Isothermal and Nonisothermal Crystallization Kinetics of Poly(ε-caprolactone) Blended with a Novel Ionic Liquid, 1-Ethyl-3-propylimidazolium Bis(trifluoromethanesulfonyl)imide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Blend Preparation
2.2. Instruments and Experiments
3. Results and Discussion
3.1. Phase Morphology and Thermal Analysis of the [EPrI][TFSI]/PCL Blends
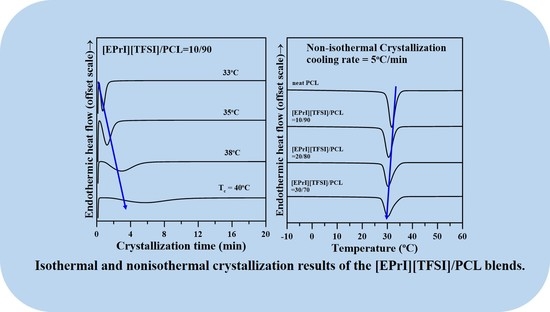
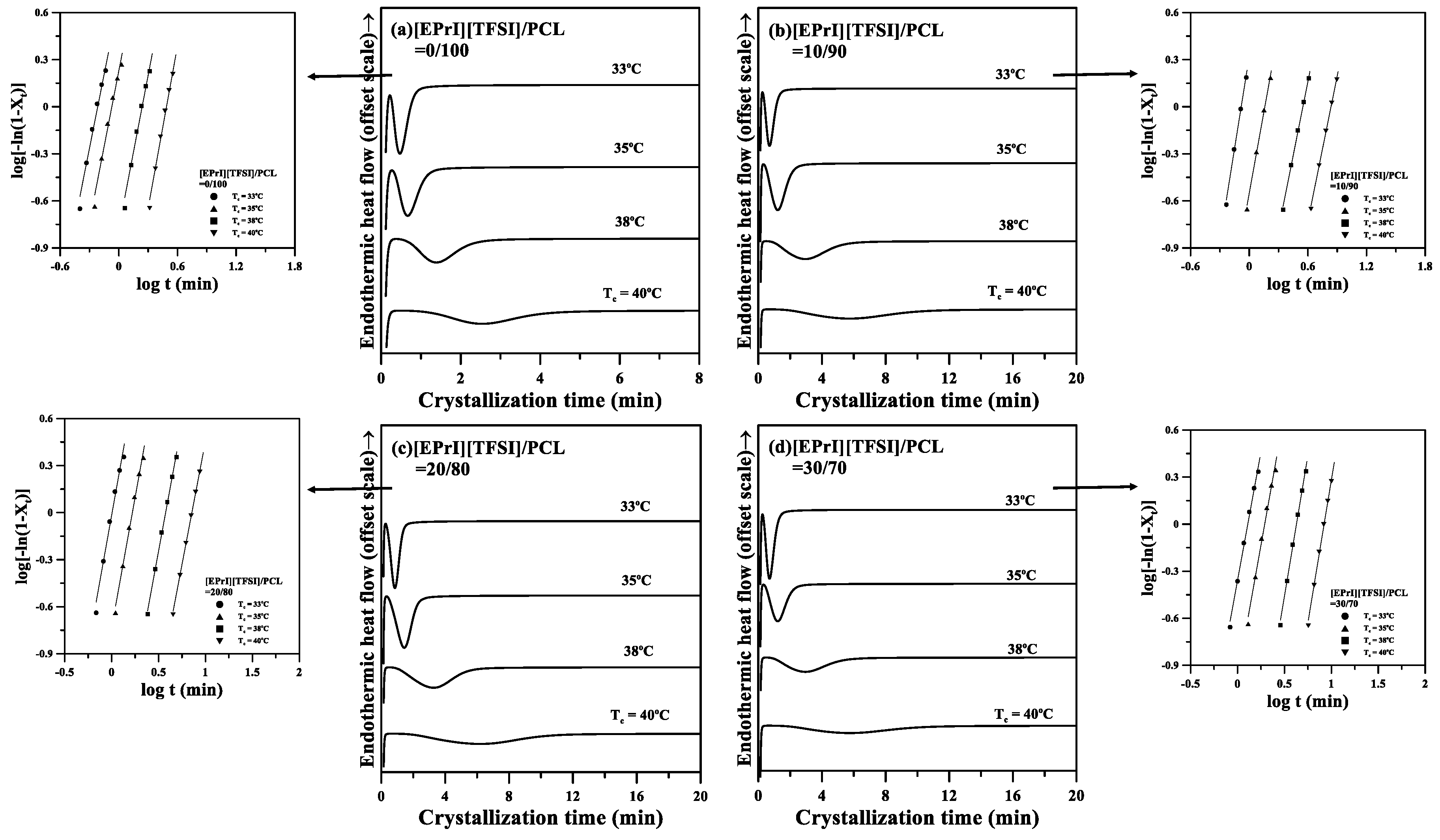
3.2. Isothermal Crystallization Kinetics of the [EPrI][TFSI]/PCL Blends
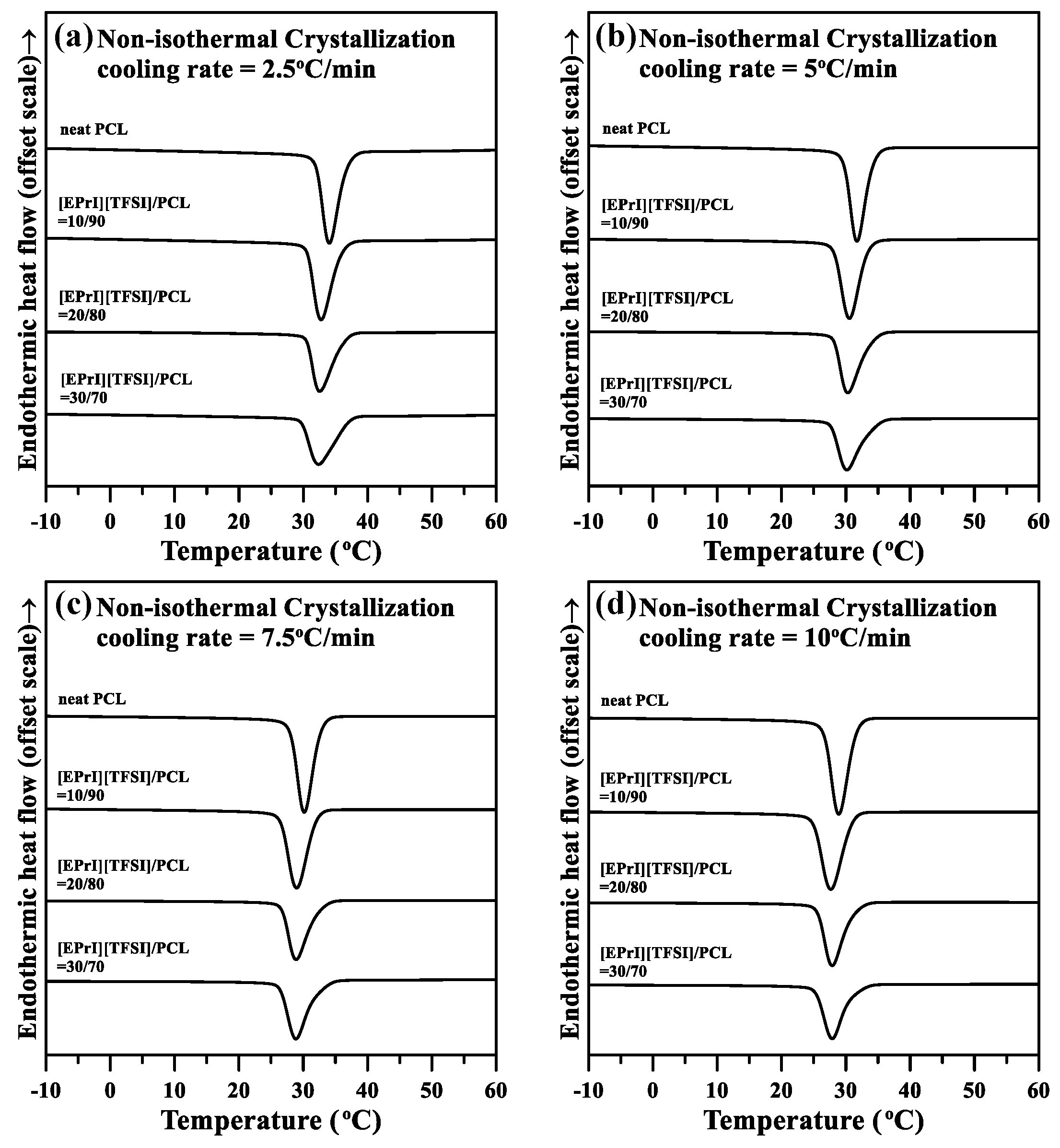
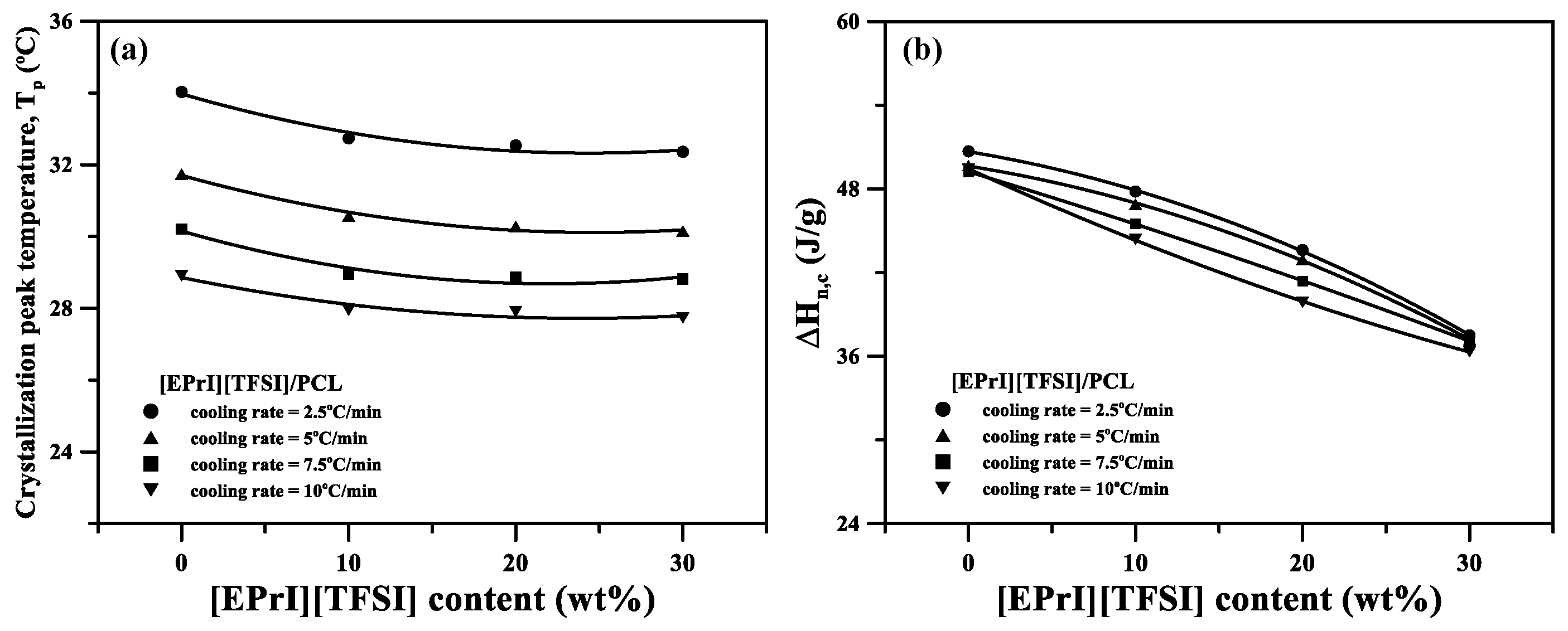
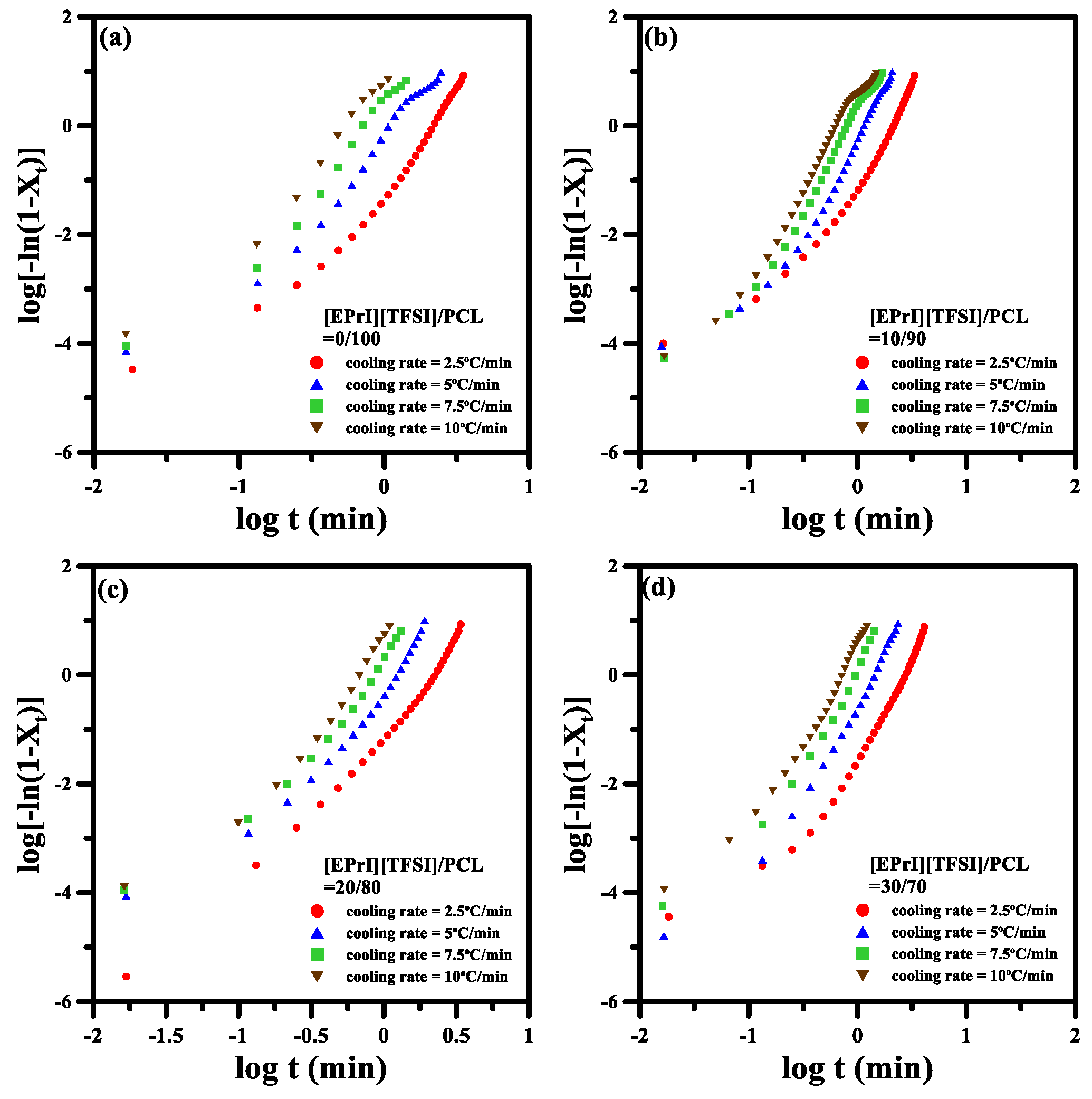
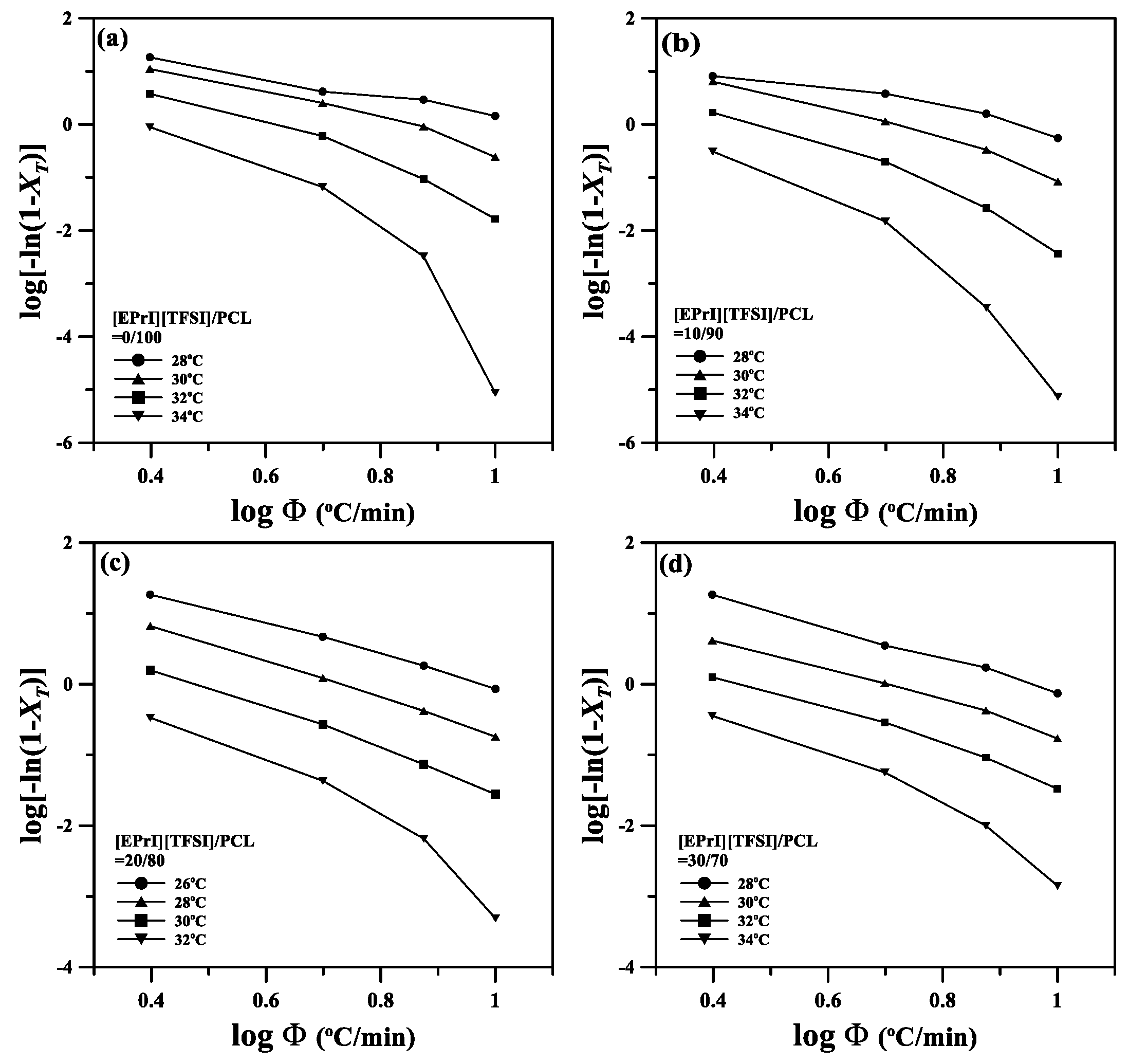
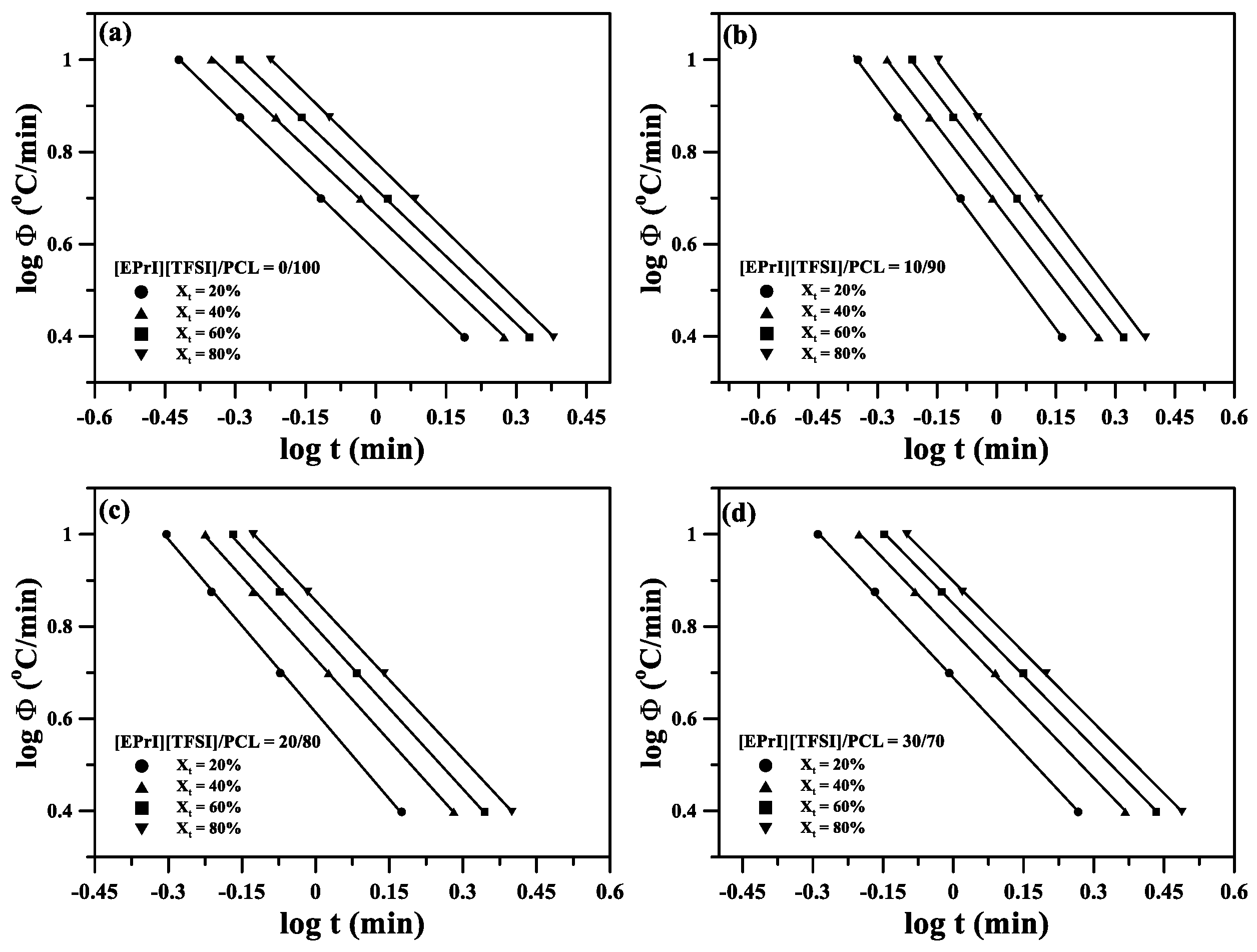
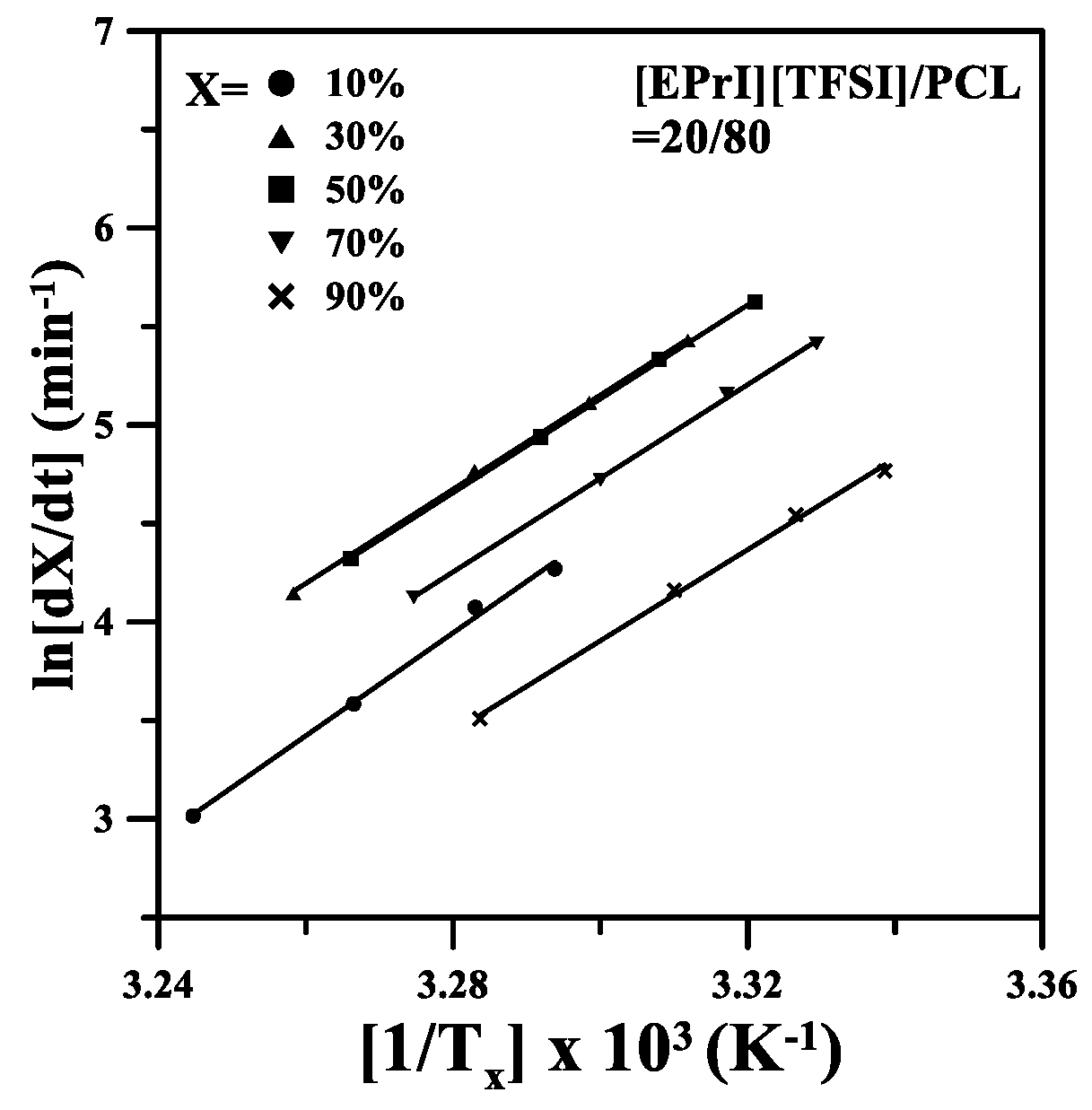
3.3. Nonisothermal Crystallization Kinetics of the [EPrI][TFSI]/PCL Blends
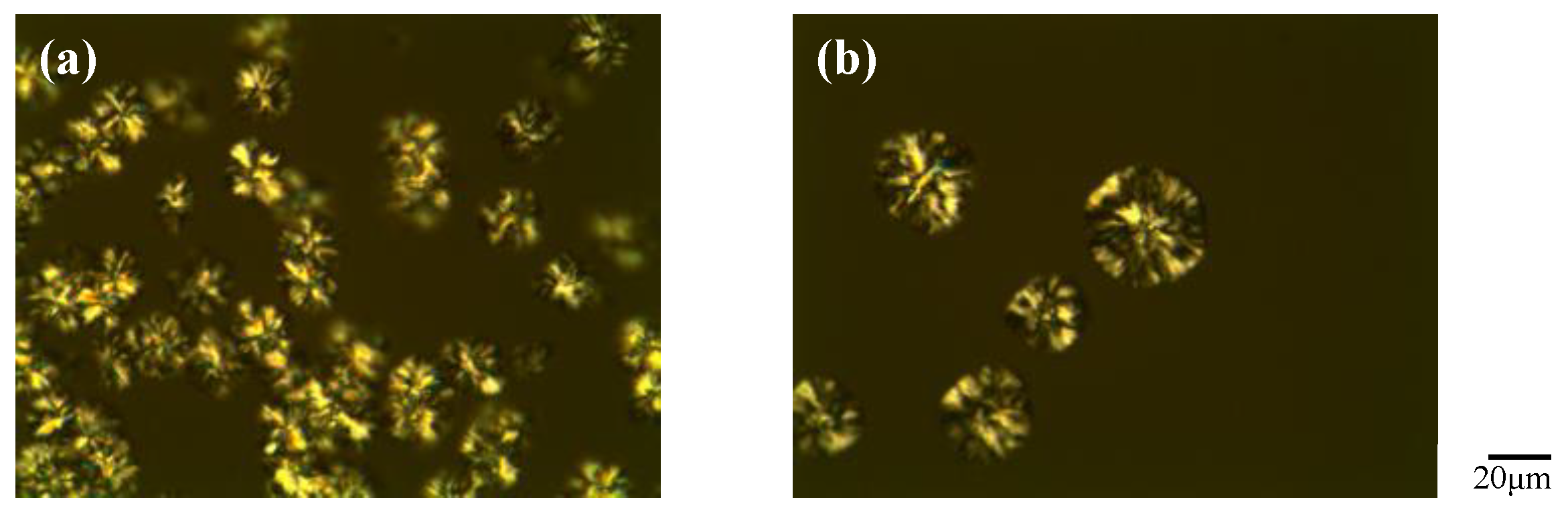
3.4. Polarizing Optical Microscopy (POM) Observations for [EPrI][TFSI]/PCL Blends under Cooling
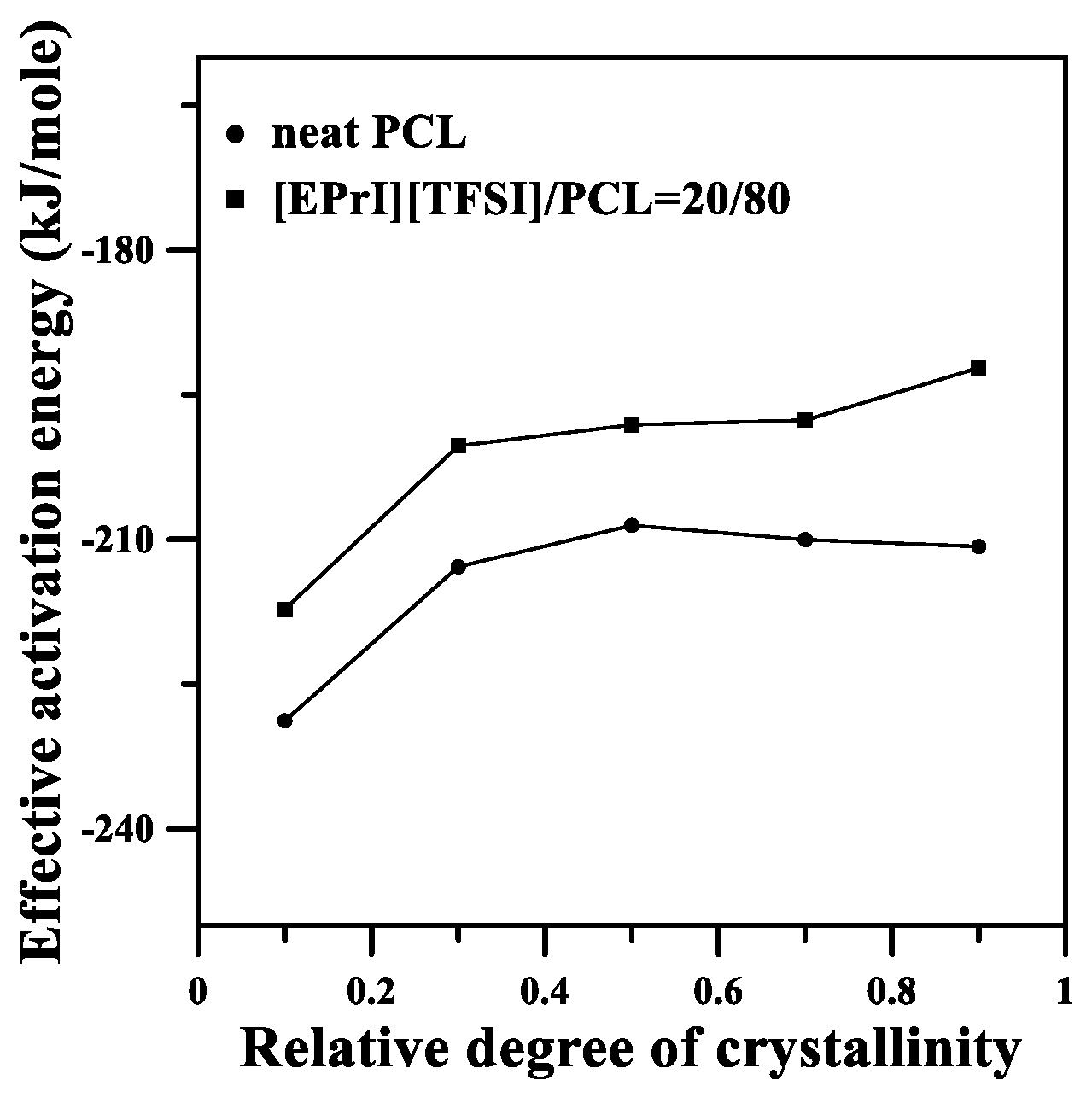
3.5. Studies of Effective Ectivation Energy
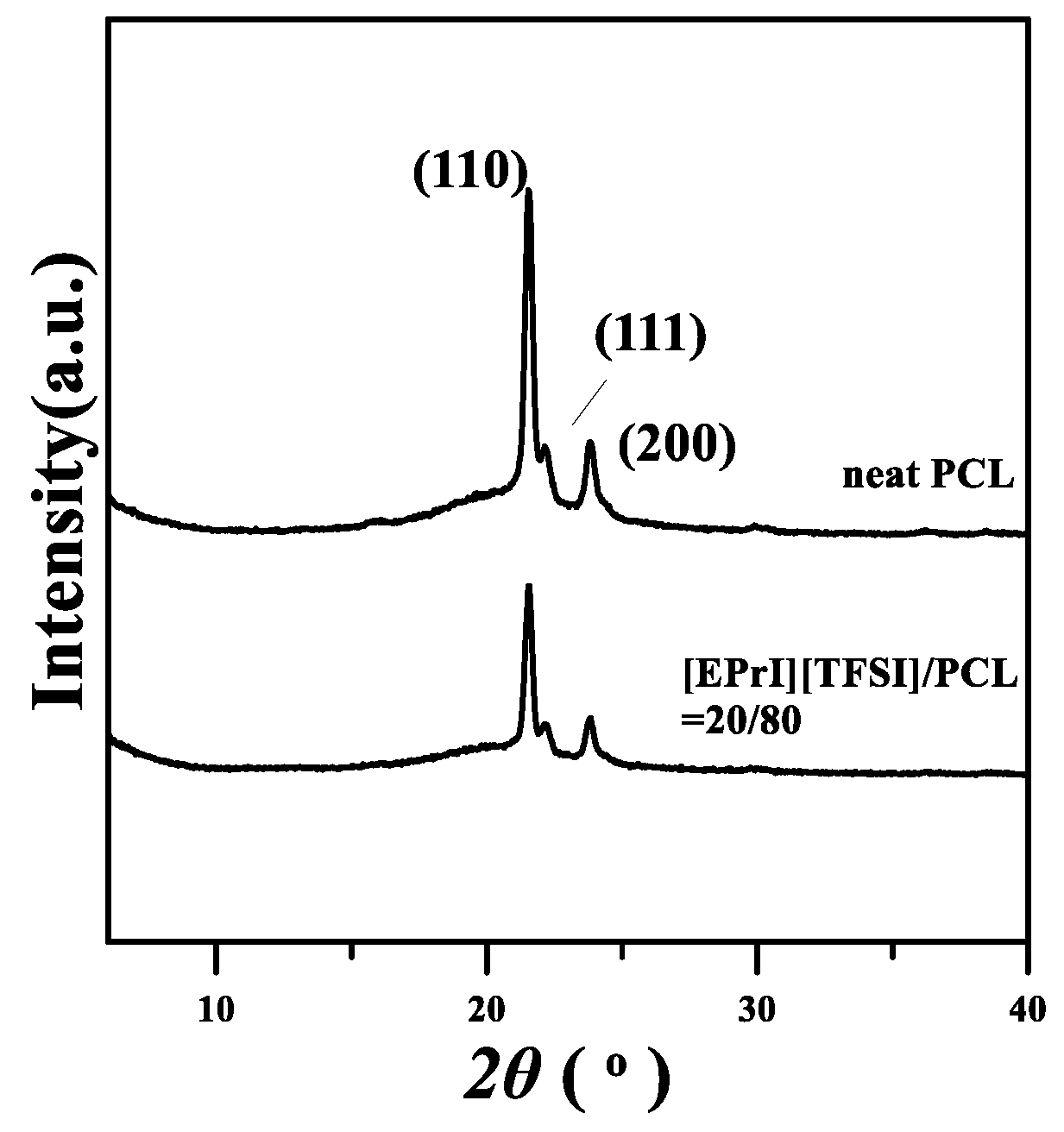
3.6. Wide-Angle X-ray Diffraction (WAXD) Analyzes for Crystal Structures of [EPrI][TFSI]/PCL Blends
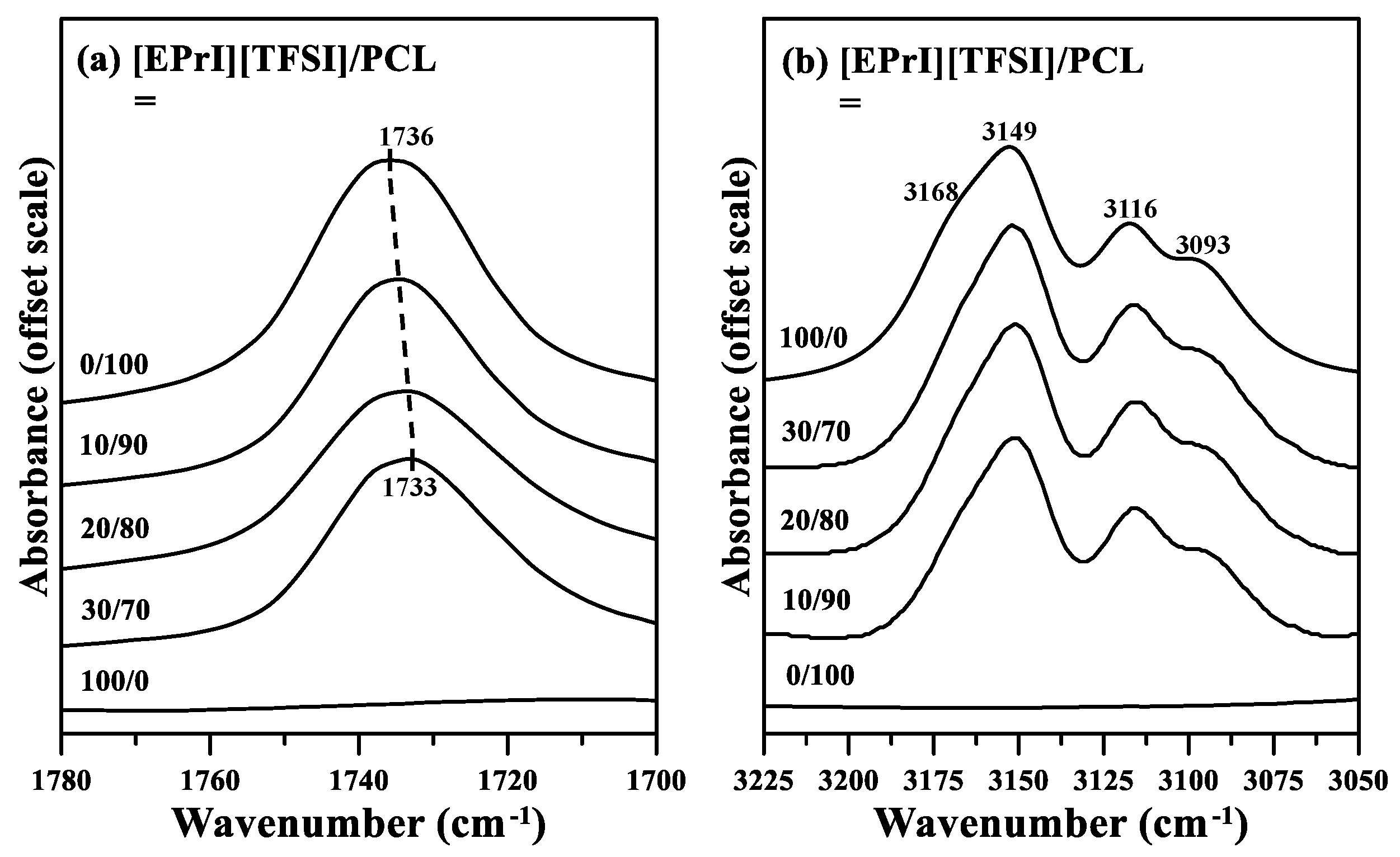
3.7. Possible Interactions between [EPrI][TFSI] and PCL in the Blends
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Xiao, H.; Lu, W.; Yeh, J.T. Effect of plasticizer on the crystallization behavior of poly(lactic acid). J. Appl. Polym. Sci. 2009, 113, 112–121. [Google Scholar] [CrossRef]
- Qiu, Z.; Ikehara, T.; Nishi, T. Crystallization behaviour of biodegradable poly(ethylene succinate) from the amorphous state. Polymer 2003, 44, 5429–5437. [Google Scholar] [CrossRef]
- Chandra, R.; Rustgi, R. Biodegradable polymers. Prog. Polym. Sci. 1998, 23, 1273–1335. [Google Scholar] [CrossRef]
- Gross, R.A.; Kalra, B. Biodegradable polymers for the environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Dhanvijay, P.U.; Shertukde, V.V.; Kalkar, A.K. Isothermal and nonisothermal crystallization kinetics of poly(ε-caprolactone). J. Appl. Polym. Sci. 2012, 124, 1333–1343. [Google Scholar] [CrossRef]
- Xing, Z.; Zha, L.; Yang, G. Thermomechanical behavior and nonisothermal crystallization kinetics of poly(ε-caprolactone) and poly(ε-caprolactone)/poly(N-vinylpyrrolidone) blends. e-Polymers 2010, 121, 1–13. [Google Scholar] [CrossRef]
- Liu, Q.; Deng, B.; Zhu, M.; Shyr, T.W.; Shan, G. Nonisothermal crystallization kinetics of poly(ε-caprolactone)/zinc oxide nanocomposites with high zinc oxide content. J. Macromol. Sci. Part B Phys. 2011, 50, 2366–2375. [Google Scholar] [CrossRef]
- Hua, L.; Kai, W.H.; Inoue, Y. Crystallization behavior of poly(ε-caprolactone)/graphite oxide composites. J. Appl. Polym. Sci. 2007, 106, 4225–4232. [Google Scholar] [CrossRef]
- Piao, L.; Dai, Z.; Deng, M.; Chen, X.; Jing, X. Synthesis and characterization of PCL/PEG/PCL triblock copolymers by using calcium catalyst. Polymer 2003, 44, 2025–2031. [Google Scholar] [CrossRef]
- Shin, K.; Dong, T.; He, Y.; Taguchi, Y.; Oishi, A.; Nishida, H.; Inoue, Y. Inclusion complex formation between ε-Cyclodextrin and biodegradable aliphatic polyesters. Macromol. Biosci. 2004, 4, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Prud’homme, R.E. Crystallization of poly(ε-caprolactone)/poly(vinyl chloride) miscible blends under strain: The role of molecular weight. Macromol. Rapid Commun. 2006, 27, 1565–1571. [Google Scholar] [CrossRef]
- Chen, X.; Gross, R.A. Versatile copolymers from [l]-lactide and [d]-xylofuranose. Macromolecules 1999, 32, 308–314. [Google Scholar] [CrossRef]
- Seoane, I.T.; Manfredi, L.B.; Cyras, V.P. Effect of two different plasticizers on the properties of poly(3-hydroxybutyrate) binary and ternary blends. J. Appl. Polym. Sci. 2018, 135, 46016. [Google Scholar] [CrossRef]
- Shibita, A.; Mizumura, Y.; Shibata, M. Stereocomplex crystallization behavior and physical properties of polyesterurethane networks incorporating diglycerol-based enantiomeric 4-armed lactide oligomers and a 1,3-propanediol-based 2-armed rac-lactide oligomer. Polym. Bull. 2017, 74, 3139–3160. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Bikiaris, D.N.; Guigo, N.; Exarhopoulos, S.; Papageorgiou, D.G.; Sbirrazzuoli, N.; Papageorgiou, G.Z. Synthesis, properties and thermal behavior of poly(decylene-2,5-furanoate): A biobased polyester from 2,5-furan dicarboxylic acid. RSC Adv. 2015, 5, 74592–74604. [Google Scholar] [CrossRef]
- Chang, L.; Woo, E.M. Crystallization of poly(3-hydroxybutyrate) with stereocomplexed polylactide as biodegradable nucleation agent. Polym. Eng. Sci. 2012, 52, 1413–1419. [Google Scholar] [CrossRef]
- Cui, Z.; Qiu, Z. Thermal properties and crystallization kinetics of poly(butylene suberate). Polymer 2015, 67, 12–19. [Google Scholar] [CrossRef]
- Palacios, J.K.; Zhao, J.; Hadjichristidis, N.; Müller, A.J. How the Complex Interplay between Different Blocks Determines the Isothermal Crystallization Kinetics of Triple-Crystalline PEO-b-PCL-b-PLLA Triblock Terpolymers. Macromolecules 2017, 50, 9683–9695. [Google Scholar] [CrossRef]
- Lai, W.C.; Liau, W.B.; Lin, T.T. The effect of end groups of PEG on the crystallization behaviors of binary crystalline polymer blends PEG/PLLA. Polymer 2004, 45, 3073–3080. [Google Scholar] [CrossRef]
- Maiz, J.; Schäfer, H.; Rengarajan, G.T.; Hartmann-Azanza, B.; Eickmeier, H.; Haase, M.; Mijangos, C.; Steinhart, M. How gold nanoparticles influence crystallization of polyethylene in rigid cylindrical nanopores. Macromolecules 2013, 46, 403–412. [Google Scholar] [CrossRef]
- Yuan, Q.; Awate, S.; Misra, R. Nonisothermal crystallization behavior of polypropylene-clay nanocomposites. Eur. Polym. J. 2006, 42, 1994–2003. [Google Scholar] [CrossRef]
- Joshi, M.; Butola, B. Studies on nonisothermal crystallization of HDPE/POSS nanocomposites. Polymer 2004, 45, 4953–4968. [Google Scholar] [CrossRef]
- Choi, J.; Chun, S.W.; Kwak, S.Y. Non-Isothermal crystallization of hyperbranched poly(ε-caprolactone)s and their linear counterpart. Macromol. Chem. Phys. 2006, 207, 1166–1173. [Google Scholar] [CrossRef]
- Long, Y.; Shanks, R.A.; Stachurski, Z.H. Kinetics of polymer crystallisation. Prog. Polym. Sci. 1995, 20, 651–701. [Google Scholar] [CrossRef]
- Tadmor, Z.; Gogos, C.G. Principles of Polymer Processing, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Seddon, K.R.; Stark, A.; Torres, M.J. Influence of chloride, water, and organic solvents on the physical properties of ionic liquids. Pure Appl. Chem. 2000, 72, 2275–2287. [Google Scholar] [CrossRef]
- Holbrey, J.; Seddon, K. Ionic liquids. Clean Technol. Environ. Policy 1999, 1, 223–236. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Ye, Y.S.; Rick, J.; Hwang, B.J. Ionic liquid polymer electrolytes. J. Mater. Chem. A 2013, 1, 2719–2743. [Google Scholar] [CrossRef]
- Earle, M.J.; Seddon, K.R. Ionic liquids. Green solvents for the future. Pure Appl. Chem. 2000, 72, 1391–1398. [Google Scholar] [CrossRef]
- Earle, M.J.; Esperança, J.M.; Gilea, M.A.; Lopes, J.N.C.; Rebelo, L.P.; Magee, J.W.; Seddon, K.R.; Widegren, J.A. The distillation and volatility of ionic liquids. Nature 2006, 439, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Fredlake, C.P.; Crosthwaite, J.M.; Hert, D.G.; Aki, S.N.; Brennecke, J.F. Thermophysical properties of imidazolium-based ionic liquids. J. Chem. Eng. Data 2004, 49, 954–964. [Google Scholar] [CrossRef]
- Chaurasia, S.K.; Singh, R.K.; Chandra, S. Ion-polymer and ion-ion interaction in PEO-based polymer electrolytes having complexing salt LiClO4 and/or ionic liquid, [BMIM][PF6]. J. Raman Spectrosc. 2011, 42, 2168–2172. [Google Scholar] [CrossRef]
- Schäfer, T.; Di Paolo, R.E.; Franco, R.; Crespo, J.G. Elucidating interactions of ionic liquids with polymer films using confocal Raman spectroscopy. Chem. Commun. 2005, 50, 2594–2596. [Google Scholar]
- Wu, T.Y.; Chen, B.K.; Kuo, C.W.; Hao, L.; Peng, Y.C.; Sun, I.W. Standard entropy, surface excess entropy, surface enthalpy, molar enthalpy of vaporization, and critical temperature of bis(trifluoromethanesulfonyl) imide-based ionic liquids. J. Taiwan Inst. Chem. Eng. 2012, 43, 860–867. [Google Scholar] [CrossRef]
- Pizzoli, M.; Scandola, M.; Ceccorulli, G. Crystallization and melting of isotactic poly(3-hydroxy butyrate) in the presence of a low molecular weight diluent. Macromolecules 2002, 35, 3937–3941. [Google Scholar] [CrossRef]
- Hoffman, J.D.; Weeks, J.J. Melting process and the equilibrium melting temperature of polychlorotrifluoroethylene. Res. Natl. Bur. Stand. A 1962, 66, 13–28. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change II Transformation-time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change III Granulation, phase change, and microstructure. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Balsamo, V.; Calzadilla, N.; Mora, G.; Müller, A.J. Thermal characterization of polycarbonate/polycaprolactone blends. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 771–785. [Google Scholar] [CrossRef]
- L’abee, R.; Van Duin, M.; Goossens, H. Crystallization kinetics and crystalline morphology of poly(ε-caprolactone) in blends with grafted rubber particles. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1438–1448. [Google Scholar] [CrossRef]
- Remiro, P.M.; Cortazar, M.M.; Calahorra, M.E.; Calafel, M.M. Miscibility and crystallization of an amine-cured epoxy resin modified with crystalline poly(ε-caprolactone). Macromol. Chem. Phys. 2001, 202, 1077–1088. [Google Scholar] [CrossRef]
- Wunderlich, B. Macromolecular Physics; Academic Press: New York, NY, USA, 1976; Volume 2, p. 147. [Google Scholar]
- Jeziorny, A. Parameters characterizing the kinetics of the non-isothermal crystallization of poly(ethylene terephthalate) determined by d.s.c. Polymer 1978, 19, 1142–1144. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetics of non-isothermal crystallization. Polymer 1971, 12, 150–158. [Google Scholar] [CrossRef]
- Liu, T.X.; Mo, Z.S.; Wang, S.G.; Zhang, H.F. Nonisothermal melt and cold crystallization kinetics of poly(aryl ether ether ketone ketone). Polym. Eng. Sci. 1997, 37, 568–575. [Google Scholar] [CrossRef]
- Su, Z.; Guo, W.; Liu, Y.; Li, Q.; Wu, C. Non-isothermal crystallization kinetics of poly(lactic acid)/modified carbon black composite. Polym. Bull. 2009, 62, 629–642. [Google Scholar] [CrossRef]
- Mohsen-Nia, M.; Memarzadeh, M.R. Characterization and non-isothermal crystallization behavior of biodegradable poly(ethylene sebacate)/SiO2 nanocomposites. Polym. Bull. 2013, 70, 2471–2491. [Google Scholar] [CrossRef]
- Auliawan, A.; Woo, E.M. Crystallization kinetics and degradation of nanocomposites based on ternary blend of poly(l-lactic acid), poly(methyl methacrylate), and poly(ethylene oxide) with two different organoclays. J. Appl. Polym. Sci. 2012, 125, E444–E458. [Google Scholar] [CrossRef]
- Friedman, H. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Vassiliou, A.A.; Papageorgiou, G.Z.; Achilias, D.S.; Bikiaris, D.N. Non-isothermal crystallisation kinetics of in situ prepared poly(ε-caprolactone)/surface-treated SiO2 nanocomposites. Macromol. Chem. Phys. 2007, 208, 364–376. [Google Scholar] [CrossRef]
- Papadimitriou, S.A.; Papageorgiou, G.Z.; Bikiaris, D.N. Crystallization and enzymatic degradation of novel poly(ε-caprolactone-co-propylene succinate) copolymers. Eur. Polym. J. 2008, 44, 2356–2366. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, R.K. Development of ion conducting polymer gel electrolyte membranes based on polymer PVdF-HFP, BMIMTFSI ionic liquid and the Li-salt with improved electrical, thermal and structural properties. J. Mater. Chem. C 2015, 3, 7305–7318. [Google Scholar]
- Lim, J.S.; Noda, I.; Im, S.S. Effects of metal ion-carbonyl interaction on miscibility and crystallization kinetic of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)/lightly ionized PBS. Eur. Polym. J. 2008, 44, 1428–1440. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Bikiaris, D.N.; Panayiotou, C.G. Novel miscible poly(ethylene sebacate)/poly(4-vinyl phenol) blends: Miscibility, melting behavior and crystallization study. Polymer 2011, 52, 4553–4561. [Google Scholar] [CrossRef]



| [EPrI][TFSI]/PCL (wt %) | ΔHm (J/g) | Tm (°C) |
|---|---|---|
| 0/100 | 52.06 | 56.83 |
| 10/90 | 49.61 | 55.76 |
| 20/80 | 43.04 | 54.83 |
| 30/70 | 39.38 | 53.18 |
| [EPrI][TFSI]/PCL (wt %) | Tc (°C) | n | k (min−n) | t0.5 (min) | 1/t0.5 (min−1) |
|---|---|---|---|---|---|
| 0/100 | 33 | 3.14 | 4.775 | 0.54 | 1.85 |
| 35 | 3.21 | 1.694 | 0.76 | 1.32 | |
| 38 | 3.26 | 0.167 | 1.55 | 0.65 | |
| 40 | 3.44 | 0.021 | 2.76 | 0.36 | |
| 10/90 | 33 | 3.10 | 1.466 | 0.79 | 1.27 |
| 35 | 3.45 | 0.28 | 1.29 | 0.77 | |
| 38 | 3.12 | 0.019 | 3.15 | 0.32 | |
| 40 | 3.08 | 2.6 10−3 | 6.09 | 0.16 | |
| 20/80 | 33 | 3.35 | 0.962 | 0.91 | 1.10 |
| 35 | 3.32 | 0.185 | 1.49 | 0.67 | |
| 38 | 3.26 | 0.013 | 3.39 | 0.30 | |
| 40 | 3.15 | 2.046 10−3 | 6.36 | 0.16 | |
| 30/70 | 33 | 3.28 | 0.438 | 1.15 | 0.87 |
| 35 | 3.36 | 0.106 | 1.75 | 0.57 | |
| 38 | 3.58 | 5.585 10−3 | 3.85 | 0.26 | |
| 40 | 3.63 | 4.406 10−4 | 7.60 | 0.13 |
| [EPrI][TFSI]/PCL (wt %) | Xt (%) | a | F(T) |
|---|---|---|---|
| 0/100 | 20 | 0.99 | 3.80 |
| 40 | 0.97 | 4.62 | |
| 60 | 0.97 | 5.25 | |
| 80 | 1.00 | 5.99 | |
| 10/90 | 20 | 1.16 | 3.90 |
| 40 | 1.13 | 4.87 | |
| 60 | 1.12 | 5.72 | |
| 80 | 1.15 | 6.69 | |
| 20/80 | 20 | 1.25 | 4.11 |
| 40 | 1.18 | 5.37 | |
| 60 | 1.17 | 6.27 | |
| 80 | 1.14 | 7.18 | |
| 30/70 | 20 | 1.09 | 4.88 |
| 40 | 1.06 | 6.15 | |
| 60 | 1.04 | 7.07 | |
| 80 | 1.02 | 7.90 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-T.; Lee, L.-T.; Wu, T.-Y. Isothermal and Nonisothermal Crystallization Kinetics of Poly(ε-caprolactone) Blended with a Novel Ionic Liquid, 1-Ethyl-3-propylimidazolium Bis(trifluoromethanesulfonyl)imide. Polymers 2018, 10, 543. https://doi.org/10.3390/polym10050543
Yang C-T, Lee L-T, Wu T-Y. Isothermal and Nonisothermal Crystallization Kinetics of Poly(ε-caprolactone) Blended with a Novel Ionic Liquid, 1-Ethyl-3-propylimidazolium Bis(trifluoromethanesulfonyl)imide. Polymers. 2018; 10(5):543. https://doi.org/10.3390/polym10050543
Chicago/Turabian StyleYang, Chun-Ting, Li-Ting Lee, and Tzi-Yi Wu. 2018. "Isothermal and Nonisothermal Crystallization Kinetics of Poly(ε-caprolactone) Blended with a Novel Ionic Liquid, 1-Ethyl-3-propylimidazolium Bis(trifluoromethanesulfonyl)imide" Polymers 10, no. 5: 543. https://doi.org/10.3390/polym10050543