Lateral Order and Self-Organized Morphology of Diblock Copolymer Micellar Films
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization
2.3. Modeling of GISAXS Data
3. Results and Discussion
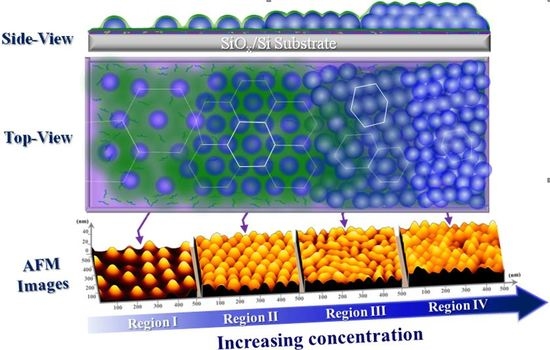
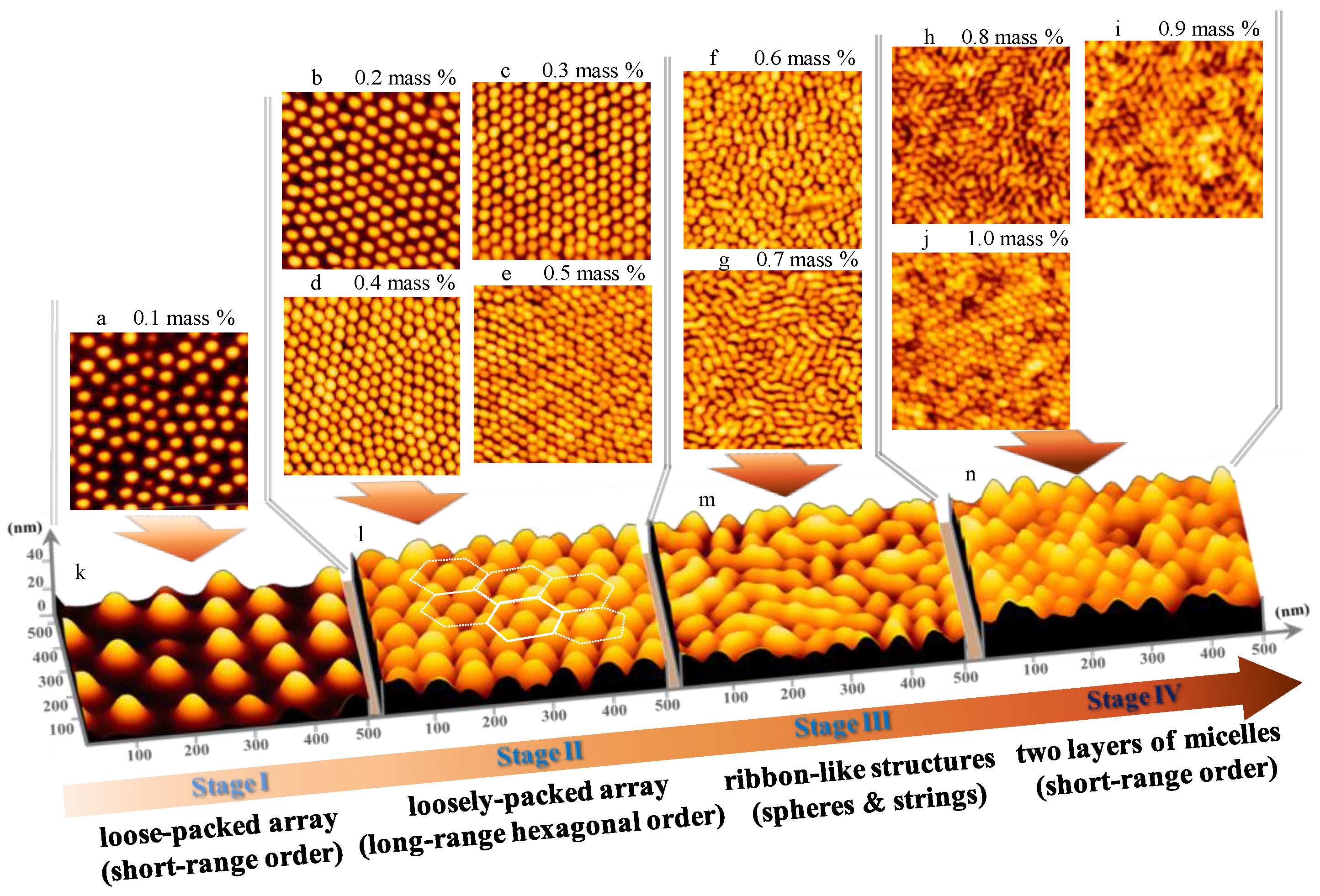
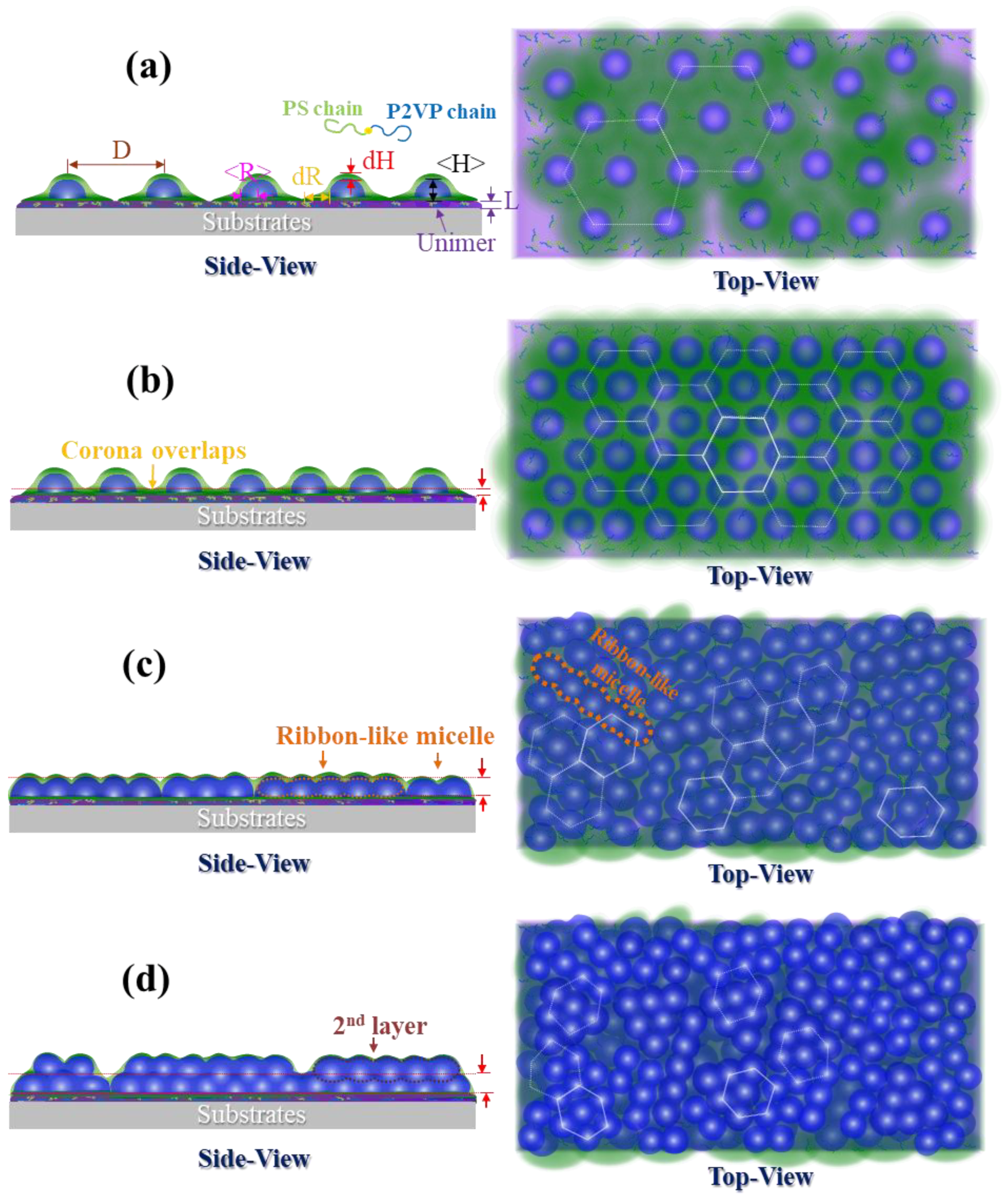
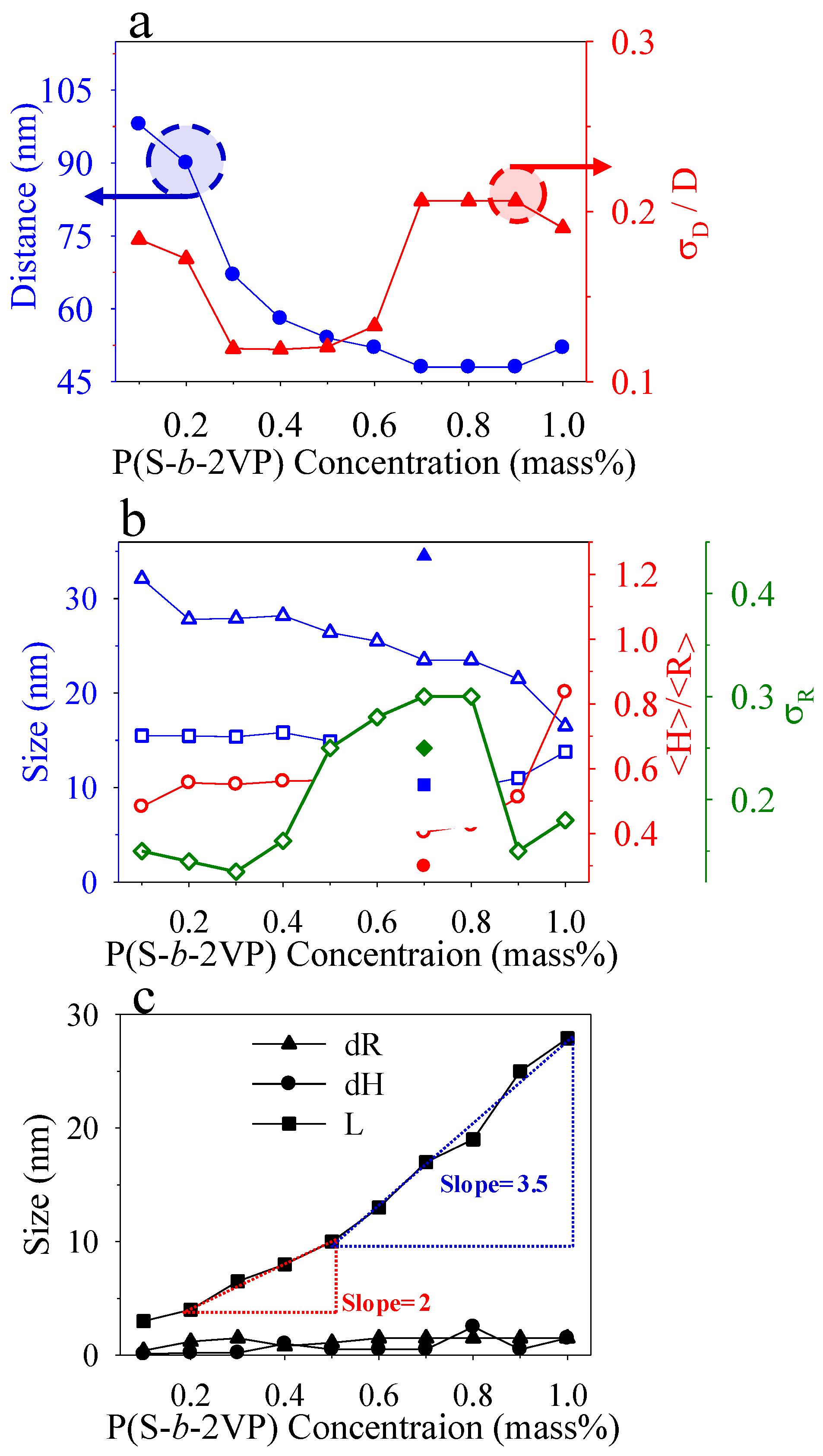
3.1. Surface Morphology of P(S-b-2VP) Micelles as Spun from Toluene onto SiOx/Si
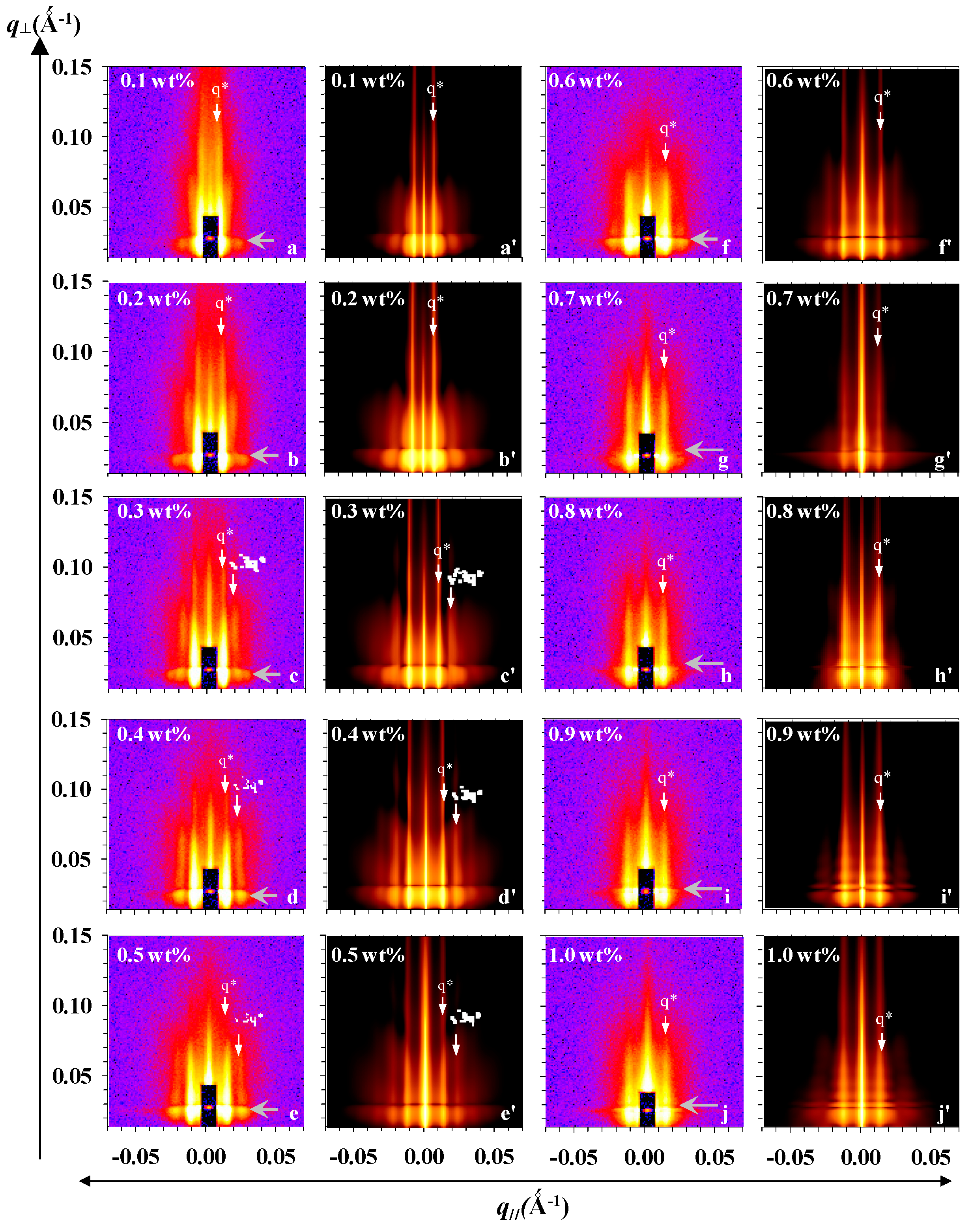
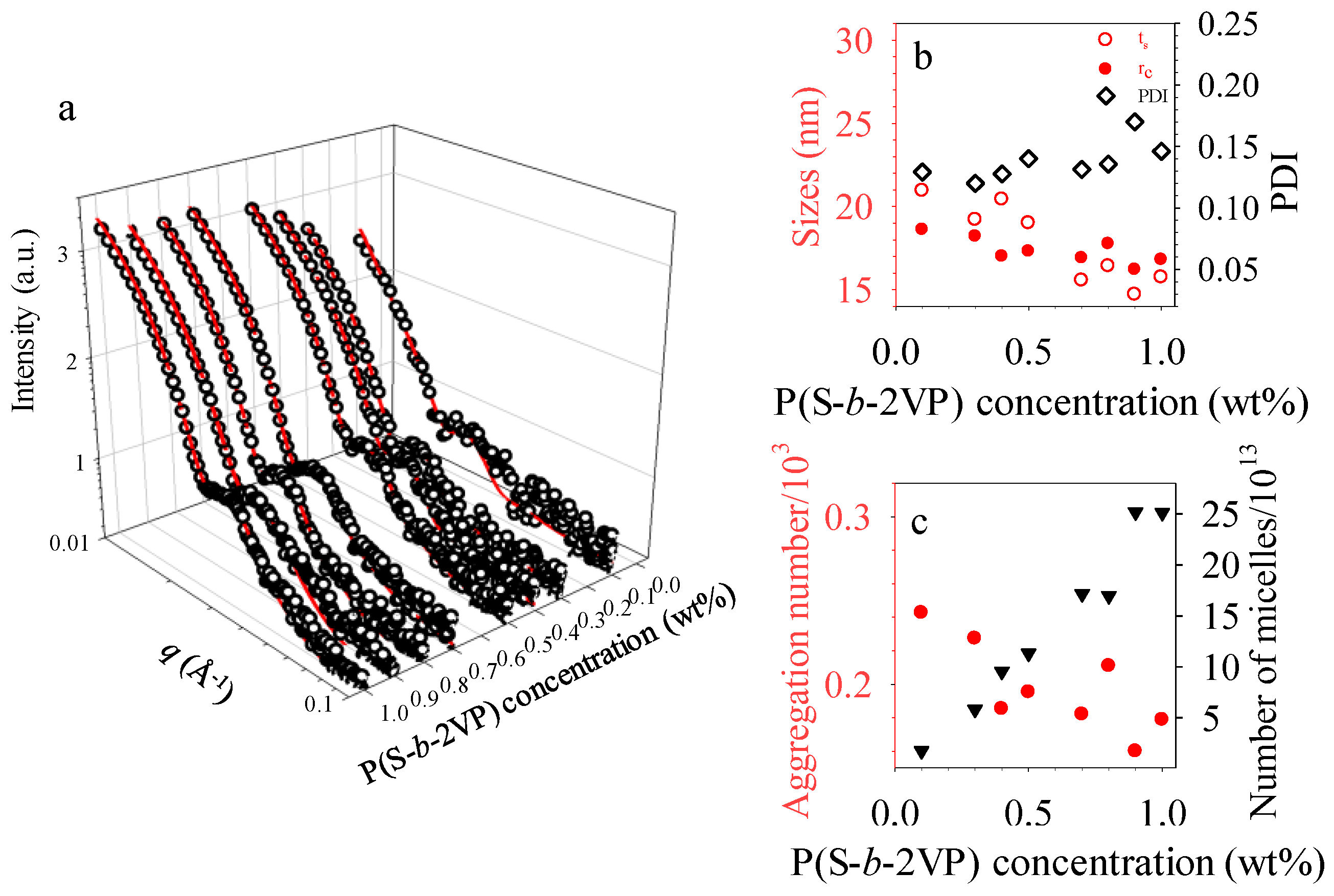
3.2. Experimental and Simulated GISAXS Patterns of Micellar Films on SiOx/Si
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lo, T.Y.; Ho, R.M.; Georgopanos, P.; Avgeropoulos, A.; Hashimoto, T. Direct Visualization of Order-Order Transitions in Silicon-Containing Block Copolymers by Electron Tomography. ACS Macro Lett. 2013, 2, 190–194. [Google Scholar] [CrossRef]
- Georgopanos, P.; Lo, T.Y.; Ho, R.M.; Avgeropoulos, A. Synthesis, Molecular Characterization and Self-Assembly of (PS-b-PDMS)n Type Linear (n = 1, 2) and Star (n = 3, 4) Block Copolymers. Polym. Chem. 2017, 8, 843–850. [Google Scholar] [CrossRef]
- Georgopanos, P.; Handge, U.A.; Abetz, C.; Abetz, V. Influnence of Block Sequence and Molecular Weight on Morphological, Rheological and Dielectric Properties of Weakly and Stongly Segregated Styrene-Isoprene Triblock Copolymers. Polymer 2016, 104, 279–295. [Google Scholar] [CrossRef]
- Dami, S.; Abetz, C.; Fischer, B.; Radjabian, M.; Georgopanos, P.; Abetz, V. A Correlation between Structural Features of an Amphiphilic Diblock Copolymer in Solusion and the Structure of the Porous Surface in an Integral Asymmetric Membrane. Polymer 2017, 126, 376–385. [Google Scholar] [CrossRef]
- Carrasco, P.; Ruiz de Luzuriaga, A.; Kirsten, M.; Constantinou, M.; Georgopanos, P.; Rangou, S.; Avgeropoulos, A.; Zafeiropoulos, N.E.; Stamm, M.; Grande, H.; et al. Self-Assembled Thermoset Materials by Modification with Polystyrene-Block-Poly(2-vinylpyridine). J. Mater. Sci. 2012, 47, 4348–4353. [Google Scholar] [CrossRef]
- Liang, C.; Hong, K.; Guiochon, G.A.; Mays, J.W.; Dai, S. Synthesis of a Large-Scale Highly Ordered Porous Carbon Film by Self-Assembly of Block Copolymers. Angew. Chem. Int. Ed. 2004, 43, 5785–5789. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.; Eisenberg, A. Self-Assembly of Block Copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Chen, Z.; Zhong, S.; Wooley, K.L.; Pochan, D.J. Block Copolymer Assembly via Kinetic Control. Science 2007, 317, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Riess, G. Micellization of block copolymers. Prog. Polym. Sci. 2003, 28, 1107–1170. [Google Scholar] [CrossRef]
- Förster, S.; Antonietti, M. Amphiphilic Block Copolymers in Structure-Controlled Nanomaterial Hybrids. Adv. Mater. 1998, 10, 195–217. [Google Scholar] [CrossRef]
- Chen, J.-C.; Liu, C.-L.; Sun, Y.-S.; Tung, S.-H.; Chen, W.-C. Tunable electrical memory characteristics by the morphology of self-assembled block copolymers: PCBM nanocomposite films. Soft Matter 2012, 8, 526–535. [Google Scholar] [CrossRef]
- Wang, Y.; Becker, M.; Wang, L.; Liu, J.; Scholz, R.; Peng, J.; Gösele, U.; Christiansen, S.; Kim, D.H.; Steinhart, M. Nanostructured Gold Films for SERS by Block Copolymer-Templated Galvanic Displacement Reactions. Nano Lett. 2009, 9, 2384–2389. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, J.; Jang, Y.J.; Lee, J.; Jang, Y.H.; Kochuveedu, S.T.; Lee, S.S.; Kim, D.H. Plasmonic nano-necklace arrays via reconstruction of diblock copolymer inverse micelle nanotemplates. Soft Matter 2011, 7, 57–60. [Google Scholar] [CrossRef]
- Liu, Y.; He, L.; Xu, C.; Han, M. Photochemical fabrication of hierarchical Ag nanoparticle arrays from domain-selective Ag+-loading on block copolymer templates. Chem. Commun. 2009, 43, 6566–6568. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, J.; Jang, Y.J.; Lee, J.; Jang, Y.H.; Kochuveedu, S.T.; Park, C.; Kim, D.H. Controlling the composition of plasmonic nanoparticle arrays via galvanic displacement reactions on block copolymer nanotemplates. Chem. Commun. 2011, 47, 1782–1784. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Lee, S.Y.; Briber, R.M.; Rabin, O. Self-Assembled SERS Substrates with Tunable Surface Plasmon Resonances. Adv. Func. Mater. 2011, 21, 3424–3429. [Google Scholar] [CrossRef]
- Cho, W.J.; Kim, Y.; Kim, J.K. Ultrahigh-Density Array of Silver Nanoclusters for SERS Substrate with High Sensitivity and Excellent Reproducibility. ACS Nano 2012, 6, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Mistark, P.A.; Park, S.; Yalcin, S.E.; Lee, D.H.; Yavuzcetin, O.; Tuominen, M.T.; Russell, T.P.; Achermann, M. Block-Copolymer-Based Plasmonic Nanostructures. ACS Nano 2009, 3, 3987–3992. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, M.; Buriak, J.M. Block Copolymer Templated Chemistry for the Formation of Metallic Nanoparticle Arrays on Semiconductor Surfaces. Chem. Mater. 2007, 19, 5090–5101. [Google Scholar] [CrossRef]
- Chai, J.; Buriak, J.M. Using Cylindrical Domains of Block Copolymers To Self-Assemble and Align Metallic Nanowires. ACS Nano 2008, 2, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.; Spatz, J.P.; Roescher, A. Gold nanoparticles in micellar poly(styrene)-b-poly(ethylene oxide) films—Size and interparticle distance control in monoparticulate films. Adv. Mater. 1996, 8, 337–340. [Google Scholar] [CrossRef]
- Kästle, G.; Boyen, H.G.; Weigl, F.; Lengl, G.; Herzog, T.; Ziemann, P.; Riethmüller, S.; Mayer, O.; Hartmann, C.; Spatz, J.P.; et al. Micellar Nanoreactors—Preparation and Characterization of Hexagonally Ordered Arrays of Metallic Nanodots. Adv. Funct. Mater. 2003, 13, 853–861. [Google Scholar] [CrossRef]
- Glass, R.; Arnold, M.; Blümmel, J.; Küller, A.; Möller, M.; Spatz, J.P. Micro-Nanostructured Interfaces Fabricated by the Use of Inorganic Block Copolymer Micellar Monolayers as Negative Resist for Electron-Beam Lithography. Adv. Funct. Mater. 2003, 13, 569–575. [Google Scholar] [CrossRef]
- Frömsdorf, A.; Kornowski, A.; Pütter, S.; Stillrich, H.; Lee, L.T. Highly Ordered Nanostructured Surfaces Obtained with Silica-Filled Diblock-Copolymer Micelles as Templates. Small 2007, 3, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Han, H.; Kim, Y.; Lee, W.; Alexe, M.; Baik, S.; Kim, J.K. Ultrahigh Density Array of Epitaxial Ferroelectric Nanoislands on Conducting Substrates. Nano Lett. 2010, 10, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, W.; Liu, Y.; Rafailovich, M.H.; Sokolov, J.; Khougaz, K.; Eisenberg, A.; Lennox, R.B.; Krausch, G. Self-Ordering of Diblock Copolymers from Solution. J. Am. Chem. Soc. 1996, 118, 10892–10893. [Google Scholar] [CrossRef]
- Meiners, J.C.; Ritzi, A.; Rafailovich, M.H.; Sokolov, J.; Mlynek, J.; Krausch, G. Two-dimensional micelle formation of polystyrene-poly(vinylpyridine) diblock copolymers on mice surfaces. Appl. Phys. 1995, 61, 519–524. [Google Scholar] [CrossRef]
- Meiners, J.C.; Elbs, H.; Ritzi, A.; Mlynek, J.; Krausch, G. Chemically functionalized surfaces from ultrathin block-copolymer films. J. Appl. Phys. 1996, 80, 2224–2227. [Google Scholar] [CrossRef]
- Meiners, J.C.; Quintel-Ritzi, A.; Mlynek, J.; Elbs, H.; Krausch, G. Adsorption of Block-Copolymer Micelles from a Selective Solvent. Macromolecules 1997, 30, 4945–4951. [Google Scholar] [CrossRef]
- Spatz, J.P.; Sheiko, S.; Möller, M. Ion-Stabilized Block Copolymer Micelles: Film Formation and Intermicellar Interaction. Macromolecules 1996, 29, 3220–3226. [Google Scholar] [CrossRef]
- Spatz, J.P.; Eibeck, P.; Mössmer, S.; Möller, M.; Kramarenko, E.Y.; Khalatur, P.G.; Potemkin, I.I.; Khokhlov, A.R.; Winkler, R.G.; Reineker, P. Order−Disorder Transition in Surface-Induced Nanopattern of Diblock Copolymer Films. Macromolecules 2000, 33, 150–157. [Google Scholar] [CrossRef]
- Spatz, J.P.; Möller, M.; Noeske, M.; Behm, R.J.; Pietralla, M. Nanomosaic Surfaces by Lateral Phase Separation of a Diblock Copolymer. Macromolecules 1997, 30, 3874–3880. [Google Scholar] [CrossRef]
- Mössmer, S.; Spatz, J.P.; Möller, M.; Aberle, T.; Schmidt, J.; Burchard, W. Solution Behavior of Poly(styrene)-block-poly(2-vinylpyridine) Micelles Containing Gold Nanoparticles. Macromolecules 2000, 33, 4791–4798. [Google Scholar] [CrossRef]
- Kim, T.H.; Huh, J.; Hwang, J.; Kim, H.-C.; Kim, S.H.; Sohn, B.-H.; Park, C. Ordered Arrays of PS-b-P4VP Micelles by Fusion and Fission Process upon Solvent Annealing. Macromolecules 2009, 42, 6688–6697. [Google Scholar] [CrossRef]
- Park, S.; Wang, J.-Y.; Kim, B.; Chen, W.; Russell, T.P. Solvent-Induced Transition from Micelles in Solution to Cylindrical Microdomains in Diblock Copolymer Thin Films. Macromolecules 2007, 40, 9059–9063. [Google Scholar] [CrossRef]
- Park, S.; Lee, D.H.; Xu, J.; Kim, B.; Hong, S.W.; Jeong, U.; Xu, T.; Russell, T.P. Macroscopic 10-Terabit–per–Square-Inch Arrays from Block Copolymers with Lateral Order. Science 2009, 323, 1030–1033. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Tracz, A.; Kruk, M.; Zhang, R.; Smilgies, D.-M.; Matyjaszewski, K.; Kowalewski, T. Long-Range Ordered Thin Films of Block Copolymers Prepared by Zone-Casting and Their Thermal Conversion into Ordered Nanostructured Carbon. J. Am. Chem. Soc. 2005, 127, 6918–6919. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Wu, P.-J.; Sun, Y.-S. Kinetically controlled self-assembly of monolayered micelle films of P(S-b-4VP) on bare and PS-grafted substrates. Soft Matter 2011, 7, 9140–9147. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Lee, Y.-C.; Wu, P.-J.; Liou, J.-Y.; Sun, Y.-S.; Ko, B.-T. Micellar Transitions in Solvent-Annealed Thin Films of an Amphiphilic Block Copolymer Controlled with Tunable Surface Fields. Langmuir 2011, 27, 14545–14553. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.-Y.; Sun, Y.-S. Monolayers of Diblock Copolymer Micelles by Spin-Coating from o-Xylene on SiOx/Si Studied in Real and Reciprocal Space. Macromolecules 2012, 45, 1963–1971. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Pugin, R.; Brugger, J.; Heinzelmann, H.; Hinderling, C. Tuning the Dimensions and Periodicities of Nanostructures Starting from the Same Polystyrene-block-poly(2-vinylpyridine) Diblock Copolymer. Adv. Funct. Mater. 2006, 16, 1469–1475. [Google Scholar] [CrossRef]
- Kline, S. Reduction and analysis of SANS and USANS data using IGOR Pro. J. Appl. Cryst. 2006, 39, 895–900. [Google Scholar] [CrossRef]
- Lazzari, R. IsGISAXS: A program for grazing-incidence small-angle X-ray scattering analysis of supported islands. J. Appl. Cryst. 2002, 35, 406–421. [Google Scholar] [CrossRef]
- Renaud, G.; Lazzari, R.; Leroy, F. Probing surface and interface morphology with Grazing Incidence Small Angle X-ray Scattering. Surf. Sci. Rep. 2009, 64, 255–380. [Google Scholar] [CrossRef]
- Müller-Buschbaum, P. Grazing incidence small-angle X-ray scattering: An advanced scattering technique for the investigation of nanostructured polymer films. Anal. Bioanal. Chem. 2003, 376, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Müller-Buschbaum, P. Polymer Surfaces and Interfaces: Characterization and Applications; Stamm, M., Ed.; Springer: Berlin, Germany, 2008; pp. 17–46. [Google Scholar]
- Müller-Buschbaum, P. Applications of Synchrotron Light to Noncrystalline Diffraction in Materials and Life Sciences; Ezquerra, T.A., Garcia-Gutierrez, M., Nogales, A., Gomez, M., Eds.; Springer: Berlin, Germany, 2009; pp. 61–89. [Google Scholar]
- Esselink, F.J.; Dormidontova, E.E.; Hadziioannou, G. Redistribution of Block Copolymer Chains between Mixed Micelles in Solution. Macromolecules 1998, 31, 4873–4878. [Google Scholar] [CrossRef] [PubMed]
- Esselink, F.J.; Dormidontova, E.; Hadziioannou, G. Evolution of Block Copolymer Micellar Size and Structure Evidenced with Cryo Electron Microscopy. Macromolecules 1998, 31, 2925–2932. [Google Scholar] [CrossRef]
- Sun, Y.-S.; Chien, S.-W.; Wu, P.-J. Effects of Film Instability on Roughness Correlation and Nanodomain Ordering in Ultrathin Films of Asymmetric Block Copolymers. Macromolecules 2010, 43, 5016–5023. [Google Scholar] [CrossRef]
- Sun, Y.-S.; Chien, S.-W.; Liou, J.-Y. Probing Relief Terraces in Destabilized Thin Films of an Asymmetric Block Copolymer with Grazing-Incidence Small-Angle X-ray Scattering. Macromolecules 2010, 43, 7250–7260. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liou, J.-Y.; Sun, Y.-S. Lateral Order and Self-Organized Morphology of Diblock Copolymer Micellar Films. Polymers 2018, 10, 597. https://doi.org/10.3390/polym10060597
Liou J-Y, Sun Y-S. Lateral Order and Self-Organized Morphology of Diblock Copolymer Micellar Films. Polymers. 2018; 10(6):597. https://doi.org/10.3390/polym10060597
Chicago/Turabian StyleLiou, Jiun-You, and Ya-Sen Sun. 2018. "Lateral Order and Self-Organized Morphology of Diblock Copolymer Micellar Films" Polymers 10, no. 6: 597. https://doi.org/10.3390/polym10060597