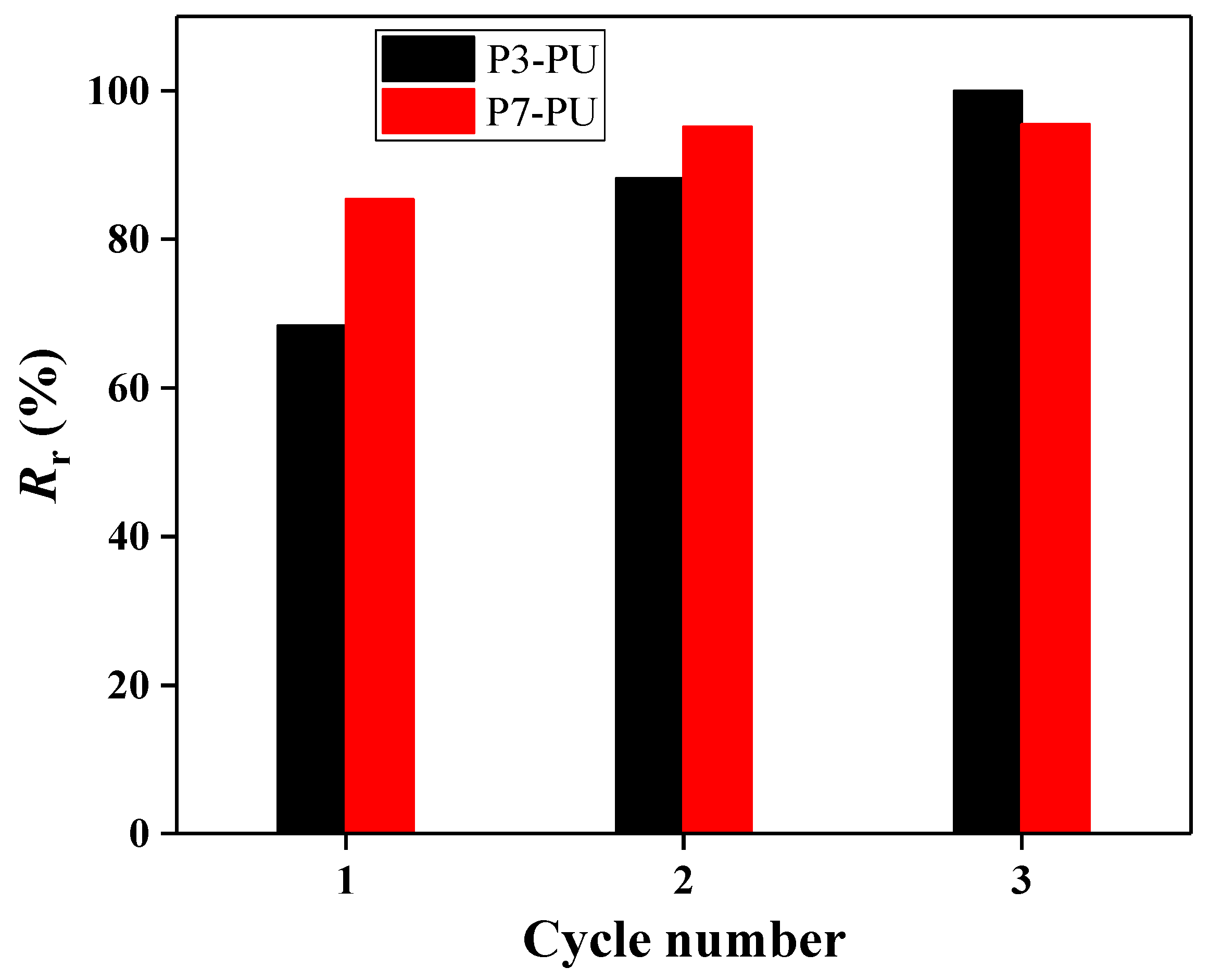
3. Results and Discussion
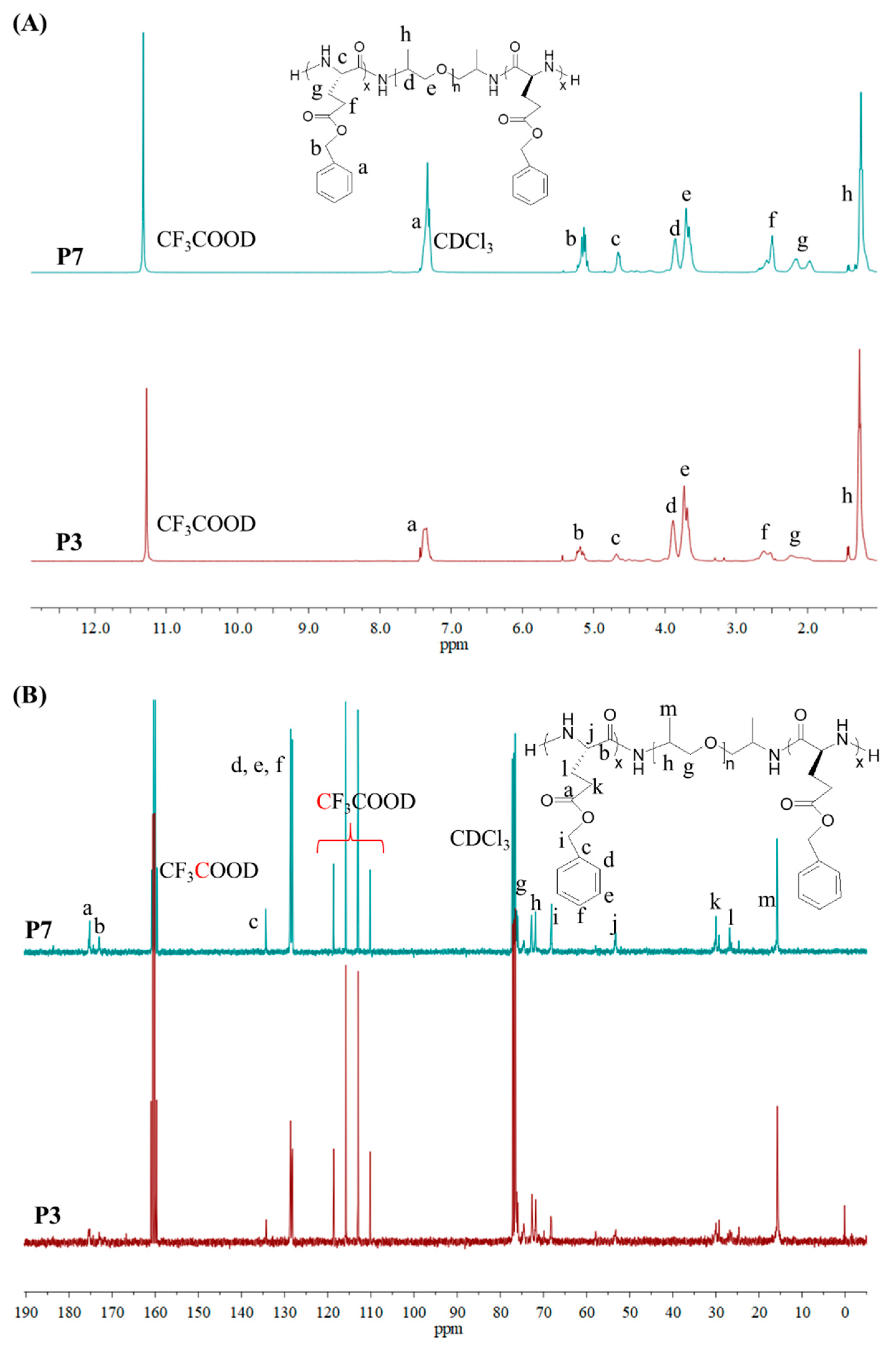
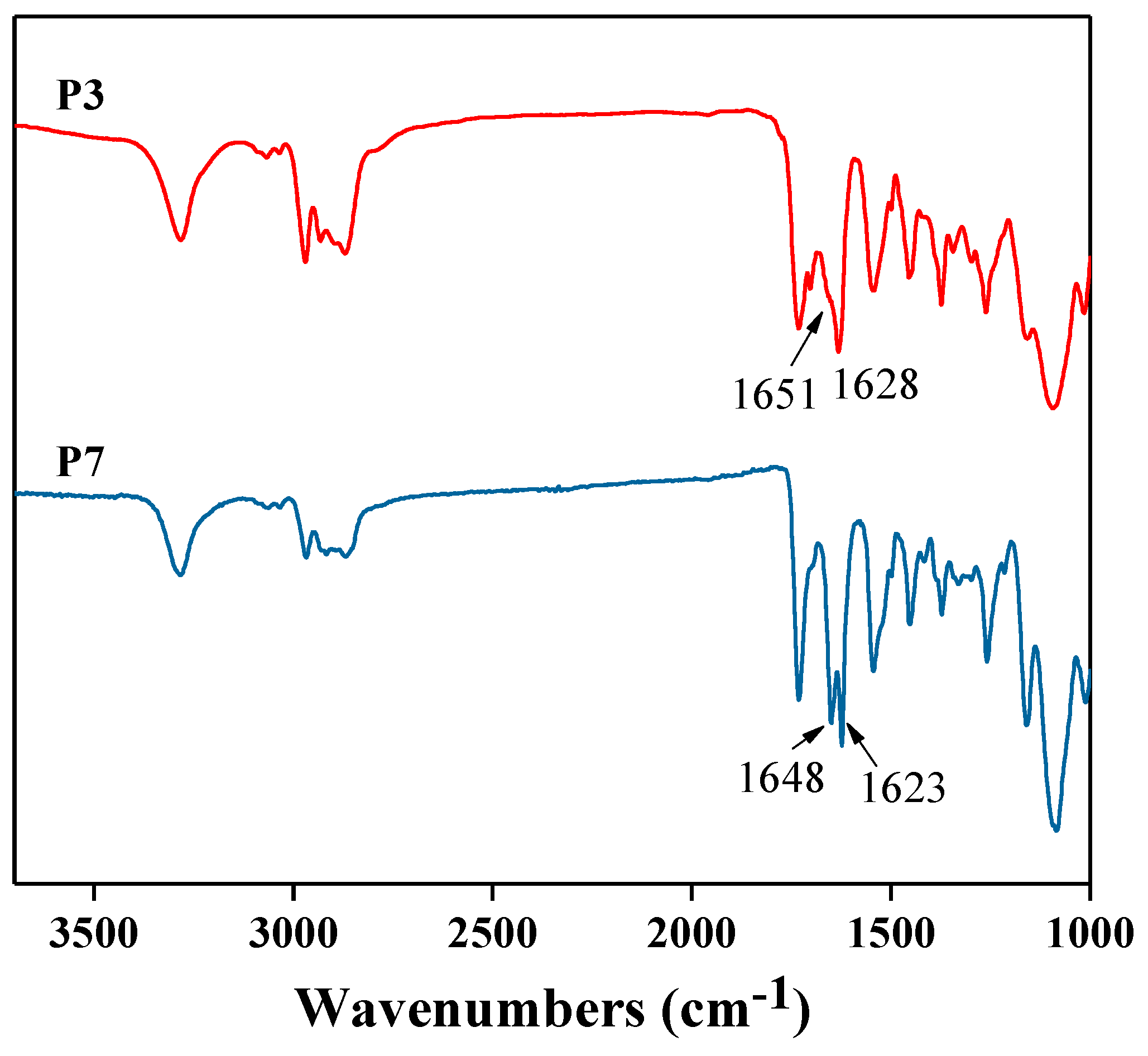
3.1. Synthesis and Characterization of Peptidic Triblocks
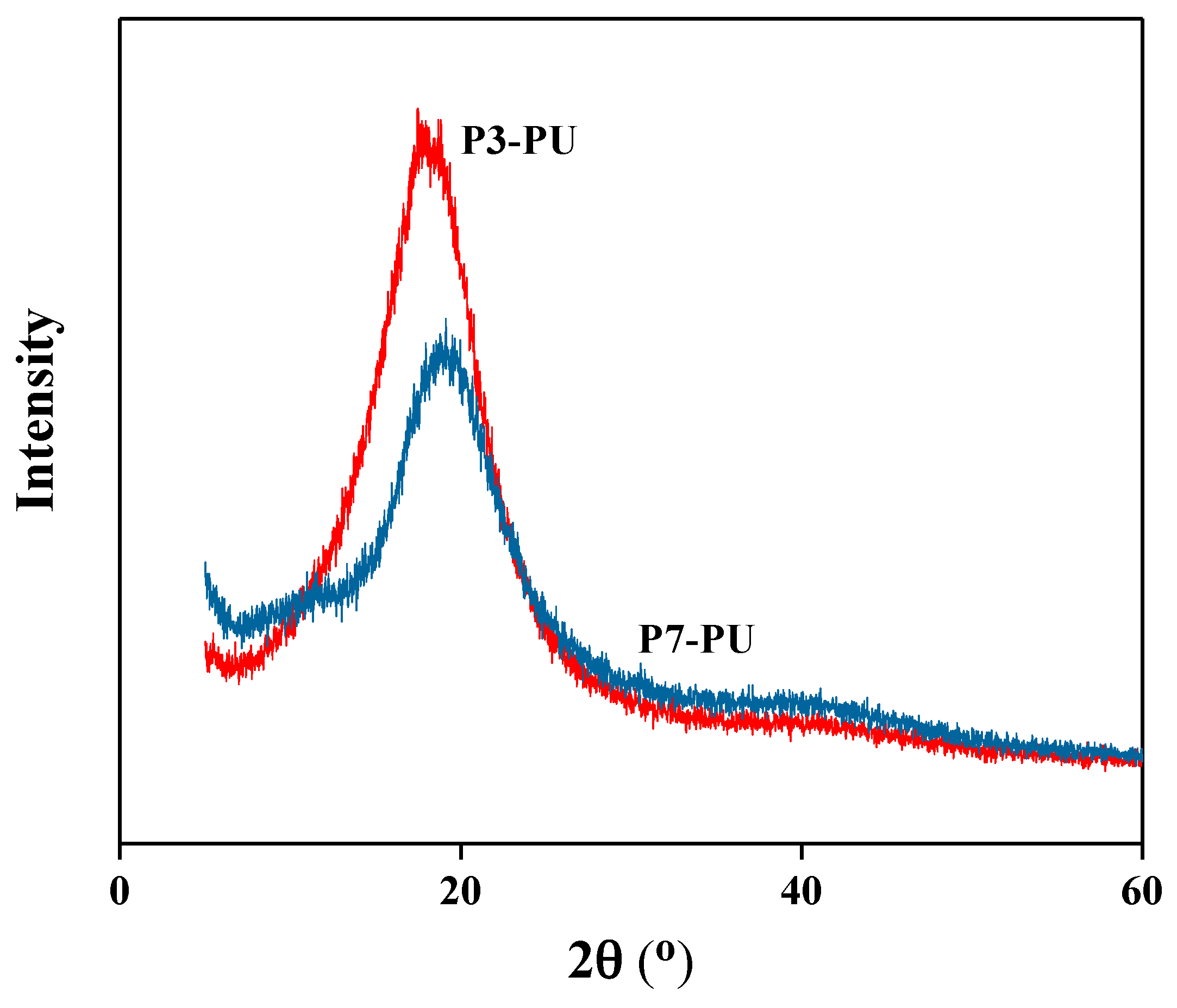
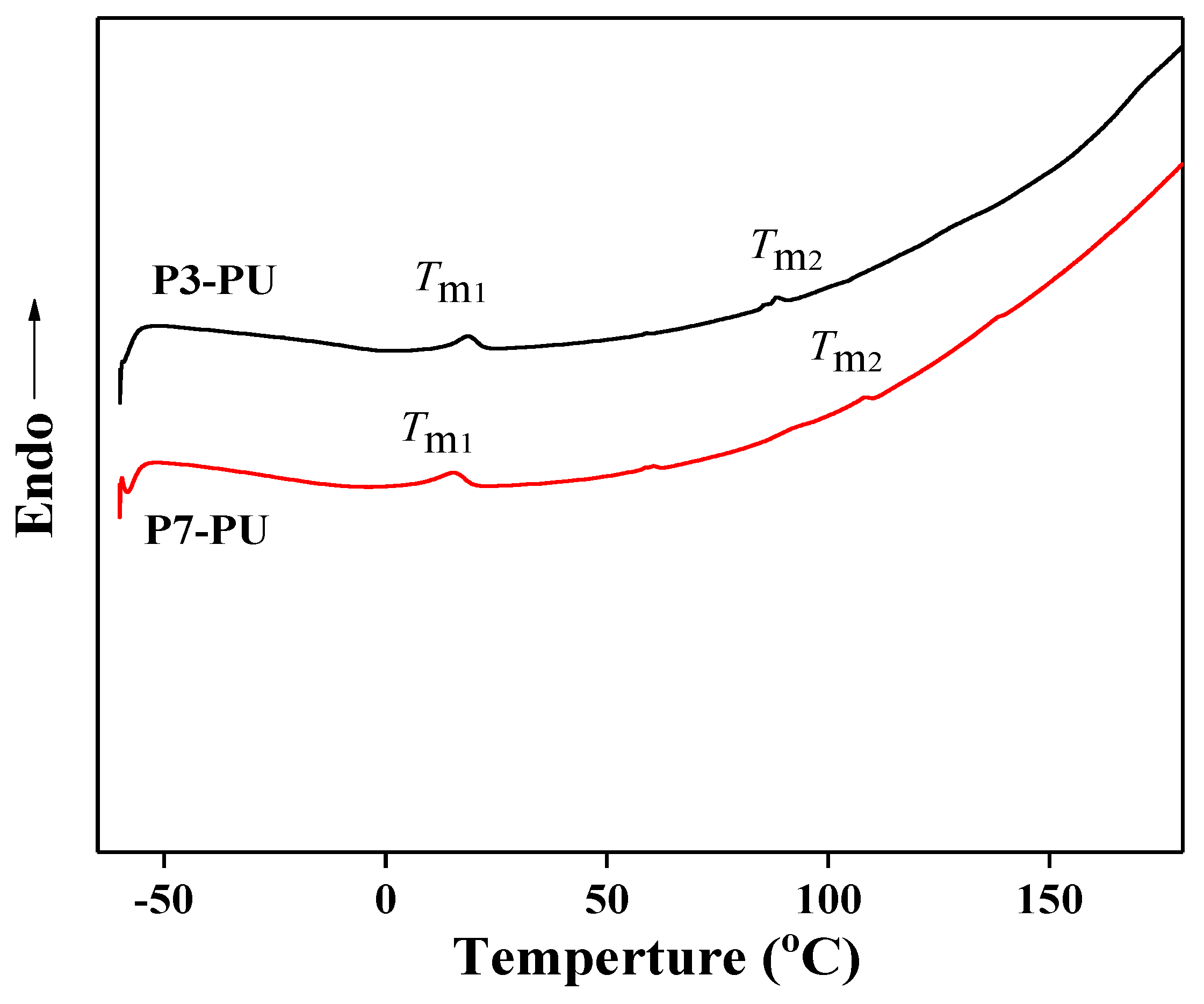
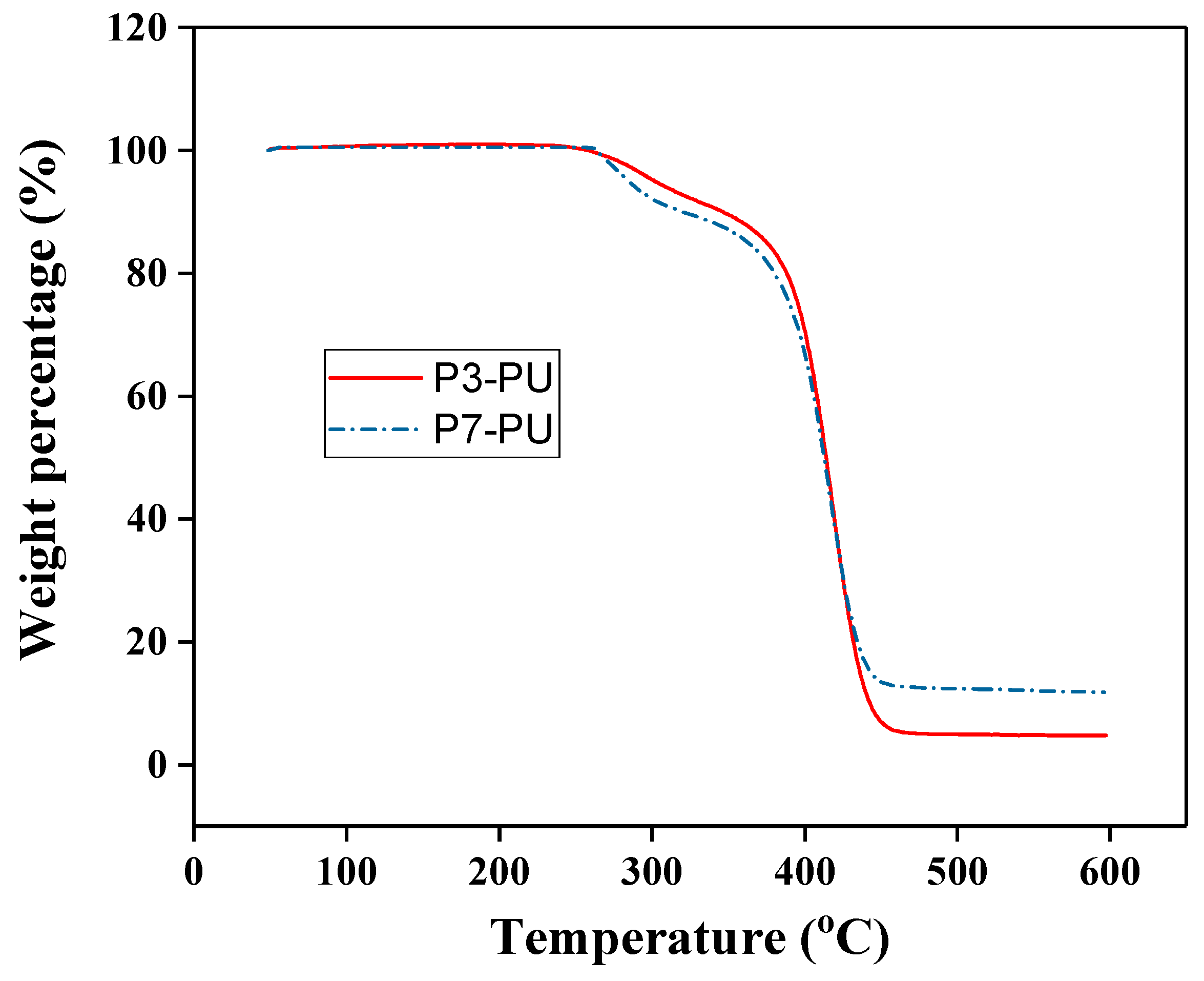
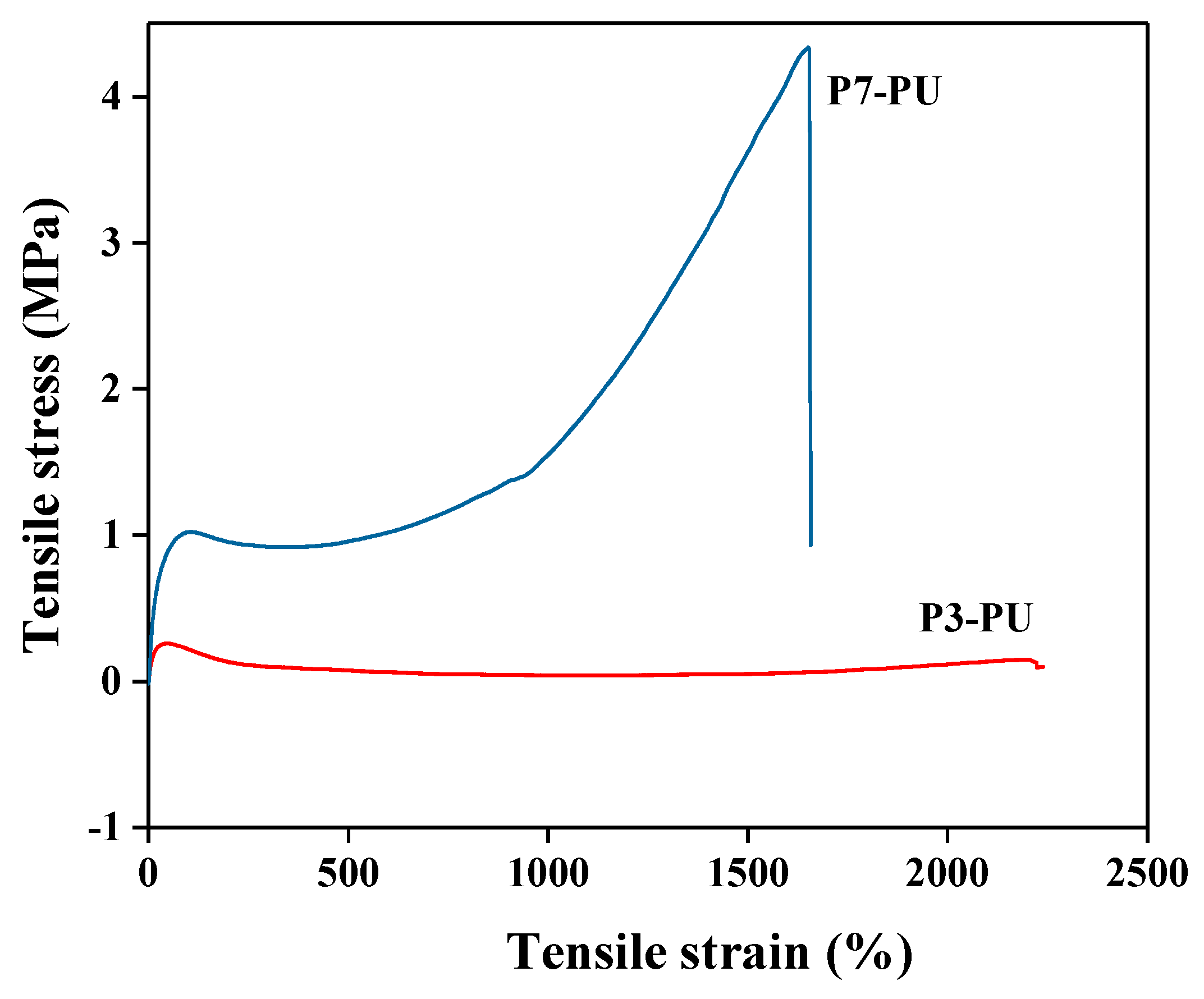
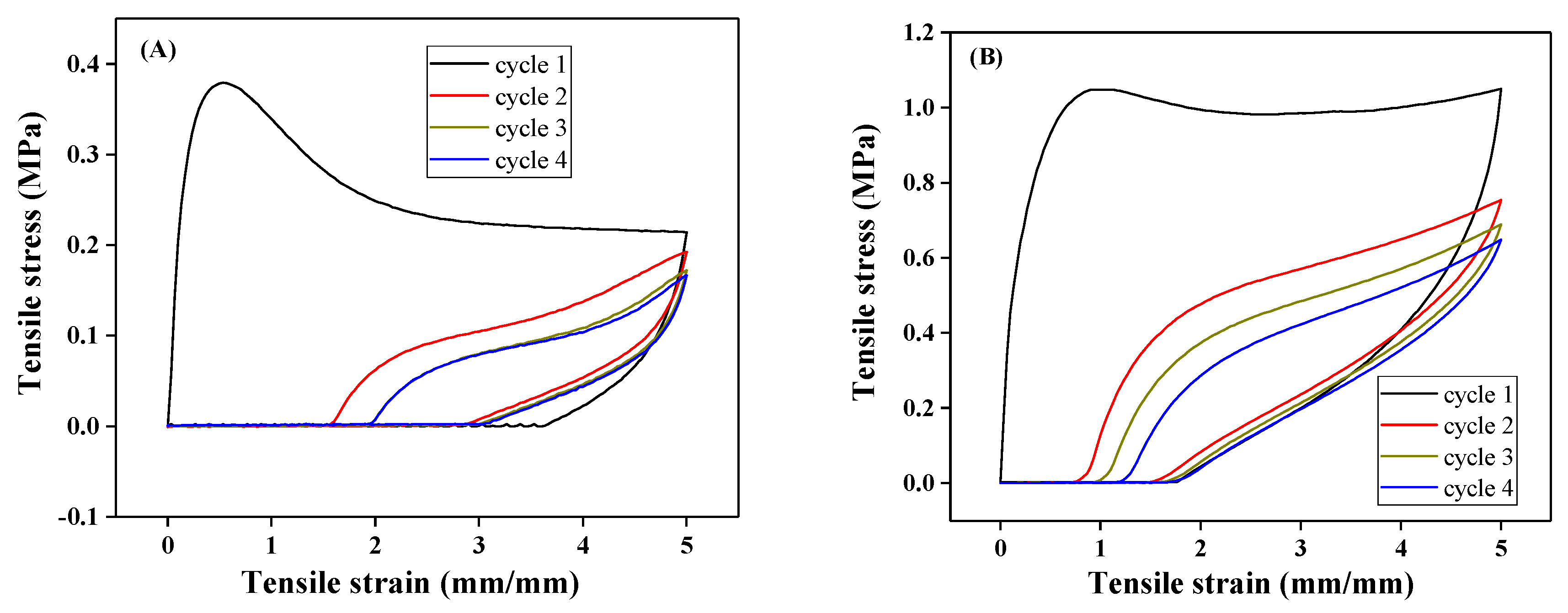
3.2. Preparation and Properties of Peptide-Polyurethane/Ureas
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ritchie, R.O. The conflicts between strength and toughness. Nat. Mater. 2011, 10, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Meyers, M.A.; McKittrick, J.; Chen, P.-Y. Structural Biological Materials: Critical Mechanics-Materials Connections. Science 2013, 339, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.C.; Korley, L.T.J. Enhanced mechanical pathways through nature’s building blocks: Amino acids. Soft Matter 2012, 8, 11431–11442. [Google Scholar] [CrossRef]
- Olsen, B.D. Engineering materials from proteins. AIChE J. 2013, 59, 3558–3568. [Google Scholar] [CrossRef]
- Anton, A.M.; Heidebrecht, A.; Mahmood, N.; Beiner, M.; Scheibel, T.; Kremer, F. Foundation of the Outstanding Toughness in Biomimetic and Natural Spider Silk. Biomacromolecules 2017, 18, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.A.; Vierra, C.A.; Moore, A.M.F. Molecular and Mechanical Properties of Major Ampullate Silk of the Black Widow Spider, Latrodectus hesperus. Biomacromolecules 2004, 5, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Scavuzzo, J.J.; Yan, X.; Zhao, Y.; Scherger, J.D.; Chen, J.; Zhang, S.; Liu, H.; Gao, M.; Li, T.; Zhao, X.; et al. Supramolecular Elastomers. Particulate β-Sheet Nanocrystal-Reinforced Synthetic Elastic Networks. Macromolecules 2016, 49, 2688–2697. [Google Scholar] [CrossRef]
- Mithieux, S.M.; Rasko, J.E.J.; Weiss, A.S. Synthetic elastin hydrogels derived from massive elastic assemblies of self-organized human protein monomers. Biomaterials 2004, 25, 4921–4927. [Google Scholar] [CrossRef] [PubMed]
- Bonduelle, C. Secondary structures of synthetic polypeptide polymers. Polym. Chem. 2018, 9, 1517–1529. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Numata, K. Chemical Synthesis of Multiblock Copolypeptides Inspired by Spider Dragline Silk Proteins. ACS Macro Lett. 2017, 6, 103–106. [Google Scholar] [CrossRef]
- Winningham, M.J.; Sogah, D.Y. A Modular Approach to Polymer Architecture Control via Catenation of Prefabricated Biomolecular Segments: Polymers Containing Parallel β-Sheets Templated by a Phenoxathiin-Based Reverse Turn Mimic. Macromolecules 1997, 30, 862–876. [Google Scholar] [CrossRef]
- Rathore, O.; Sogah, D.Y. Self-Assembly of β-Sheets into Nanostructures by Poly(alanine) Segments Incorporated in Multiblock Copolymers Inspired by Spider Silk. J. Am. Chem. Soc. 2001, 123, 5231–5239. [Google Scholar] [CrossRef] [PubMed]
- Rathore, O.; Sogah, D.Y. Nanostructure Formation through β-Sheet Self-Assembly in Silk-Based Materials. Macromolecules 2001, 34, 1477–1486. [Google Scholar] [CrossRef]
- Martino, M.; Perri, T.; Tamburro, A.M. Elastin-Based Biopolymers: Chemical Synthesis and Structural Characterization of Linear and Cross-Linked Poly(OrnGlyGlyOrnGly). Biomacromolecules 2002, 3, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.C.; Wanasekara, N.D.; Korley, L.T.J. Utilizing Peptidic Ordering in the Design of Hierarchical Polyurethane/Ureas. Biomacromolecules 2012, 13, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.E.; Pashuck, E.T.; Bertazzo, S.; Weaver, J.V.M.; Stevens, M.M. Self-Healing, Self-Assembled β-Sheet Peptide–Poly(γ-glutamic acid) Hybrid Hydrogels. J. Am. Chem. Soc. 2017, 139, 7250–7255. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Hu, J.; Zhu, Y. Shape-memory biopolymers based on beta-sheet structures of polyalanine segments inspired by spider silks. Macromol. Biosci. 2013, 13, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Ogura, A.; Kaneko, T.; Murata, Y.; Akashi, M. Precise Synthesis of ABA Triblock Copolymers Comprised of Poly(ethylene oxide) and Poly(β-benzyl-l-aspartate): A Hierarchical Structure Inducing Excellent Elasticity. Macromolecules 2004, 37, 1370–1377. [Google Scholar] [CrossRef]
- Johnson, J.C.; Wanasekara, N.D.; Korley, L.T.J. Influence of secondary structure and hydrogen-bonding arrangement on the mechanical properties of peptidic-polyurea hybrids. J. Mater. Chem. B 2014, 2, 2554–2561. [Google Scholar] [CrossRef]
- Matolyak, L.; Keum, J.; Korley, L.T.J. Molecular Design: Network Architecture and Its Impact on the Organization and Mechanics of Peptide-Polyurea Hybrids. Biomacromolecules 2016, 17, 3931–3939. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Jiang, Y.; Hu, J. Synthesis and Properties of Shape Memory Poly(γ-Benzyl-l-Glutamate)-b-Poly(Propylene Glycol)-b-Poly(γ-Benzyl-l-Glutamate). Appl. Sci. 2017, 7, 1258. [Google Scholar] [CrossRef]
- Guo, A.-R.; Yang, W.-X.; Yang, F.; Yu, R.; Wu, Y.-X. Well-Defined Poly(γ-benzyl-l-glutamate)-g-Polytetrahydrofuran: Synthesis, Characterization, and Properties. Macromolecules 2014, 47, 5450–5461. [Google Scholar] [CrossRef]
- Gu, L.; Wu, Q.-Y. Recyclable bio-based crosslinked polyurethanes with self-healing ability. J. Appl. Polym. Sci. 2018, 135, 46272. [Google Scholar] [CrossRef]
- Gu, L.; Cui, B.; Wu, Q.-Y.; Yu, H. Bio-based polyurethanes with shape memory behavior at body temperature: Effect of different chain extenders. RSC Adv. 2016, 6, 17888–17895. [Google Scholar] [CrossRef]
- Cui, B.; Wu, Q.-Y.; Gu, L.; Shen, L.; Yu, H.-B. High performance bio-based polyurethane elastomers: Effect of different soft and hard segments. Chin. J. Polym. Sci. 2016, 34, 901–909. [Google Scholar] [CrossRef]
- Lee, N.H.; Frank, C.W. Surface-Initiated Vapor Polymerization of Various α-Amino Acids. Langmuir 2003, 19, 1295–1303. [Google Scholar] [CrossRef]
- Kotharangannagari, V.K.; Sánchez-Ferrer, A.; Ruokolainen, J.; Mezzenga, R. Thermoreversible Gel–Sol Behavior of Rod–Coil–Rod Peptide-Based Triblock Copolymers. Macromolecules 2012, 45, 1982–1990. [Google Scholar] [CrossRef]
- Sánchez-Ferrer, A.; Mezzenga, R. Secondary Structure-Induced Micro- and Macrophase Separation in Rod-Coil Polypeptide Diblock, Triblock, and Star-Block Copolymers. Macromolecules 2010, 43, 1093–1100. [Google Scholar] [CrossRef] [Green Version]
- Wei, M.-J.; Guo, A.-R.; Wu, Y.-X. Microstructure and Micromorphology of Poly (γ-benzyl-l-glutamate)-g-(Polytetrahydrofuran-b-Polyisobutylene) Copolymer. Acta Polym. Sin. 2017, 3, 506–515. [Google Scholar]
- Wang, Q.; Yanzhang, R.; Wu, Y.; Zhu, H.; Zhang, J.; Du, M.; Zhang, M.; Wang, L.; Zhang, X.; Liang, X. Silk-derived graphene-like carbon with high electrocatalytic activity for oxygen reduction reaction. RSC Adv. 2016, 6, 34219–34224. [Google Scholar] [CrossRef]
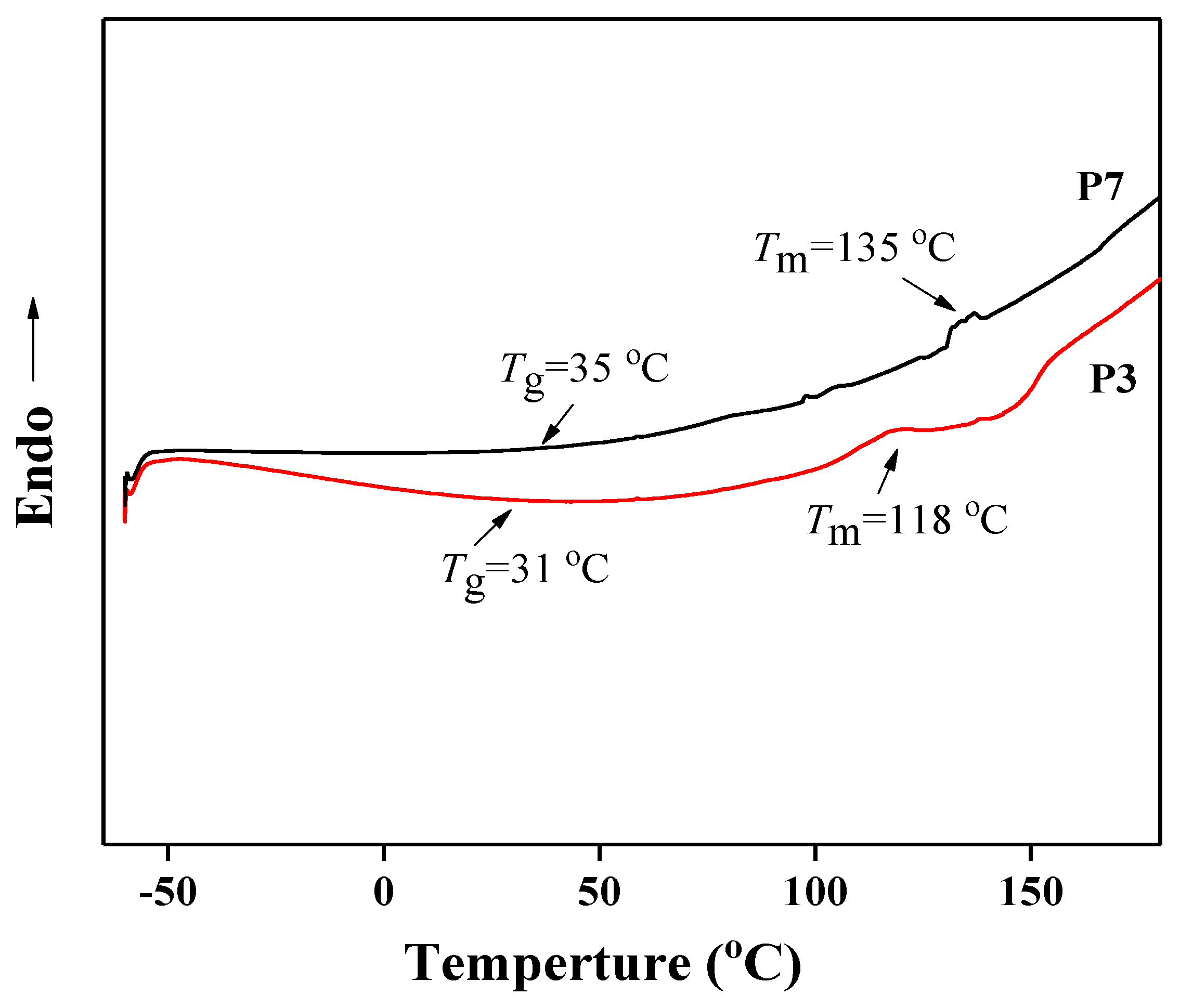

| Samples | Peptide Content (%) | β-Sheet Content (%) | Tg (°C) | Tm (°C) |
|---|---|---|---|---|
| P3 | 39.6 | 83.0 | 31 | 118 |
| P7 | 60.5 | 48.4 | 35 | 135 |
| Samples | Tm1 (°C) | Tm2 (°C) | Tensile Strength (MPa) | Elongation (%) | Young’s Modulus (KPa) |
|---|---|---|---|---|---|
| P3-PU | 18.6 | 88.3 | 0.25 | 2210 | 226 |
| P7-PU | 15.5 | 108.6 | 4.69 | 1650 | 513 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, L.; Jiang, Y.; Hu, J. Facile Preparation of Highly Stretchable and Recovery Peptide-Polyurethane/Ureas. Polymers 2018, 10, 637. https://doi.org/10.3390/polym10060637
Gu L, Jiang Y, Hu J. Facile Preparation of Highly Stretchable and Recovery Peptide-Polyurethane/Ureas. Polymers. 2018; 10(6):637. https://doi.org/10.3390/polym10060637
Chicago/Turabian StyleGu, Lin, Yuanzhang Jiang, and Jinlian Hu. 2018. "Facile Preparation of Highly Stretchable and Recovery Peptide-Polyurethane/Ureas" Polymers 10, no. 6: 637. https://doi.org/10.3390/polym10060637