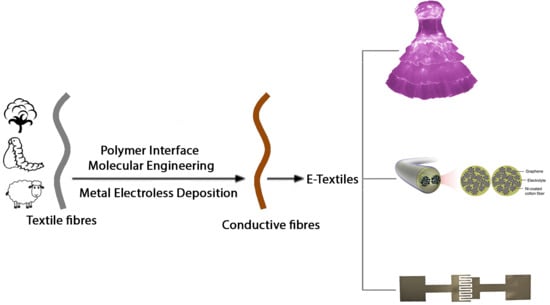
Polymer Interface Molecular Engineering for E-Textiles
Abstract
:1. Introduction
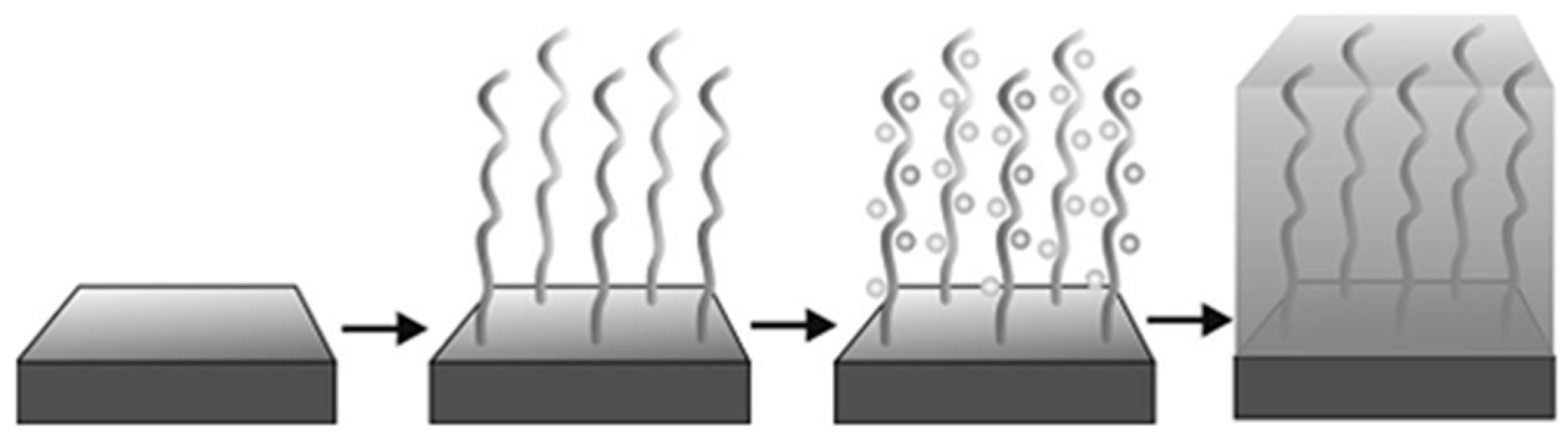
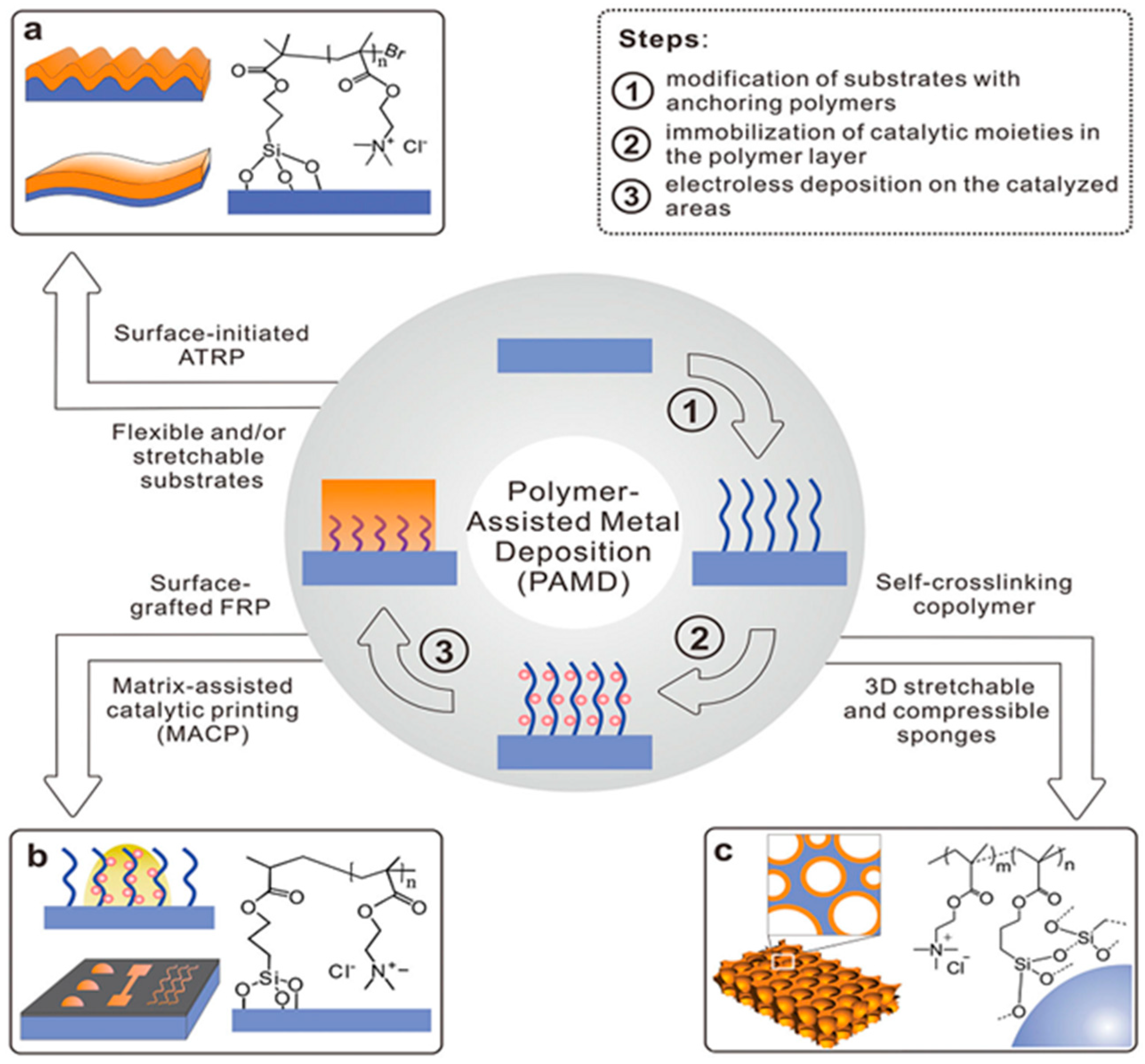
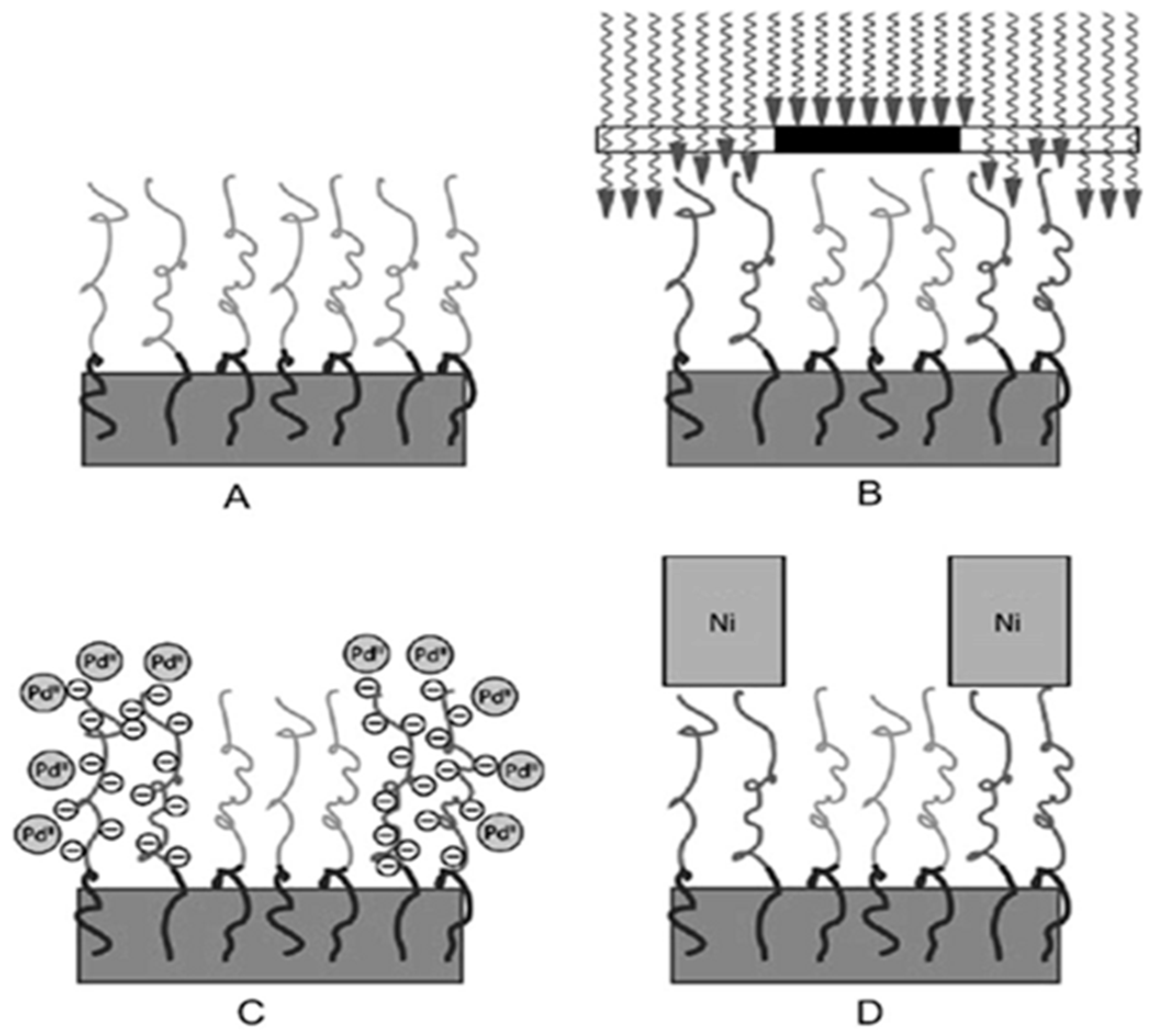
2. Polymer Interface and Catalytic Moiety
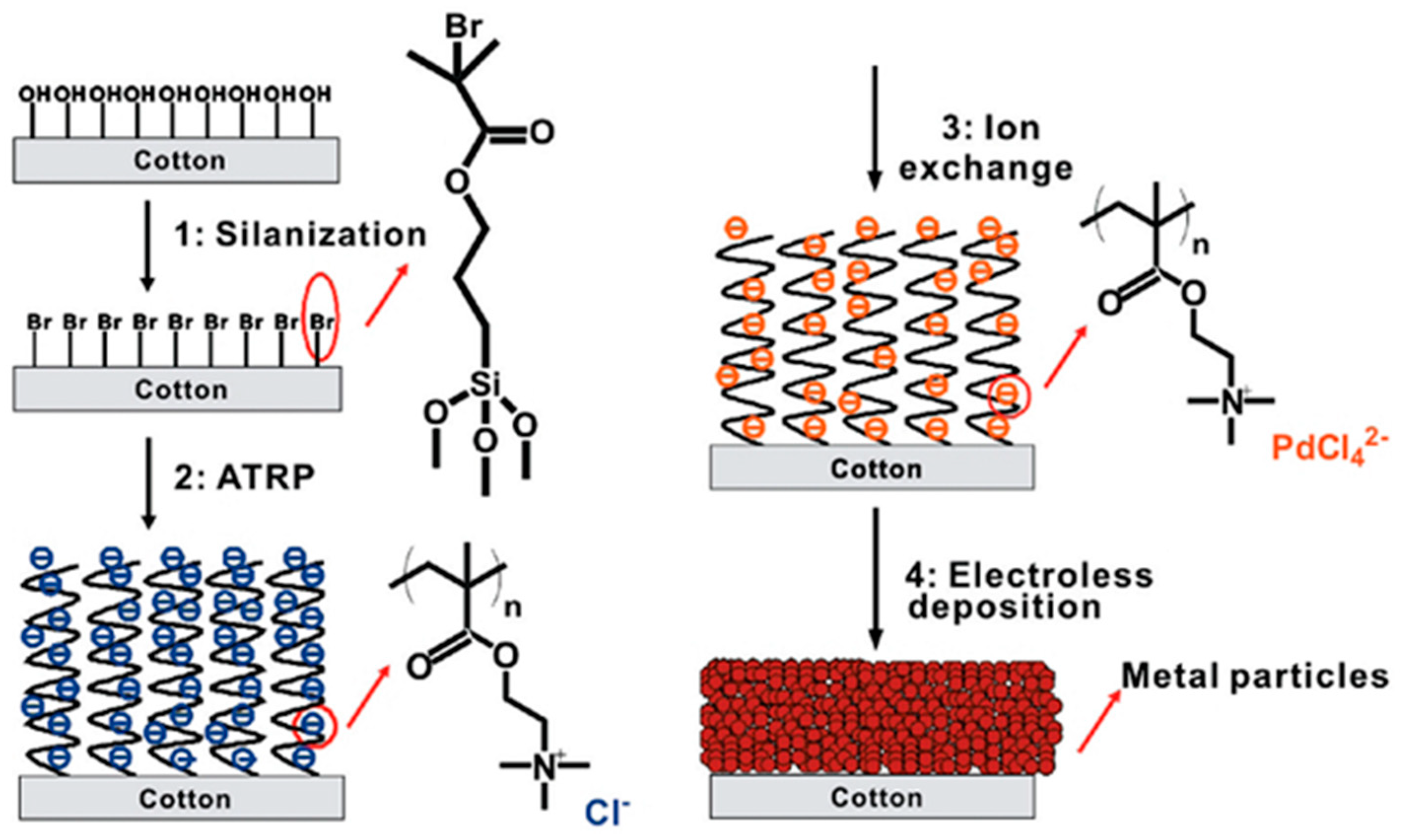
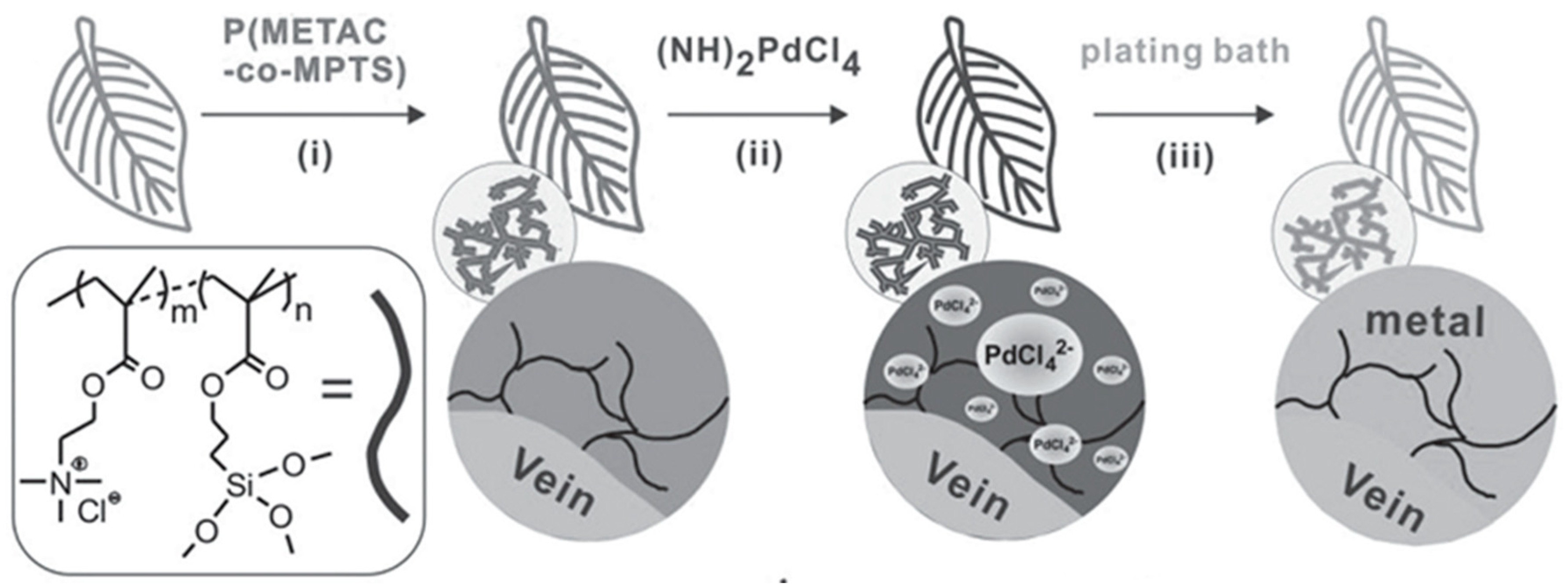
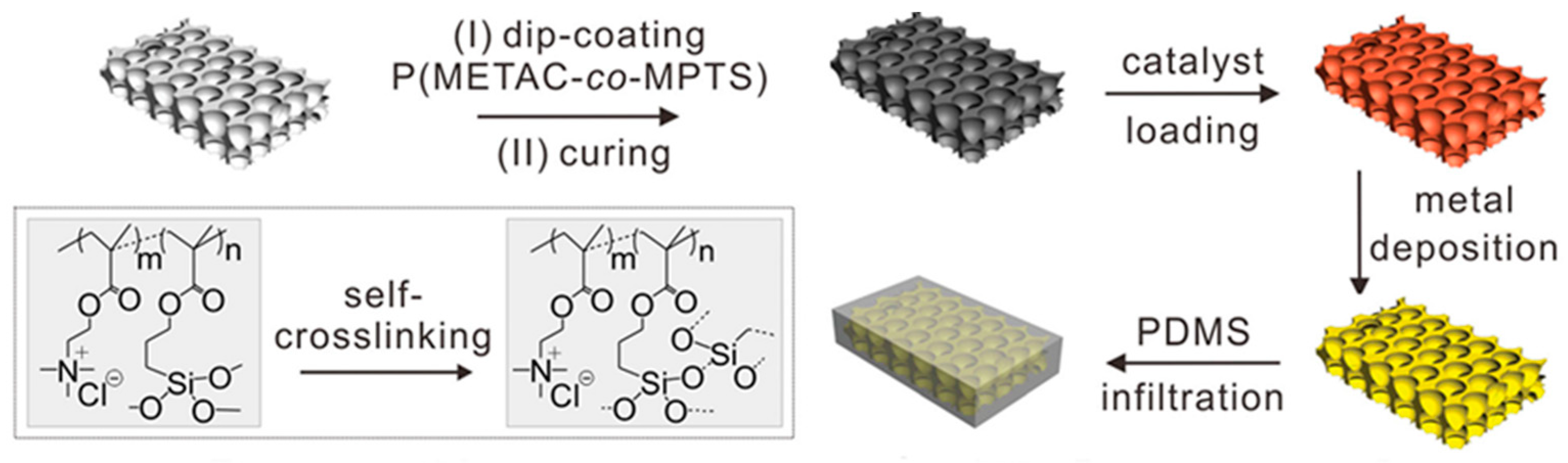
2.1. Cationic Polymer Interface and Catalytic Moiety
2.2. Anionic Polymer Matrix and Catalytic Moiety
2.3. Nonionic Polymer Interface and Catalytic Moiety
2.4. Polyphenols Interface and Catalytic Moiety
3. Substrates and Applications of ELD in Wearable Electronics
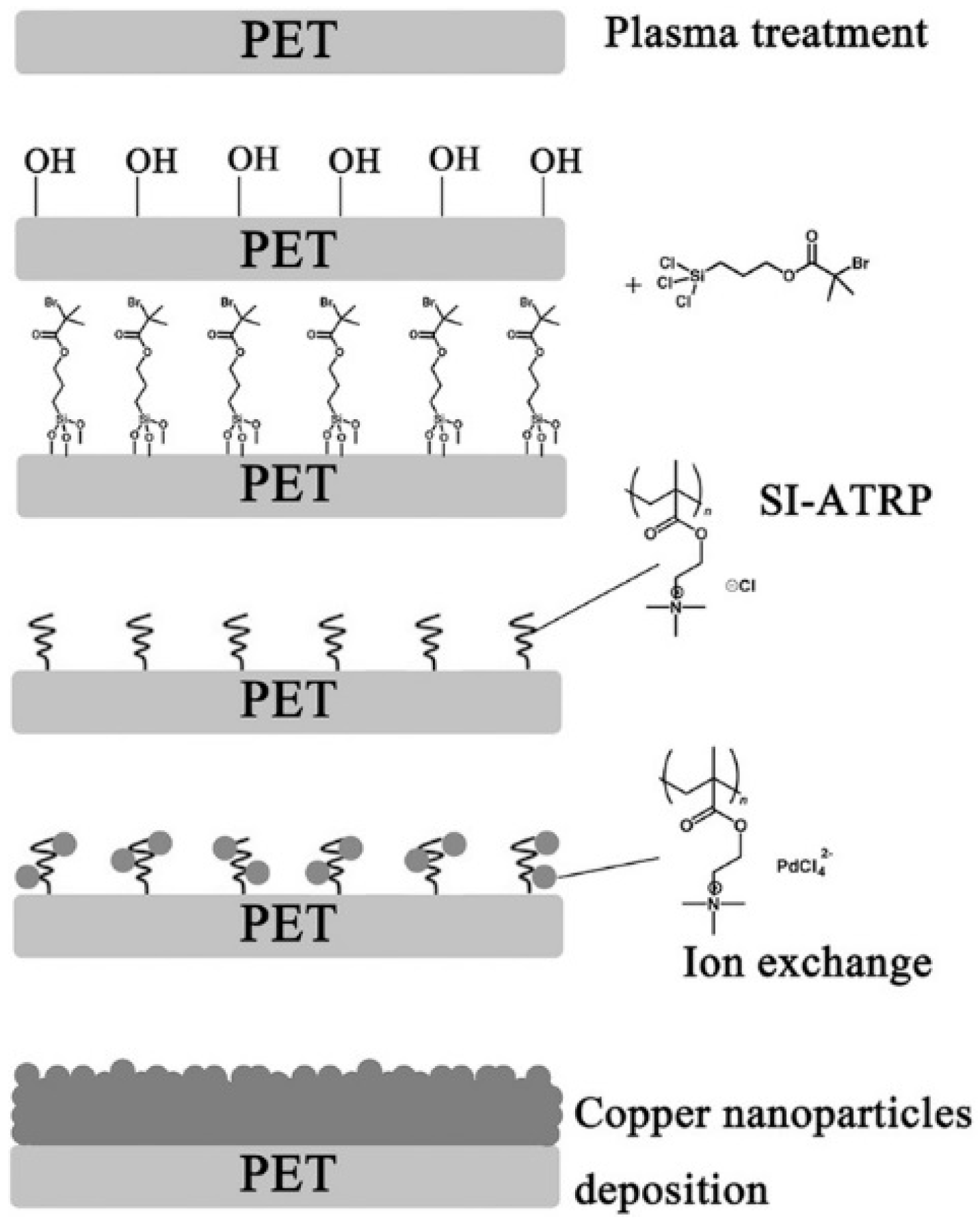
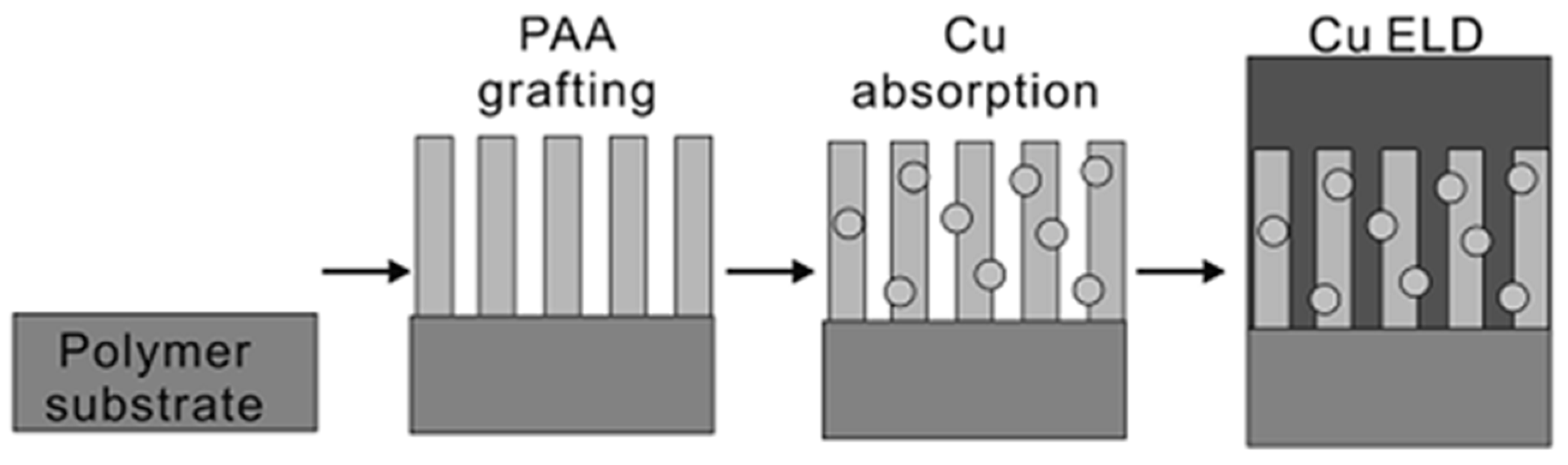
3.1. ELD on Plastic Substrates
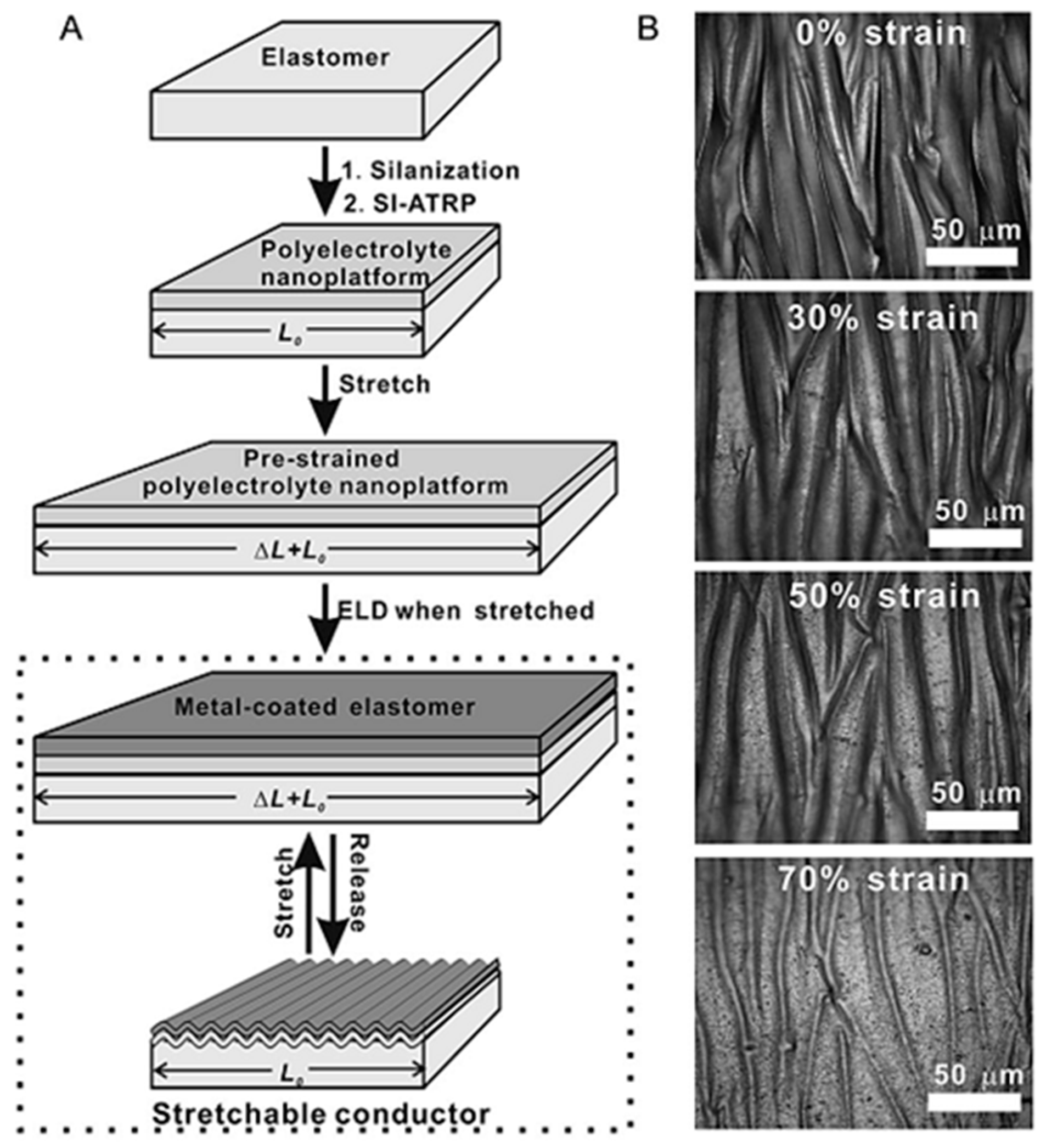
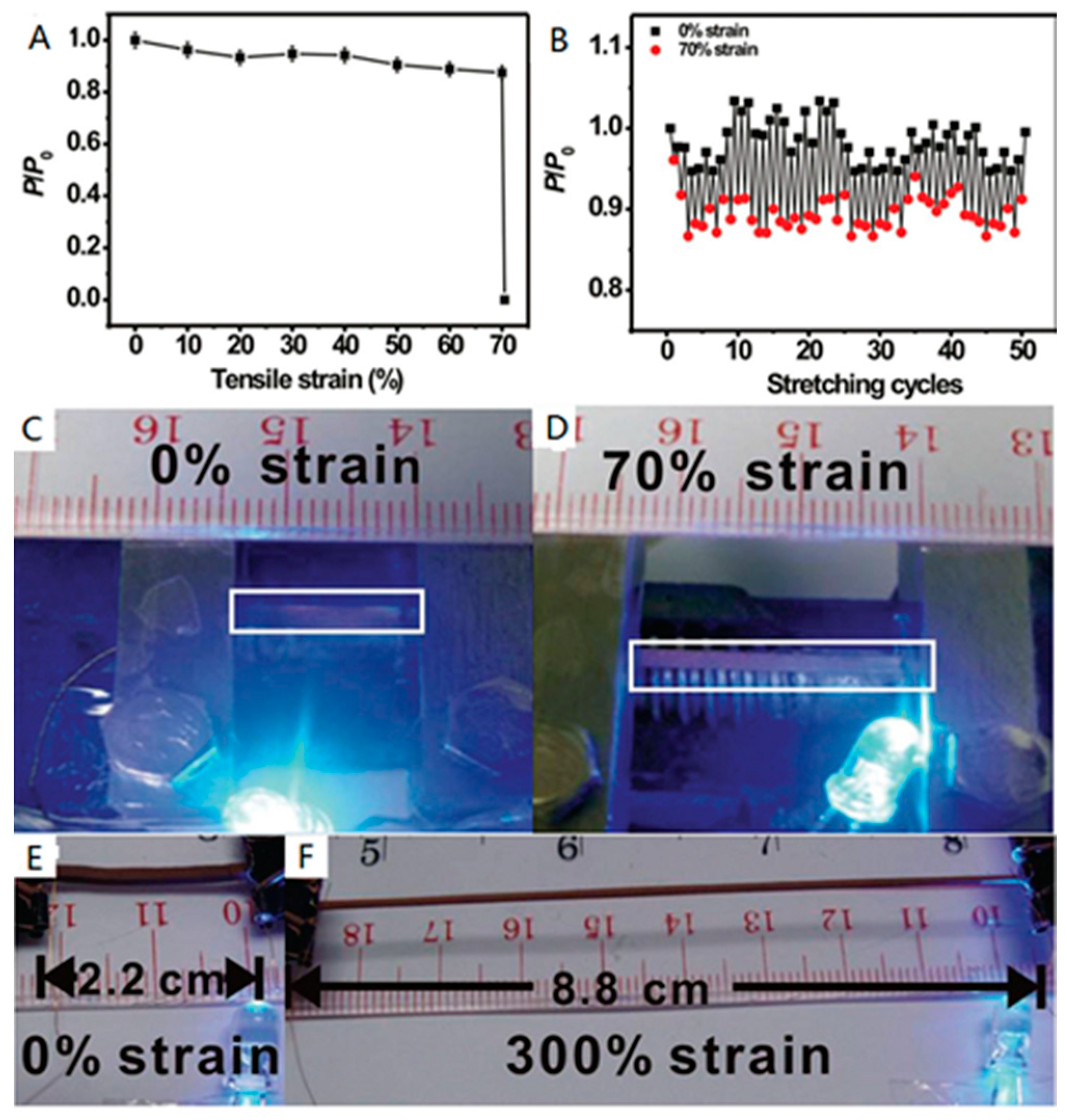
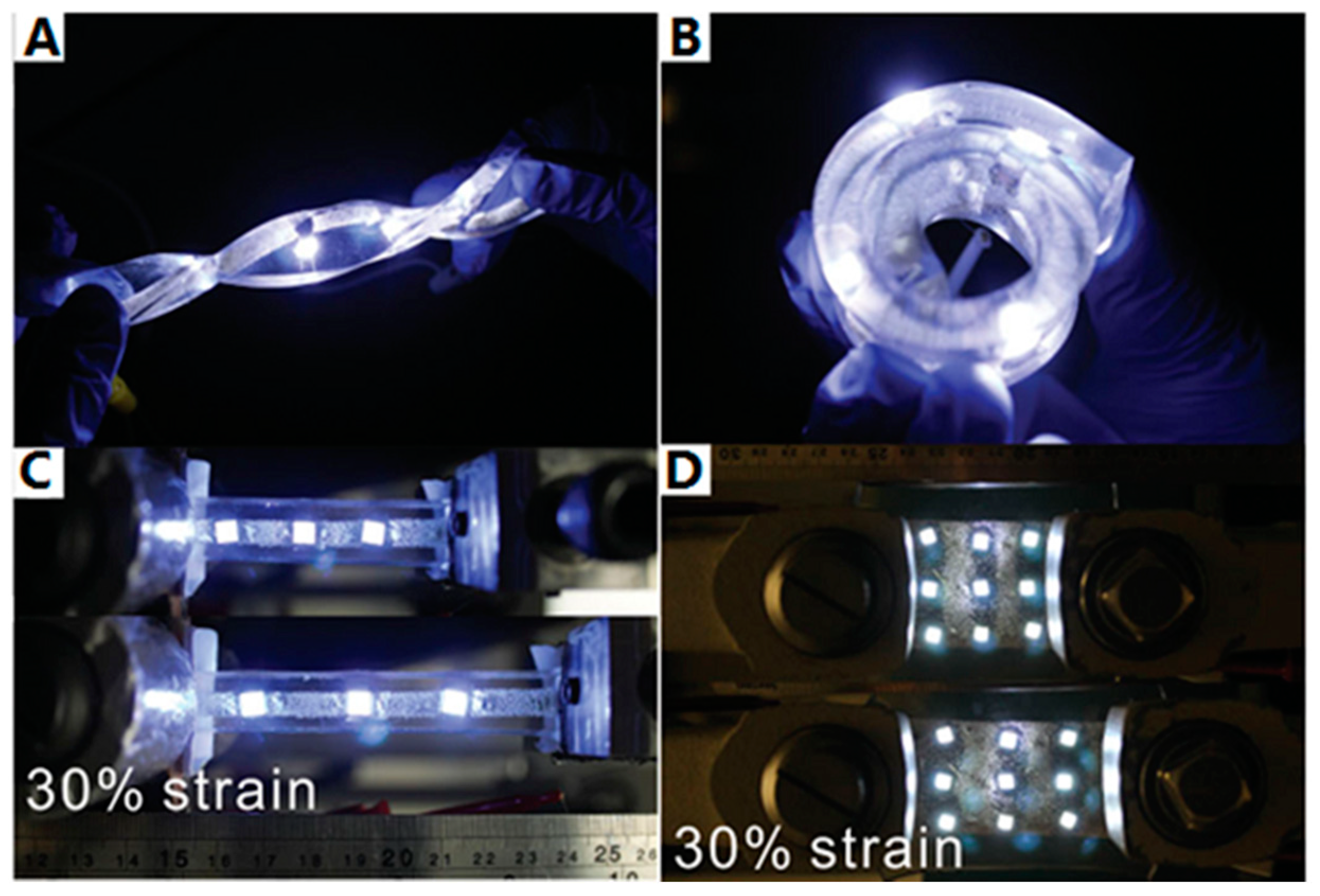
3.2. ELD on Elastomer Substrates
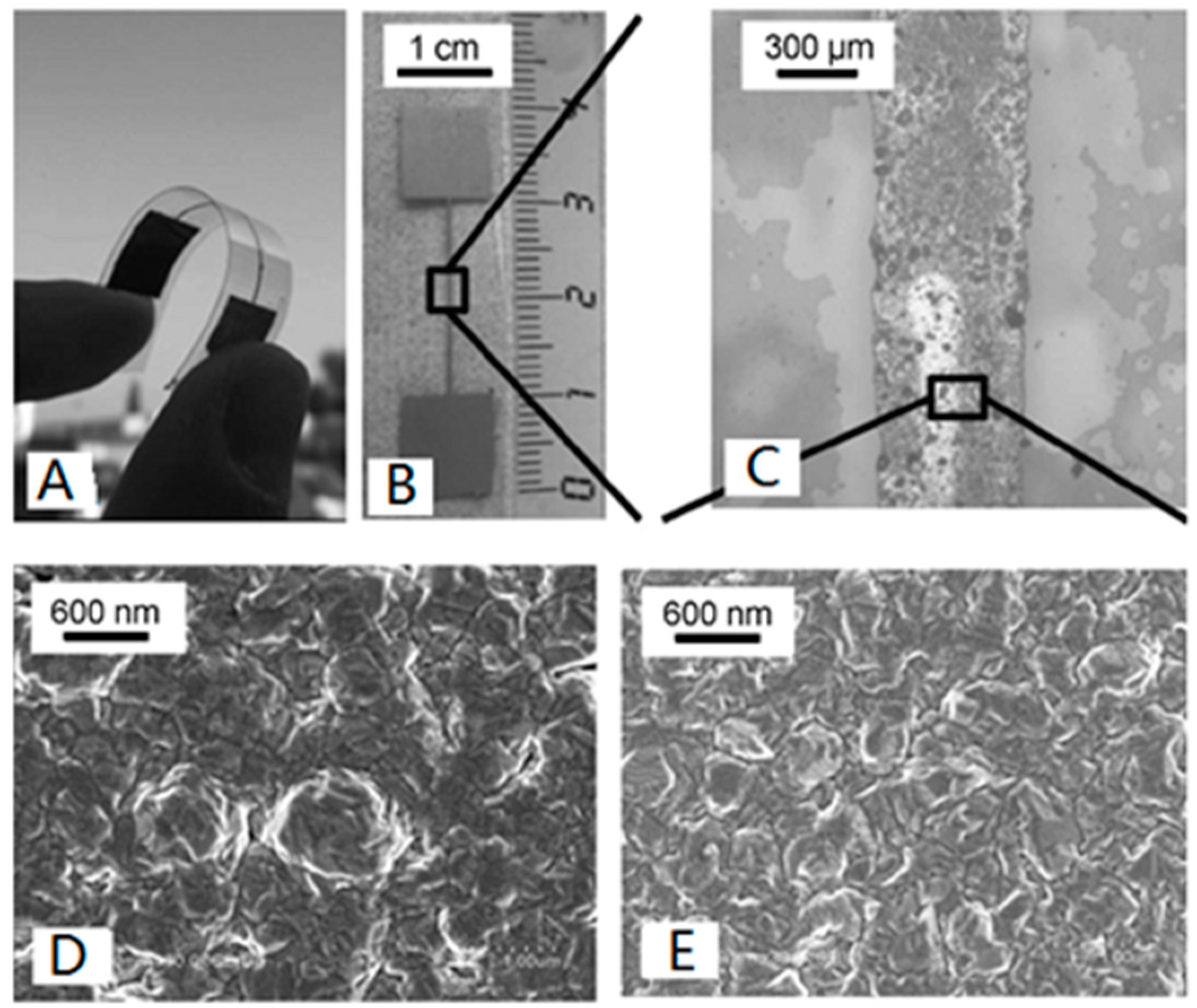
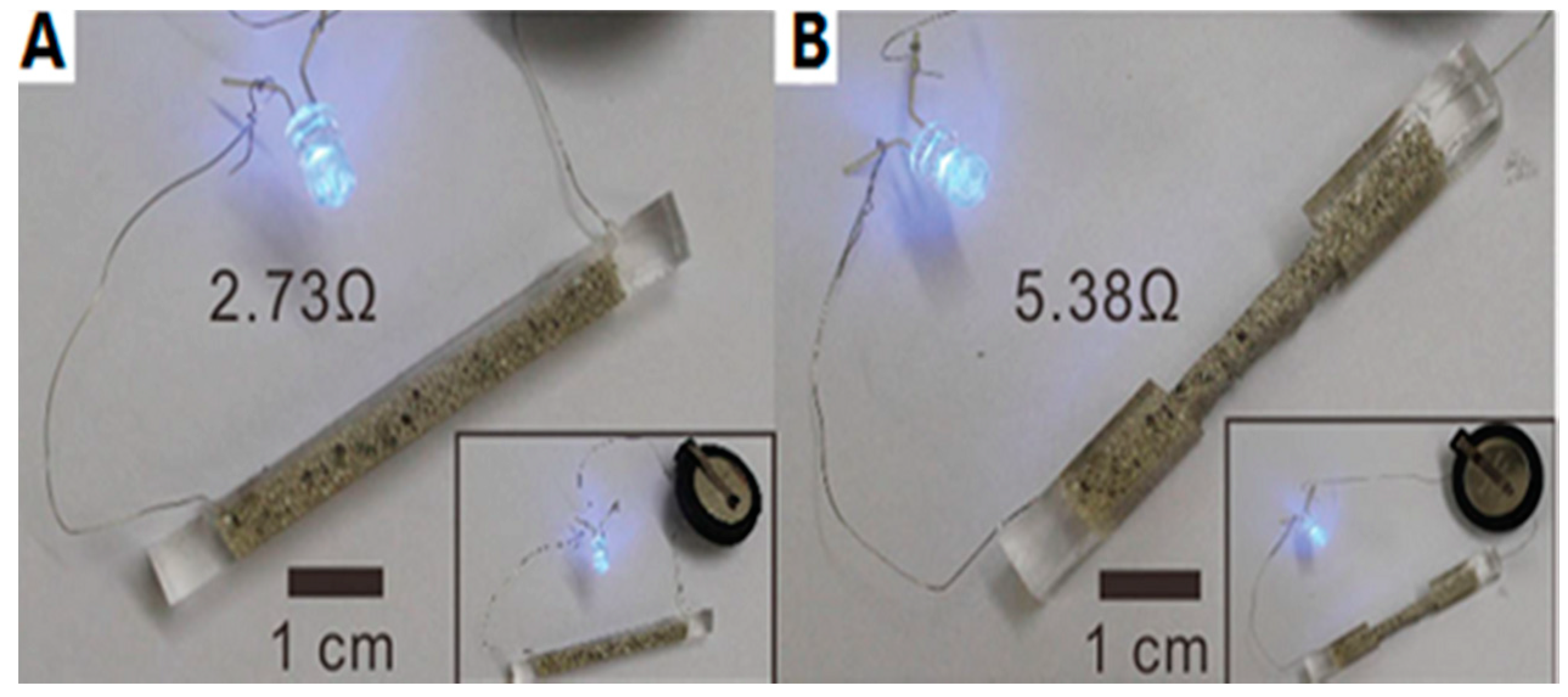
3.3. ELD on Cotton Substrates
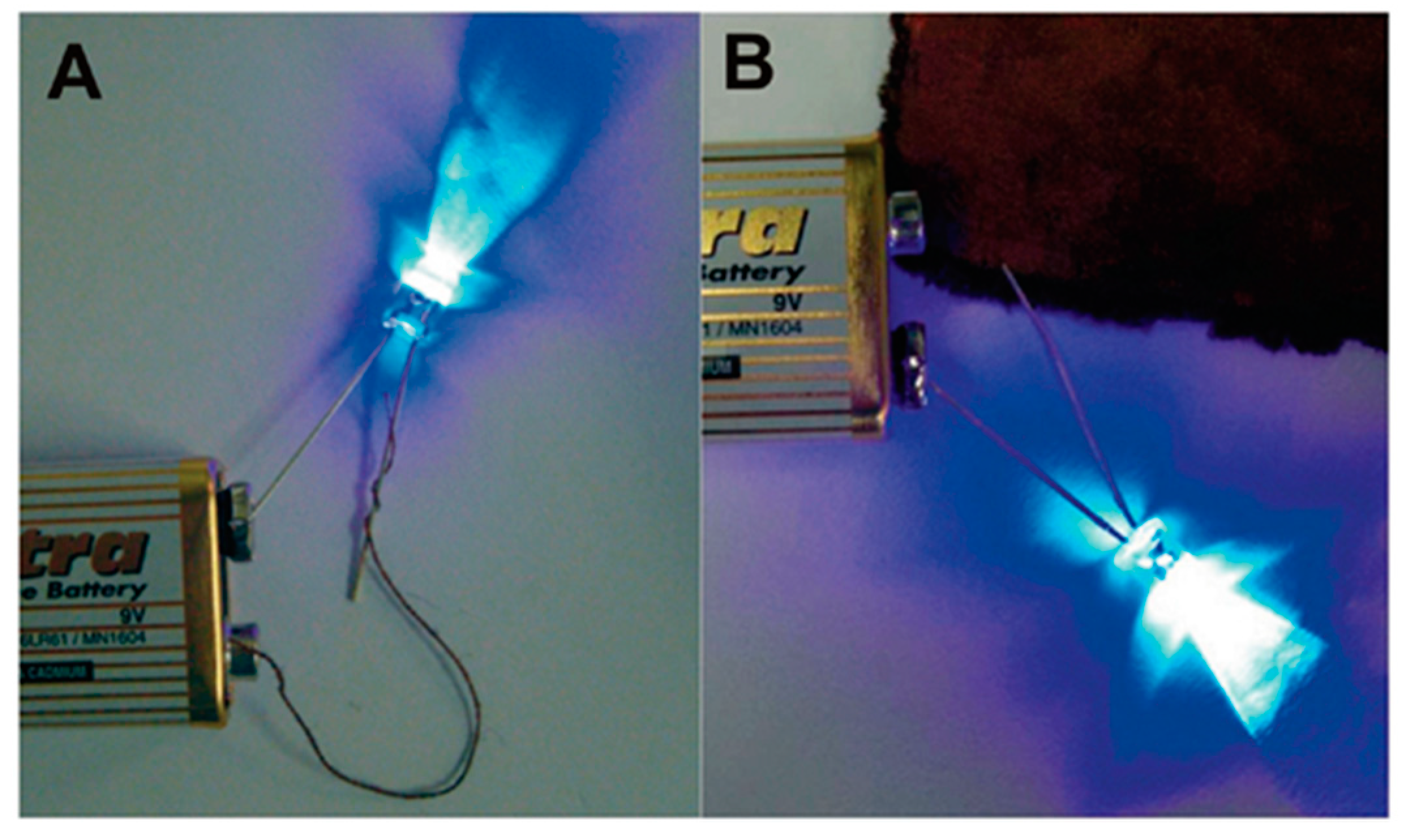
3.4. ELD on Bio-Inspired Substrates
3.5. ELD on Three-Dimensional Substrates
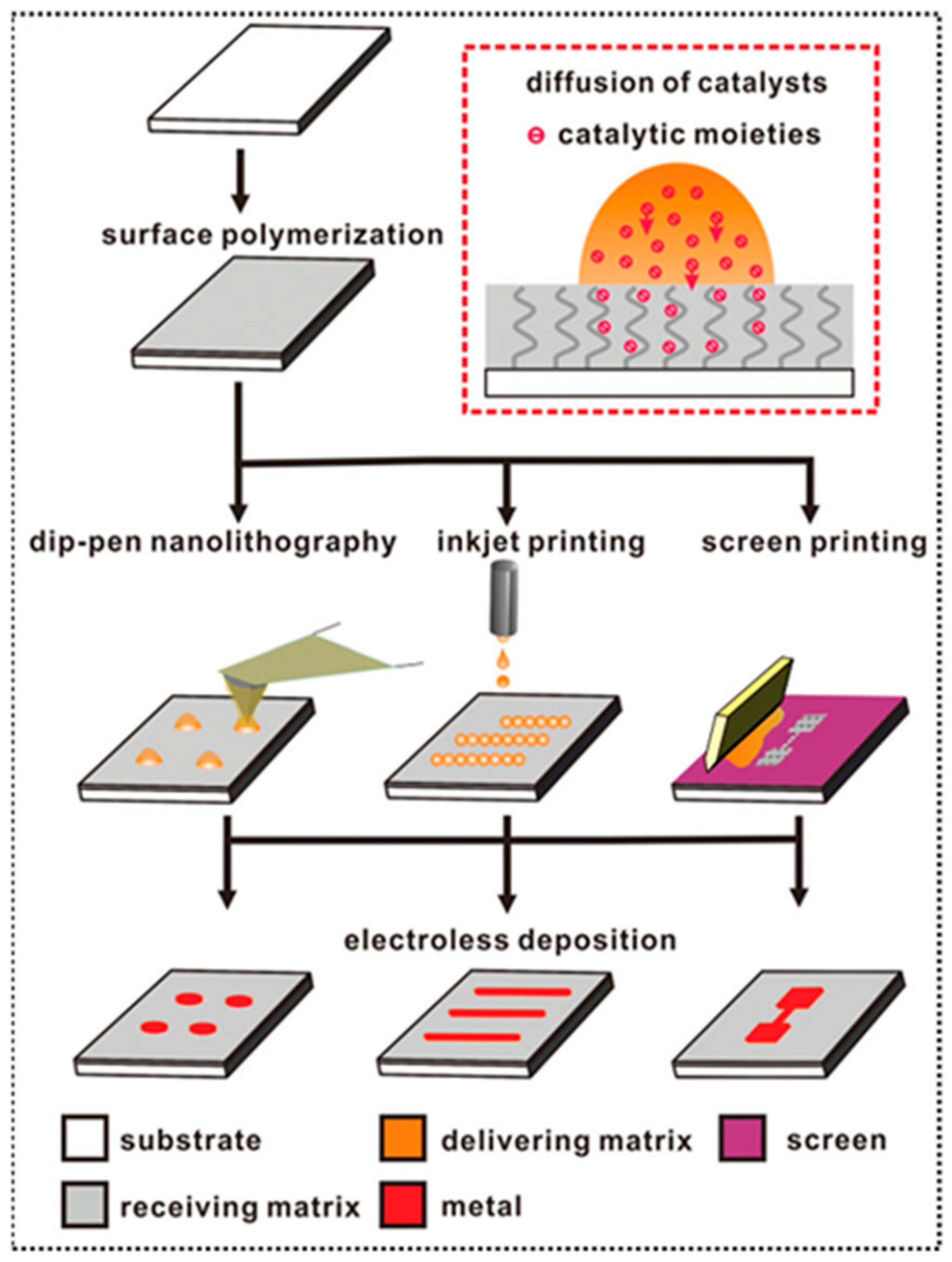
4. Surface Lithography by PIME Catalytic ELD
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Benight, S.J.; Wang, C.; Tok, J.B.; Bao, Z. Stretchable and self-healing polymers and devices for electronic skin. Prog. Polym. Sci. 2013, 38, 1961–1977. [Google Scholar] [CrossRef]
- Hu, L.; Pasta, M.; La Mantia, F.; Cui, L.; Jeong, S.; Deshazer, H.D.; Choi, J.W.; Han, S.M.; Cui, Y. Stretchable, porous, and conductive energy textiles. Nano Lett. 2010, 10, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Lu, N.; Ma, R.; Kim, Y.-S.; Kim, R.-H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A. Epidermal electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, X.; Li, Y.; Zheng, Z. Surface-Grafted Polymer-Assisted Electroless Deposition of Metals for Flexible and Stretchable Electronics. Chemistry 2012, 7, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Zeng, W.; Tao, X.-M. High stretchable MWNTs/polyurethane conductive nanocomposites. J. Mater. Chem. 2011, 21, 7274–7280. [Google Scholar] [CrossRef]
- Webb, R.C.; Bonifas, A.P.; Behnaz, A.; Zhang, Y.; Yu, K.J.; Cheng, H.; Shi, M.; Bian, Z.; Liu, Z.; Kim, Y.-S. Ultrathin conformal devices for precise and continuous thermal characterization of human skin. Nat. Mater. 2013, 12, 938–944. [Google Scholar] [CrossRef] [PubMed]
- White, M.S.; Kaltenbrunner, M.; Głowacki, E.D.; Gutnichenko, K.; Kettlgruber, G.; Graz, I.; Aazou, S.; Ulbricht, C.; Egbe, D.A.; Miron, M.C. Ultrathin, highly flexible and stretchable PLEDs. Nat. Photonics 2013, 7, 811–816. [Google Scholar] [CrossRef]
- Yeo, W.H.; Kim, Y.S.; Lee, J.; Ameen, A.; Shi, L.; Li, M.; Wang, S.; Ma, R.; Jin, S.H.; Kang, Z. Multifunctional epidermal electronics printed directly onto the skin. Adv. Mater. 2013, 25, 2773–2778. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yan, C.; Zheng, Z. Polymer-Assisted Metal Deposition (PAMD): A Full-Solution Strategy for Flexible, Stretchable, Compressible, and Wearable Metal Conductors. Adv. Mater. 2014, 26, 5508–5516. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Y.; Li, K.; Yan, C.; Zheng, Z. Bio-Inspired Chemical Fabrication of Stretchable Transparent Electrodes. Small 2015, 11, 3444–3449. [Google Scholar] [CrossRef] [PubMed]
- An, B.W.; Shin, J.H.; Kim, S.-Y.; Kim, J.; Ji, S.; Park, J.; Lee, Y.; Jang, J.; Park, Y.-G.; Cho, E. Smart Sensor Systems for Wearable Electronic Devices. Polymers 2017, 9, 303. [Google Scholar] [CrossRef]
- Genc, R.; Alas, M.O.; Harputlu, E.; Repp, S.; Kremer, N.; Castellano, M.; Colak, S.G.; Ocakoglu, K.; Erdem, E. High-Capacitance Hybrid Supercapacitor Based on Multi-Colored Fluorescent Carbon-Dots. Sci. Rep. 2017, 7, 11222. [Google Scholar] [CrossRef] [PubMed]
- Repp, S.; Harputlu, E.; Gurgen, S.; Castellano, M.; Kremer, N.; Pompe, N.; Wörner, J.; Hoffmann, A.; Thomann, R.; Emen, F.M. Synergetic effects of Fe3+ doped spinel Li4Ti5O12 nanoparticles on reduced graphene oxide for high surface electrode hybrid supercapacitors. Nanoscale 2018, 10, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Lehtimäki, S.; Suominen, M.; Damlin, P.; Tuukkanen, S.; Kvarnström, C.; Lupo, D. Preparation of supercapacitors on flexible substrates with electrodeposited PEDOT/graphene composites. ACS Appl. Mater. Interfaces 2015, 7, 22137–22147. [Google Scholar] [CrossRef] [PubMed]
- Haddara, Y.M.; Howlader, M.M. Integration of Heterogeneous Materials for Wearable Sensors. Polymers 2018, 10, 60. [Google Scholar] [CrossRef]
- Silva, R.; Franchi, D.; Leone, A.; Pilloni, L.; Masci, A.; Pozio, A. Surface conductivity and stability of metallic bipolar plate materials for polymer electrolyte fuel cells. Electrochim. Acta 2006, 51, 3592–3598. [Google Scholar] [CrossRef]
- Limb, S.J.; Gleason, K.K.; Edell, D.J.; Gleason, E.F. Flexible fluorocarbon wire coatings by pulsed plasma enhanced chemical vapor deposition. J. Vac. Sci. Technol. A 1997, 15, 1814–1818. [Google Scholar] [CrossRef]
- Sun, Y.; Rogers, J.A. Inorganic semiconductors for flexible electronics. Adv. Mater. 2007, 19, 1897–1916. [Google Scholar] [CrossRef]
- Geiser, J. Multiscale modeling of chemical vapor deposition (CVD) apparatus: Simulations and approximations. Polymers 2013, 5, 142–160. [Google Scholar] [CrossRef]
- Brosteaux, D.; Axisa, F.; Gonzalez, M.; Vanfleteren, J. Design and fabrication of elastic interconnections for stretchable electronic circuits. IEEE Electron Device Lett. 2007, 28, 552–554. [Google Scholar] [CrossRef]
- Cho, S.; Kim, S.; Lee, J.; Lee, N.-E. Micro-scale metallization of high aspect-ratio Cu and Au lines on flexible polyimide substrate by electroplating using SU-8 photoresist mask. Microelectron. Eng. 2005, 77, 116–124. [Google Scholar] [CrossRef]
- Li, X.; Pei, W.; Tang, R.; Gui, Q.; Guo, K.; Wang, Y.; Chen, H. Investigation of flexible electrodes modified by TiN, Pt black and IrO x. Sci. China Technol. Sci. 2011, 54, 2305–2309. [Google Scholar] [CrossRef]
- Cheng, K.; Yang, M.H.; Chiu, W.W.; Huang, C.Y.; Chang, J.; Ying, T.F.; Yang, Y. Ink-Jet Printing, Self-Assembled Polyelectrolytes, and Electroless Plating: Low Cost Fabrication of Circuits on a Flexible Substrate at Room Temperature. Macromol. Rapid Commun. 2005, 26, 247–264. [Google Scholar] [CrossRef]
- Zschieschang, U.; Klauk, H.; Halik, M.; Schmid, G.; Dehm, C. Flexible organic circuits with printed gate electrodes. Adv. Mater. 2003, 15, 1147–1151. [Google Scholar] [CrossRef]
- Guo, R.; Yu, Y.; Zeng, J.; Liu, X.; Zhou, X.; Niu, L.; Gao, T.; Li, K.; Yang, Y.; Zhou, F. Biomimicking Topographic Elastomeric Petals (E-Petals) for Omnidirectional Stretchable and Printable Electronics. Adv. Sci. 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yu, Y.; Yan, C.; Li, K.; Zheng, Z. Wearable energy-dense and power-dense supercapacitor yarns enabled by scalable graphene-metallic textile composite electrodes. Nat. Commun. 2015, 6, 7260. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Yu, Y.; Xie, Z.; Liu, X.; Zhou, X.; Gao, Y.; Liu, Z.; Zhou, F.; Yang, Y.; Zheng, Z. Matrix-Assisted Catalytic Printing for the Fabrication of Multiscale, Flexible, Foldable, and Stretchable Metal Conductors. Adv. Mater. 2013, 25, 3343–3350. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, S.; Osborne, V.L.; Huck, W.T. Polymer brushes via surface-initiated polymerizations. Chem. Soc. Rev. 2004, 33, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Pyun, J.; Kowalewski, T.; Matyjaszewski, K. Synthesis of polymer brushes using atom transfer radical polymerization. Macromol. Rapid Commun. 2003, 24, 1043–1059. [Google Scholar] [CrossRef]
- Deng, J.; Wang, L.; Liu, L.; Yang, W. Developments and new applications of UV-induced surface graft polymerizations. Prog. Polym. Sci. 2009, 34, 156–193. [Google Scholar] [CrossRef]
- Kang, E.T.; Zhang, Y. Surface modification of fluoropolymers via molecular design. Adv. Mater. 2000, 12, 1481–1494. [Google Scholar] [CrossRef]
- Nasef, M.M.; Hegazy, E.-S.A. Preparation and applications of ion exchange membranes by radiation-induced graft copolymerization of polar monomers onto non-polar films. Prog. Polym. Sci. 2004, 29, 499–561. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
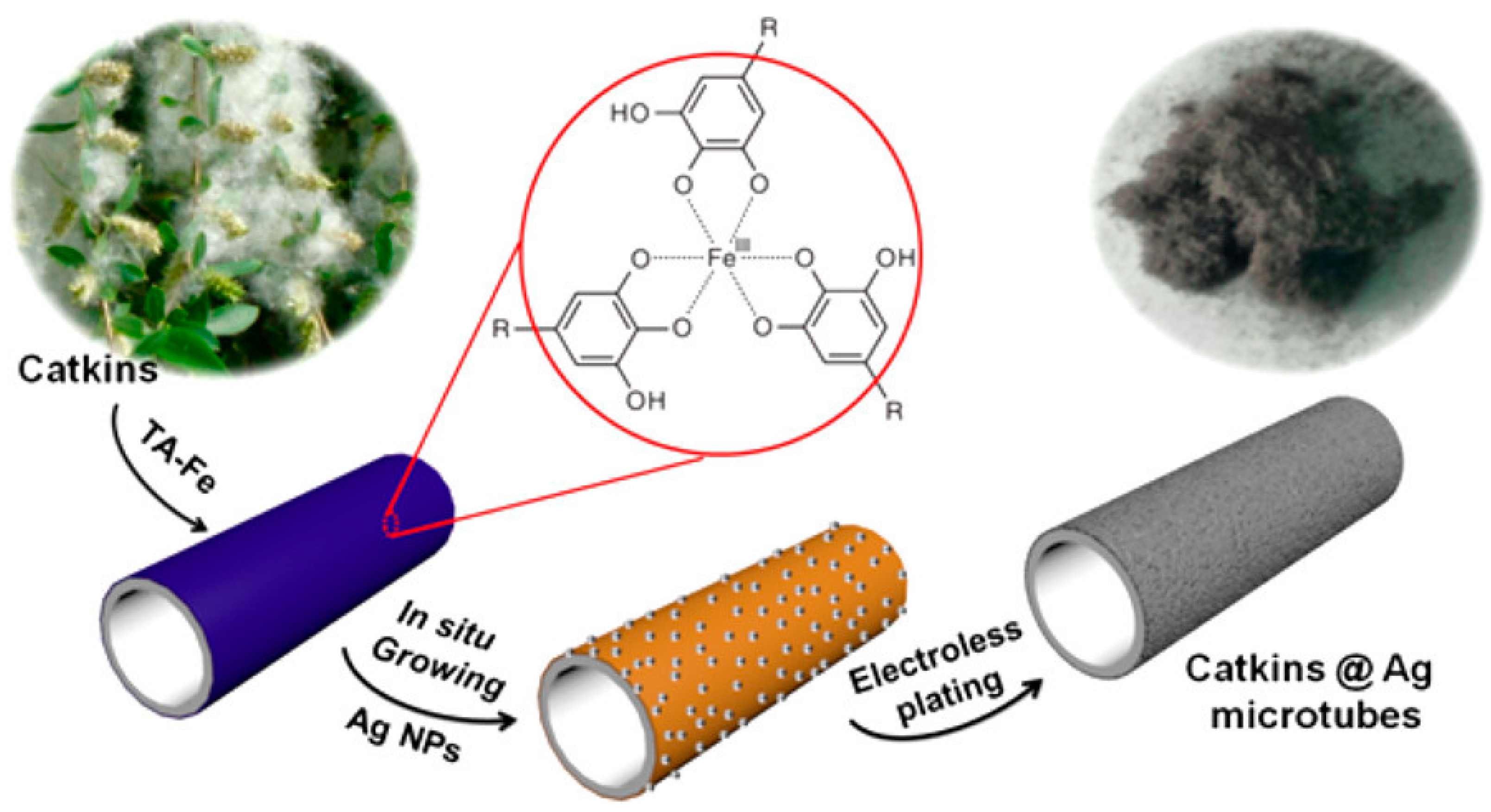
- Li, D.; Shen, H.; Cai, C.; Sun, T.; Zhao, Y.; Chen, L.; Zhao, N.; Xu, J. Fabrication of Conductive Silver Microtubes Using Natural Catkin as a Template. ACS Omega 2017, 2, 1738–1745. [Google Scholar] [CrossRef]
- Azzaroni, O.; Zheng, Z.; Yang, Z.; Huck, W.T. Polyelectrolyte brushes as efficient ultrathin platforms for site-selective copper electroless deposition. Langmuir 2006, 22, 6730–6733. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Zheng, Z. Programming nanostructures of polymer brushes by dip-pen nanodisplacement lithography (DNL). Nanoscale 2010, 2, 2614–2618. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, H.; Shen, Y.; Zhou, X.; Zheng, Z. Stretchable conductors with ultrahigh tensile strain and stable metallic conductance enabled by prestrained polyelectrolyte nanoplatforms. Adv. Mater. 2011, 23, 3090–3094. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, T.; Kobe, B.; Lau, W.M.; Yang, J. Grafting of polyelectrolytes onto hydrocarbon surfaces by high-energy hydrogen induced cross-linking for making metallized polymer films. Chem. Commun. 2013, 49, 4658–4660. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zeng, J.; Chen, C.; Xie, Z.; Guo, R.; Liu, Z.; Zhou, X.; Yang, Y.; Zheng, Z. Three-Dimensional Compressible and Stretchable Conductive Composites. Adv. Mater. 2014, 26, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Berthelot, T.; Viel, P.; Polesel-Maris, J.; Palacin, S. Microscopic study of a ligand induced electroless plating process onto polymers. ACS Appl. Mater. Interfaces 2010, 2, 3043–3051. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Hanifi, N.; Jousselme, B.; Jégou, P.; Palacin, S.; Viel, P.; Berthelot, T. Polymer grafting by inkjet printing: A direct chemical writing toolset. Adv. Funct. Mater. 2013, 23, 3668–3674. [Google Scholar] [CrossRef]
- Lancaster, J.R.; Jehani, J.; Carroll, G.T.; Chen, Y.; Turro, N.J.; Koberstein, J.T. Toward a universal method to pattern metals on a polymer. Chem. Mater. 2008, 20, 6583–6585. [Google Scholar] [CrossRef]
- Liu, X.; Guo, R.; Shi, Y.; Deng, L.; Li, Y. Durable, Washable, and Flexible Conductive PET Fabrics Designed by Fiber Interfacial Molecular Engineering. Macromol. Mater. Eng. 2016, 301, 1383–1389. [Google Scholar] [CrossRef]
- Garcia, A.; Berthelot, T.; Viel, P.; Mesnage, A.; Jégou, P.; Nekelson, F.; Roussel, S.; Palacin, S. ABS polymer electroless plating through a one-step poly (acrylic acid) covalent grafting. ACS Appl. Mater. Interfaces 2010, 2, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Polesel-Maris, J.; Viel, P.; Palacin, S.; Berthelot, T. Localized ligand induced electroless plating (LIEP) process for the fabrication of copper patterns onto flexible polymer substrates. Adv. Funct. Mater. 2011, 21, 2096–2102. [Google Scholar] [CrossRef]
- Mévellec, V.; Roussel, S.; Tessier, L.; Chancolon, J.; Mayne-L’Hermite, M.; Deniau, G.; Viel, P.; Palacin, S. Grafting polymers on surfaces: A new powerful and versatile diazonium salt-based one-step process in aqueous media. Chem. Mater. 2007, 19, 6323–6330. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, H.; Yu, B.; Chen, M.; Zheng, Z.; Zhou, F. Binary oppositely charged polyelectrolyte brushes for highly selective electroless deposition of bimetallic patterns. Electrochem. Commun. 2009, 11, 492–495. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, E.; Neoh, K.; Huang, W. Electroless metallization of glass surfaces functionalized by silanization and graft polymerization of aniline. Langmuir 2001, 17, 7425–7432. [Google Scholar] [CrossRef]
- Ma, Z.; Tan, K.; Alian, A.D.; Kang, E.; Neoh, K. Electroless plating of copper on poly (tetrafluoroethylene) films modified by NH3 plasma and surface graft copolymerization with aniline. J. Vac. Sci. Technol. A 2001, 19, 2471–2478. [Google Scholar] [CrossRef]
- Wu, S.; Kang, E.; Neoh, K.; Tan, K. Electroless deposition of copper on surface modified poly (tetrafluoroethylene) films from graft copolymerization and silanization. Langmuir 2000, 16, 5192–5198. [Google Scholar] [CrossRef]
- Yu, Z.; Kang, E.; Neoh, K. Amidoximation of the acrylonitrile polymer grafted on poly (tetrafluoroethylene-co-hexafluoropropylene) films and its relevance to the electroless plating of copper. Langmuir 2002, 18, 10221–10230. [Google Scholar] [CrossRef]
- Hao, Z.; Chen, H.; Ma, D. Preparation of micro gold devices on poly (dimethylsiloxane) chips with region-selective electroless plating. Anal. Chem. 2009, 81, 8649–8653. [Google Scholar] [CrossRef] [PubMed]
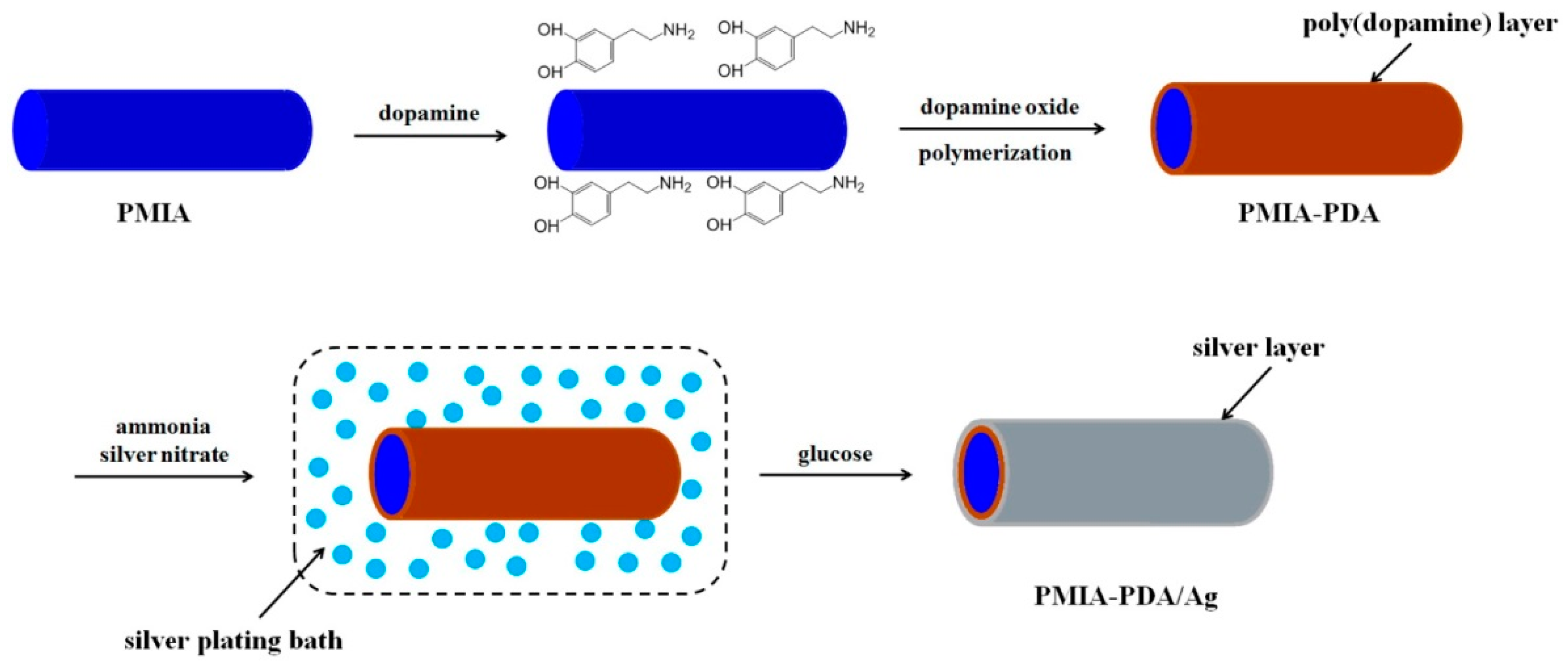
- Wang, W.; Li, R.; Tian, M.; Liu, L.; Zou, H.; Zhao, X.; Zhang, L. Surface silverized meta-aramid fibers prepared by bio-inspired poly (dopamine) functionalization. ACS Appl. Mater. Interfaces 2013, 5, 2062–2069. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chang, H.; Li, Y.; Huck, W.T.; Zheng, Z. Polyelectrolyte-bridged metal/cotton hierarchical structures for highly durable conductive yarns. ACS Appl. Mater. Interfaces 2010, 2, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Sekitani, T.; Someya, T. Stretchable, large-area organic electronics. Adv. Mater. 2010, 22, 2228–2246. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.E.H.; Aal, A.A. Conductive thin film formation onto radiation grafted polymeric surfaces using electroless plating technique. Polym. Adv. Technol. 2009, 20, 729–735. [Google Scholar] [CrossRef]
- Li, L.; Yan, G.; Wu, J.; Yu, X.; Guo, Q.; Kang, E. Electroless plating of copper on polyimide films modified by surface-initiated atom-transfer radical polymerization of 4-vinylpyridine. Appl. Surf. Sci. 2008, 254, 7331–7335. [Google Scholar] [CrossRef]
- Wang, W.; Vora, R.; Kang, E.; Neoh, K. Electroless plating of copper on fluorinated polyimide films modified by surface graft copolymerization with 1-vinylimidazole and 4-vinylpyridine. Polym. Eng. Sci. 2004, 44, 362–375. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Kang, E.; Neoh, K. Electroless deposition of copper on poly (tetrafluoroethylene) films modified by plasma-induced surface grafting of poly (4-vinylpyridine). Plasmas Polym. 2002, 7, 207–225. [Google Scholar] [CrossRef]
- Wang, W.C.; Vora, R.K.H.; Kang, E.T.; Neoh, K.G. Electroless Plating of Copper on Fluorinated Polyimide Films Modified by Plasma Graft Copolymerization and UV-induced Graft Copolymerization with 4-Vinylpyridine. Macromol. Mater. Eng. 2003, 288, 152–163. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, T.; Wang, W.C.; Zhang, L.; Jin, R. Reflective and conductive surface-silvered polyimide films prepared by surface graft copolymerization and electroless plating. Polym. Adv. Technol. 2008, 19, 335–341. [Google Scholar] [CrossRef]
- Yang, G.; Lim, C.; Tan, Y.; Zhang, Y.; Kang, E.; Neoh, K. Electroless deposition of nickel on fluoropolymers modified by surface graft copolymerization. Eur. Polym. J. 2002, 38, 2153–2160. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, Y.; Kang, E.; Neoh, K.; Wu, S.; Chow, Y. Electroless plating of copper via a Sn-free process on dielectric SiLK surface modified by UV-induced graft copolymerization with 4-vinylpyridine and 1-vinylimidazole. J. Electrochem. Soc. 2002, 149, C521–C528. [Google Scholar] [CrossRef]
- Zhu, Y.; Kang, E.; Neoh, K.; Osipowicz, T.; Chan, L. Plasma graft copolymerization of 4-vinylpyridine on dense and porous SiLK for electroless plating of copper and for retardation of copper diffusion. J. Electrochem. Soc. 2005, 152, F107–F114. [Google Scholar] [CrossRef]
- Shojaei, A.; Fathi, R.; Sheikh, N. Adhesion modification of polyethylenes for metallization using radiation-induced grafting of vinyl monomers. Surf. Coat. Technol. 2007, 201, 7519–7529. [Google Scholar] [CrossRef]
- Gates, B.D.; Xu, Q.; Stewart, M.; Ryan, D.; Willson, C.G.; Whitesides, G.M. New approaches to nanofabrication: Molding, printing, and other techniques. Chem. Rev. 2005, 105, 1171–1196. [Google Scholar] [CrossRef] [PubMed]
- Ginger, D.S.; Zhang, H.; Mirkin, C.A. Zur Entwicklung der Dip-Pen-Nanolithographie. Angew. Chem. 2004, 116, 30–46. [Google Scholar] [CrossRef]
- Haq, E.U.; Liu, Z.; Zhang, Y.; Ahmad, S.A.A.; Wong, L.-S.; Armes, S.P.; Hobbs, J.K.; Leggett, G.J.; Micklefield, J.; Roberts, C.J. Parallel scanning near-field photolithography: The snomipede. Nano Lett. 2010, 10, 4375–4380. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Kumacheva, E. Patterning surfaces with functional polymers. Nat. Mater. 2008, 7, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Boey, F.; Huo, F.; Huang, L.; Zhang, H. Chemically functionalized surface patterning. Small 2011, 7, 2273–2289. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Boey, F.; Zhang, H. Controlled growth of single-walled carbon nanotubes on patterned substrates. Chem. Soc. Rev. 2011, 40, 5221–5231. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, X.; Xie, Z.; Zheng, Z. 3D-patterned polymer brush surfaces. Nanoscale 2011, 3, 4929–4939. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, X.; Shen, Y.; Xie, Z.; Zheng, Z. Fabrication of Arbitrary Three-Dimensional Polymer Structures by Rational Control of the Spacing between Nanobrushes. Angew. Chem. Int. Ed. 2011, 50, 6506–6510. [Google Scholar] [CrossRef] [PubMed]
- Chandran, R.; Nowlin, K.; LaJeunesse, D.R. Nanosphere Lithography of Chitin and Chitosan with Colloidal and Self-Masking Patterning. Polymers 2018, 10, 218. [Google Scholar] [CrossRef]
- Nagato, K. Injection compression molding of replica molds for nanoimprint lithography. Polymers 2014, 6, 604–612. [Google Scholar] [CrossRef]




| Polymer Matrices | Polymerisation Method | Metal | Substrate |
|---|---|---|---|
| PMETAC [35,36,37,47] | SI-ATRP | Cu, Ni | Cotton, PET, PDMS, Au |
| PAA [40,42,44,45] | GraftFast, block copolymer | Cu, Ni | ABS, PET, PVDF, PAN, PB, PS |
| PVBVN [55] | Plasma grafting | Ag | PI |
| PMEP [47] | SI-ATRP | Cu, Ni | Au |
| PAA post-modified with ethylenediamine [52] | UV grafting | Au | PDMS |
| P2VP, P4VP [56,57,58,59,60,61,62,63,64] | UV, plasma, radiation grafting | Cu, Ni | PI, FPI, PTFE, silk, PVDF |
| PAM [56,65] | Radiation grafting | Cu | PP |
| PVP [65] | Radiation grafting | Cu | PE |
| PAN [51] | UV grafting | Cu | FEP |
| PHEA, PHMMAAm [50] | UV grafting | Cu | PTFE |
| PDDA [48,49] | Oxidative grafting | Cu | PTFE, glass |
| Dopamine [53] | Self-polymerisation | Ag | PIMA |
| Tannic Acid [34] | dip-coating | Ag | Catkin |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Li, Y.; Liu, X. Polymer Interface Molecular Engineering for E-Textiles. Polymers 2018, 10, 573. https://doi.org/10.3390/polym10060573
Zhu C, Li Y, Liu X. Polymer Interface Molecular Engineering for E-Textiles. Polymers. 2018; 10(6):573. https://doi.org/10.3390/polym10060573
Chicago/Turabian StyleZhu, Chuang, Yi Li, and Xuqing Liu. 2018. "Polymer Interface Molecular Engineering for E-Textiles" Polymers 10, no. 6: 573. https://doi.org/10.3390/polym10060573