Preparation, Structure, and Properties of Silk Fabric Grafted with 2-Hydroxypropyl Methacrylate Using the HRP Biocatalyzed ATRP Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
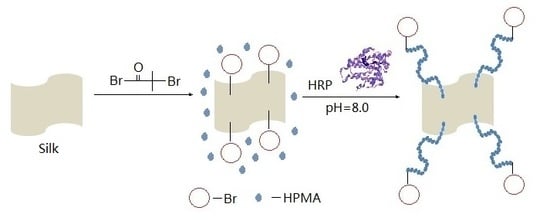
2.2. Preparation of the Silk Macroinitiator
2.3. Surface-Initiated ATRP
2.3.1. HRP-Mediated Grafting of HPMA on Silk Fabric’s Surfaces
2.3.2. CuBr-Mediated Grafting of HPMA on Silk Fabric’s Surfaces
2.3.3. Grafting Yield Calculation
2.4. Characterization and Measurements
2.4.1. Fourier Transform Infrared (FT-IR) Analysis
2.4.2. X-ray Diffraction (XRD) Analysis
2.4.3. X-ray Photoelectron Spectroscopy (XPS) Analysis
2.4.4. Thermal Properties
2.4.5. Scanning Electron Microscopy (SEM) Analysis
2.4.6. Crease-Resistant Recovery and Physical Properties Measurement
3. Results and Discussion
3.1. Fourier Transform Infrared (FTIR)Spectra and X-ray Diffraction (XRD) Curves
3.2. X-ray Photoelectron Spectroscopy (XPS)
3.3. Thermal Properties
3.4. Scanning Electron Microscopy (SEM)
3.5. Crease-Resistant Recovery
3.6. Physical Properties
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Minoura, N.; Tanioka, A. Transport of pharmaceuticals through silk fibroin membrane. Polymer 1994, 35, 2853–2856. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.T. Modification and dyeing of silk fabric treated with tetrabutyl titanate by hydrothermal method. J. Nat. Fibers 2014, 11, 25–38. [Google Scholar] [CrossRef]
- Furuzono, T.; Ishihara, K.; Nakabayashi, N.; Tamada, Y. Chemical modification of silk fibroin with 2-methacryloyloxyethyl phosphorylcholine. II. Graft-polymerization onto fabric through 2-methacryloyloxyethyl isocyanate and interaction between fabric and platelets. Biomaterials 2000, 21, 327–333. [Google Scholar] [CrossRef]
- Manickam, P.; Thilagavathi, G. A natural fungal extract for improving dyeability and antibacterial activity of silk fabric. J. Ind. Text. 2015, 44, 769–780. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, C.; Li, X.L.; Li, L.; Ren, X.H.; Liang, J.; Huang, T.S. Antibacterial efficacy of functionalized silk fabrics by radical copolymerization with quaternary ammonium salts. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, W.; Cui, Z.; He, K.; Yu, W. Studies on photoyellowing of silk fibroin and alteration of its tyrosine content. J. Text. Inst. 2016, 107, 413–419. [Google Scholar] [CrossRef]
- Shang, S.; Zhu, L.; Chen, W. Reducing silk fibrillation through MMA graft method. Fibers Polym. 2009, 10, 807–812. [Google Scholar] [CrossRef]
- Wang, P.; Yu, M.; Cui, L. Modification of Bombyx mori, silk fabrics by tyrosinase-catalyzed grafting of chitosan. Eng. Life Sci. 2014, 14, 211–217. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Tsarevsky, N.V. Nanostructured functional materials prepared by atom transfer radical polymerization. Nat. Chem. 2009, 1, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, P.V.; Averick, S.E.; Konkolewicz, D. Straightforward ARGET ATRP for the Synthesis of Primary Amine Polymethacrylate with Improved Chain-End Functionality under Mild Reaction Conditions. Macromolecules 2014, 47, 4615–4621. [Google Scholar] [CrossRef]
- Li, S.W.; Xing, T.L.; Li, Z.X.; Chen, G.Q. Structure and properties of silk grafted with acrylate fluoride monomers by ATRP. Appl. Surf. Sci. 2013, 268, 92–97. [Google Scholar] [CrossRef]
- Klaysri, R.; Wichaidit, S.; Piticharoenphun, S. Synthesis of TiO2-grafted onto PMMA film via ATRP: Using monomer as a coupling agent and reusability in photocatalytic application. Mater. Res. Bull. 2016, 83, 640–648. [Google Scholar] [CrossRef]
- Cho, H.Y.; Pawel, K.; Katarzyna, S. Synthesis of Poly(OEOMA) Using Macromonomers via “Grafting-Through” ATRP. Macromolecules 2015, 48, 6385–6395. [Google Scholar] [CrossRef]
- Teramoto, Y.; Nishio, Y. Cellulose diacetate-graft-poly (lactic acid)s: Synthesis of wide-ranging compositions and their thermal and mechanical properties. Polymer 2003, 44, 2701–2709. [Google Scholar] [CrossRef]
- Kang, H.; Liu, W.; Liu, R. A novel, amphiphilic ethyl cellulose grafting copolymer with poly (2-hydroxyethyl methacrylate) side chains and its micellization. Macromol. Chem. Phys. 2008, 209, 424–430. [Google Scholar] [CrossRef]
- Xing, T.L.; Hu, W.L.; Li, S.W. Preparation, structure and properties of multi-functional silk via ATRP method. Appl. Surf. Sci. 2012, 258, 3208–3213. [Google Scholar] [CrossRef]
- Tsarevsky, N.V.; Matyjaszewski, K. “Green” Atom Transfer Radical Polymerization: From Process Design to Preparation of Well-Defined Environmentally Friendly Polymeric Materials. Chem. Rev. 2007, 107, 2270–2299. [Google Scholar] [CrossRef] [PubMed]
- Mueller, L.; Matyjaszewski, K. Reducing Copper Concentration in Polymers Prepared via Atom Transfer Radical Polymerization. Macromol. React. Eng. 2010, 4, 180–185. [Google Scholar] [CrossRef]
- Liu, R.; Dong, A.X.; Fan, X.R. HRP-mediated polyacrylamide graft modification of raw jute fabric. J. Mol. Catal. B Enzym. 2015, 116, 29–38. [Google Scholar] [CrossRef]
- Sigg, S.J.; Seidi, F.; Renggli, K.; Silva, T.B.; Kali, G.; Bruns, N. Horseradish peroxidase as a catalyst for atom transfer radical polymerization. Macromol. Rapid Commun. 2011, 32, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Renggli, K.; Sauter, N.; Rother, M.; Nussbaumer, M.G.; Urbani, R.; Pfohl, T.; Bruns, N. Biocatalytic atom transfer radical polymerization in a protein cage nanoreactor. Polym. Chem. 2017, 8, 2133–2136. [Google Scholar] [CrossRef]
- Pan, A.; He, L.; Yang, S. The effect of side chains on the reactive rate and surface wettability of pentablock copolymers by ATRP. J. Appl. Polym. Sci. 2014, 131, 742–751. [Google Scholar] [CrossRef]
- Demirelli, K.; Coskun, M.F.; Kaya, E.; Coskun, M. Investigation of the thermal decomposition of poly(2-hydroxypropyl methacrylate). Polym. Degrad. Stab. 2002, 78, 333–339. [Google Scholar] [CrossRef]






| Silk Sample | Element Content/% | C/N | ||
|---|---|---|---|---|
| C | O | N | ||
| Control | 69.98 | 24.08 | 5.94 | 11.78 |
| silk-g-PHPMA | 71.20 | 26.31 | 2.49 | 28.59 |
| Grafting Samples | Grafting Yield/% | DCRA/° | WCRA/° |
|---|---|---|---|
| 0 | 201 | 128 | |
| HC-silk-g-PHPMA | 15.62 | 220 | 168 |
| 29.01 | 233 | 180 | |
| 38.87 | 238 | 194 | |
| CC-silk-g-PHPMA | 37.82 | 229 | 195 |
| Grafting Samples | Grafting Yield/% | Whiteness/% | Breaking Strength/N | Moisture Regain/% |
|---|---|---|---|---|
| 0 | 79.02 | 479.74 | 8.45 | |
| HC-silk-g-PHPMA | 15.62 | 75.68 | 428.25 | 8.03 |
| 29.01 | 74.26 | 415.36 | 7.96 | |
| 38.87 | 70.39 | 391.59 | 7.78 | |
| CC-silk-g-PHPMA | 37.82 | 70.23 | 396.86 | 7.98 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Lu, S.; Xing, T.; Chen, G. Preparation, Structure, and Properties of Silk Fabric Grafted with 2-Hydroxypropyl Methacrylate Using the HRP Biocatalyzed ATRP Method. Polymers 2018, 10, 557. https://doi.org/10.3390/polym10050557
Yang J, Lu S, Xing T, Chen G. Preparation, Structure, and Properties of Silk Fabric Grafted with 2-Hydroxypropyl Methacrylate Using the HRP Biocatalyzed ATRP Method. Polymers. 2018; 10(5):557. https://doi.org/10.3390/polym10050557
Chicago/Turabian StyleYang, Jinqiu, Shenzhou Lu, Tieling Xing, and Guoqiang Chen. 2018. "Preparation, Structure, and Properties of Silk Fabric Grafted with 2-Hydroxypropyl Methacrylate Using the HRP Biocatalyzed ATRP Method" Polymers 10, no. 5: 557. https://doi.org/10.3390/polym10050557