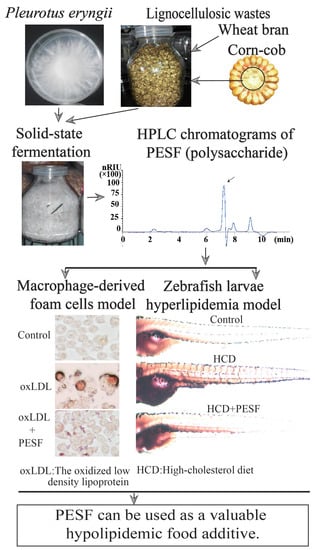
Lipid-Lowering Effect of the Pleurotus eryngii (King Oyster Mushroom) Polysaccharide from Solid-State Fermentation on Both Macrophage-Derived Foam Cells and Zebrafish Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Preparation
2.2. P. eryngii Mycelia Solid-State Fermentation, Preparation and Detection of PESF
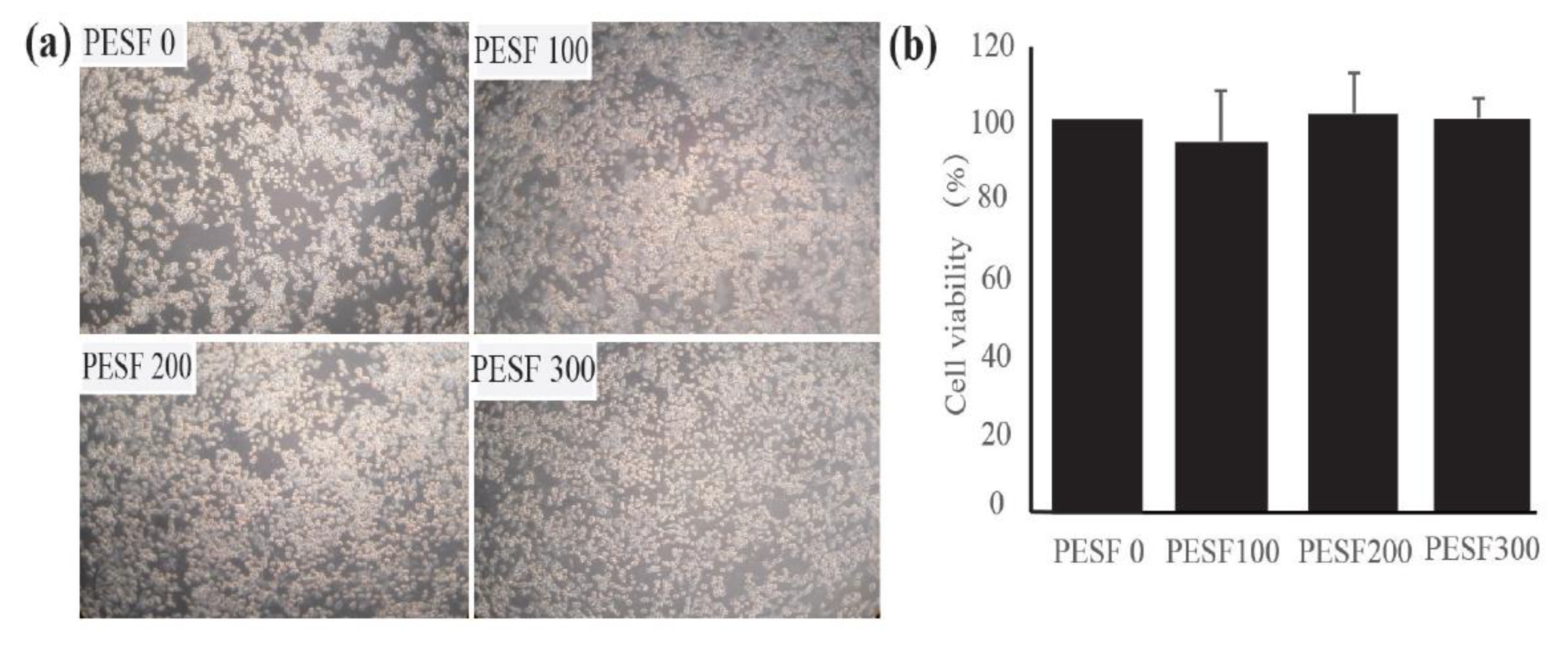
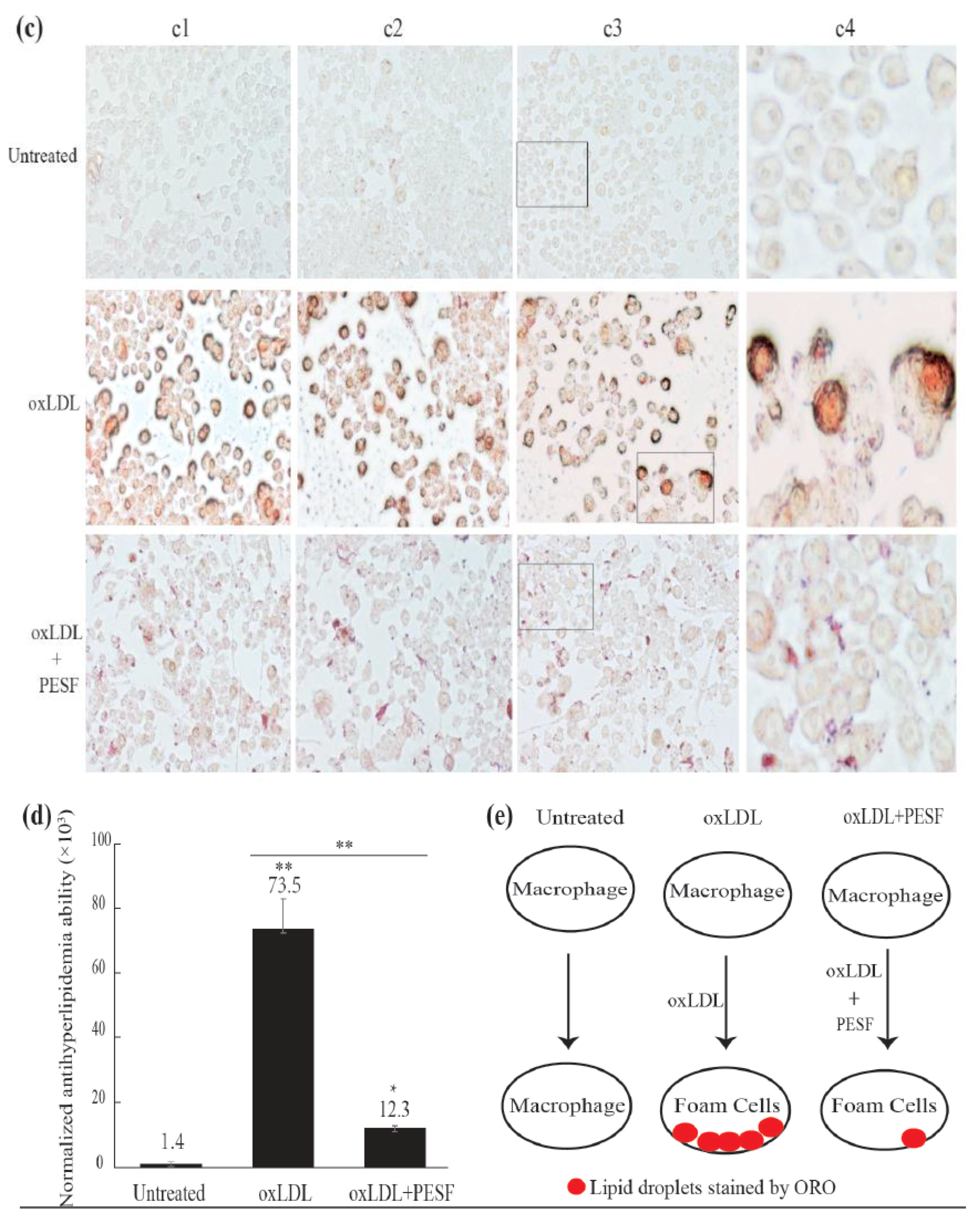
2.3. Cell Culture, Cell Oil Red O (ORO) Staining, MTT Assay
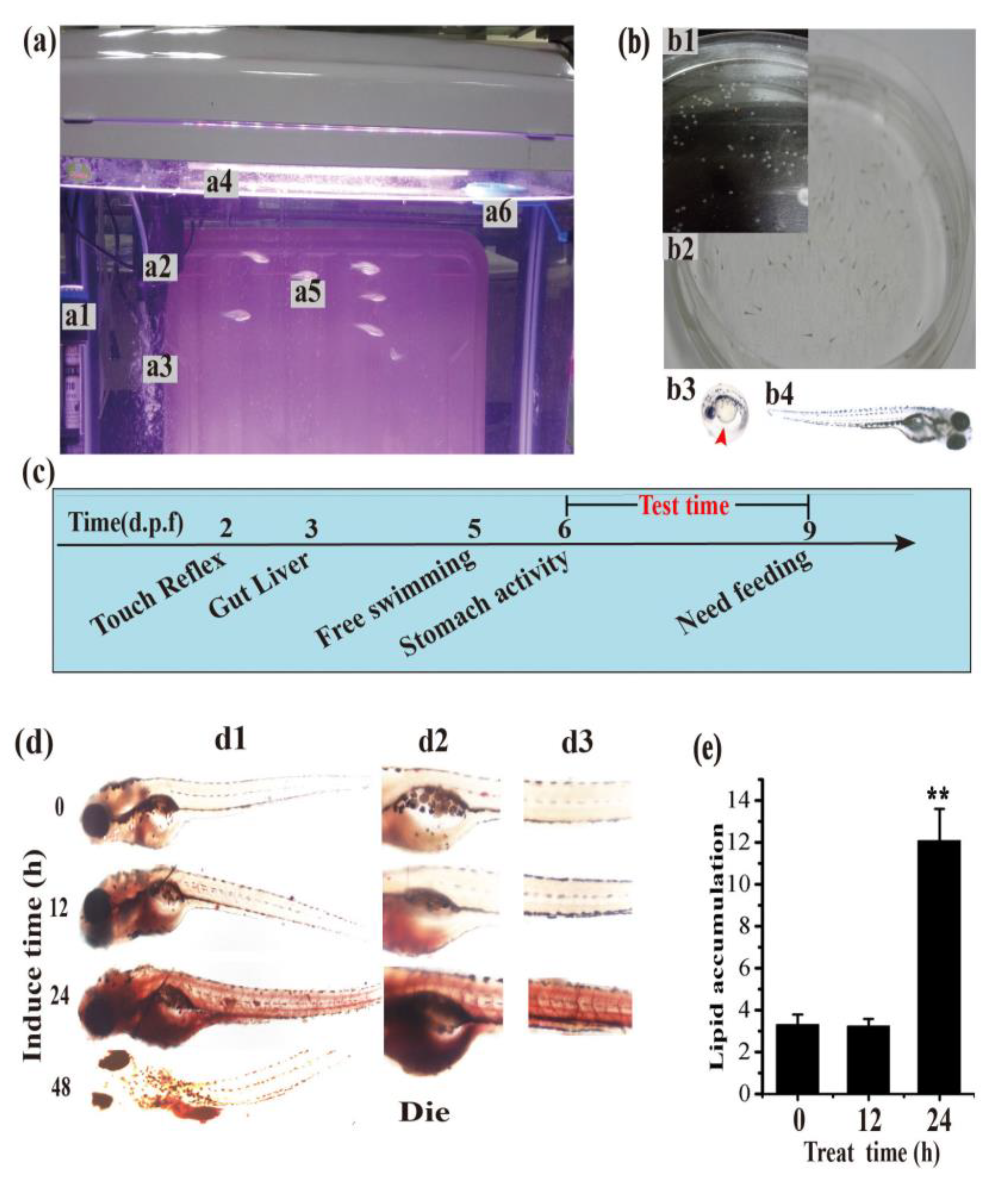
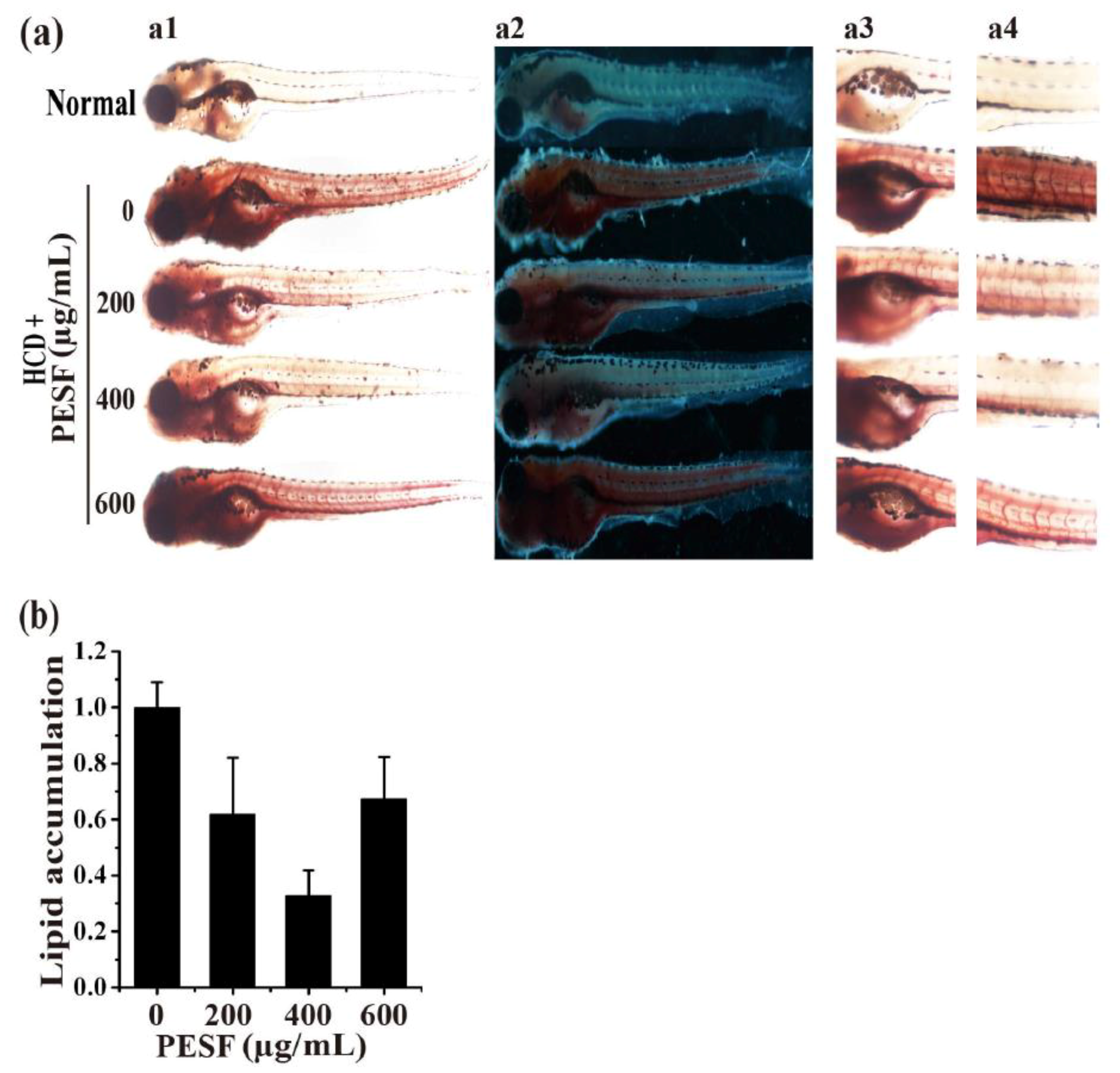
2.4. Zebrafish Handling, ORO Staining and Intensity Quantification
3. Results and Discussions
3.1. Extraction and Detection of Polysaccharides
3.2. Safety Assessment and Lipid-Lowering Effect of PESF in a Macrophage-Derived Foam Cell Model
3.3. Establishment of the Hyperlipidemia Zebrafish Model
3.4. Lipid-Lowering Effect of PESF in a Zebrafish Larvae Model
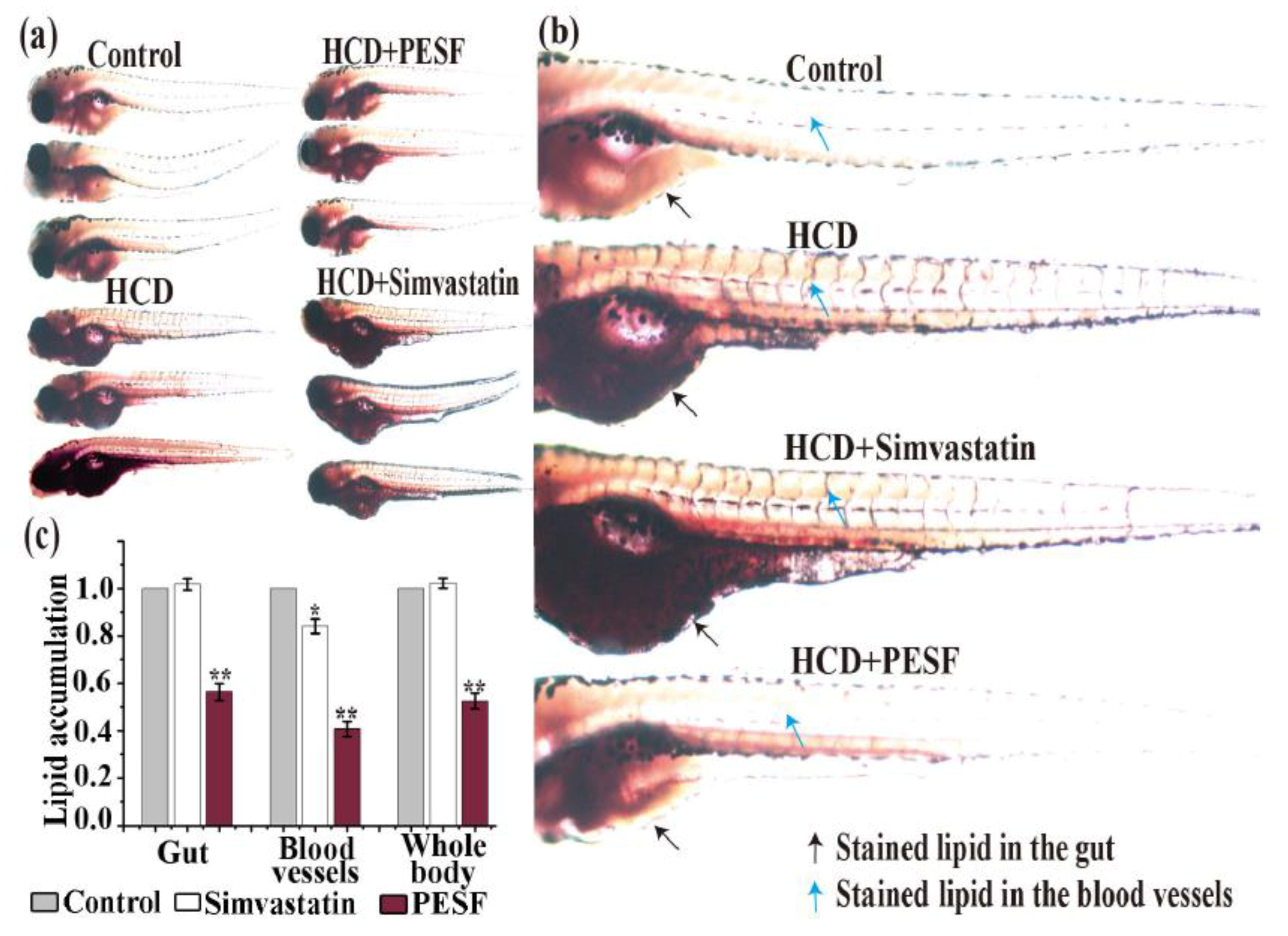
3.5. Lipid-Lowering Comparison between PESF and a Commercial Hypolipidemic Drug-Simvastatin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| d.p.f. | days post-fertilization |
| DMEM | Dulbecco’s modified eagle medium |
| HCD | high-cholesterol diet |
| MSSF | mycelia solid-state fermentation |
| ORO | oil red O |
| oxLDL | oxidized low density lipoprotein |
| OD | optical density |
| PEPE | polysaccharides from P. eryngii fruiting bodies |
| PESF | polysaccharides from P. eryngii mycelia solid-state fermentation |
| SD | standard deviation |
References
- Abramson, J.D.; Rosenberg, H.G.; Jewell, N.; Wright, J.M. Should people at low risk of cardiovascular disease take a statin? BMJ 2013, 347, f6123. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Mushroom Polysaccharides: Chemistry and Antiobesity, Antidiabetes, Anticancer, and Antibiotic Properties in Cells, Rodents, and Humans. Foods 2016, 5, e80. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yong, Y.; Xia, X.; Wang, Z.; Liang, Y.; Zhang, S.; Lu, L. The excreted polysaccharide of Pleurotus eryngii inhibits the foam-cell formation via down-regulation of CD36. Carbohydr. Polym. 2014, 112, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Lu, Y.; Fu, J.; Ning, Z.; Yang, J.; Ren, J. Preparation and Characterization of Dictyophora indusiata Polysaccharide-Zinc Complex and Its Augmented Antiproliferative Activity on Human Cancer Cells. J. Agric. Food Chem. 2015, 63, 6525–6534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Nie, S.; Huang, D.; Huang, J.; Feng, Y.; Xie, M. A polysaccharide from Ganoderma atrum inhibits tumor growth by induction of apoptosis and activation of immune response in CT26-bearing mice. J. Agric. Food Chem. 2014, 62, 9296–9304. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, L.; Li, C.; Hu, C.; Su, F.; Liu, R.; Zeng, M.; Zhao, D.; Liu, J.; Guo, Y.; et al. A mix of apple pomace polysaccharide improves mitochondrial function and reduces oxidative stress in the liver of high-fat diet-induced obese mice. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sapper, T.N.; Mah, E.; Rudraiah, S.; Schill, K.E.; Chitchumroonchokchai, C.; Moller, M.V.; McDonald, J.D.; Rohrer, P.R.; Manautou, J.E.; et al. Green tea extract provides extensive Nrf2-independent protection against lipid accumulation and NFkappaB pro-inflammatory responses during nonalcoholic steatohepatitis in mice fed a high-fat diet. Mol. Nutr. Food Res. 2016, 60, 858–870. [Google Scholar] [PubMed]
- Li, X.D.; Liu, Y.W.; Zhang, H.; Ren, L.M.; Li, Q.Y.; Li, N. Animal models for the atherosclerosis research: A review. Protein Cell 2011, 2, 189–201. [Google Scholar]
- Gratacap, R.L.; Wheeler, R.T. Utilization of zebrafish for intravital study of eukaryotic pathogen-host interactions. Dev. Comp. Immunol. 2014, 46, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Avraham-Davidi, I.; Ely, Y.; Pham, V.N.; Castranova, D.; Grunspan, M.; Malkinson, G.; Gibbs-Bar, L.; Mayseless, O.; Allmog, G.; Lo, B.; et al. ApoB-containing lipoproteins regulate angiogenesis by modulating expression of VEGF receptor 1. Nat. Med. 2012, 18, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Clifton, J.D.; Lucumi, E.; Myers, M.C.; Napper, A.; Hama, K.; Farber, S.A.; Smith, A.B., 3rd; Huryn, D.M.; Diamond, S.L.; Pack, M. Identification of novel inhibitors of dietary lipid absorption using zebrafish. PLoS ONE 2010, 5, e12386. [Google Scholar]
- Cruz-Garcia, L.; Schlegel, A. Lxr-driven enterocyte lipid droplet formation delays transport of ingested lipids. J. Lipid Res. 2014, 55, 1944–1958. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, Y.Q.; Guo, S.Y.; Li, C.Q. Rapid analysis of hypolipidemic drugs in a live zebrafish assay. J. Pharmacol. Toxicol. Methods 2015, 72, 47–52. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, E.J.; Soukas, A.A.; Carr, C.E.; Ruvkun, G. C. elegans major fats are stored in vesicles distinct from lysosome-related organelles. Cell Metab. 2009, 10, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Hamid, R.; Rotshteyn, Y.; Rabadi, L.; Parikh, R.; Bullock, P. Comparison of alamar blue and MTT assays for high through-put screening. Toxicol. In Vitro 2004, 18, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Mao, D.; Yong, Y.Y.; Li, J.L.; Wei, H.; Lu, L. Hepatoprotective and hypolipidemic effects of water-soluble polysaccharidic extract of Pleurotus eryngii. Food Chem. 2012, 130, 687–694. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, H.; Yue, S.; Zhang, S.; Lu, L. Lipid-Lowering Effect of the Pleurotus eryngii (King Oyster Mushroom) Polysaccharide from Solid-State Fermentation on Both Macrophage-Derived Foam Cells and Zebrafish Models. Polymers 2018, 10, 492. https://doi.org/10.3390/polym10050492
Wei H, Yue S, Zhang S, Lu L. Lipid-Lowering Effect of the Pleurotus eryngii (King Oyster Mushroom) Polysaccharide from Solid-State Fermentation on Both Macrophage-Derived Foam Cells and Zebrafish Models. Polymers. 2018; 10(5):492. https://doi.org/10.3390/polym10050492
Chicago/Turabian StyleWei, Hua, Shang Yue, Shizhu Zhang, and Ling Lu. 2018. "Lipid-Lowering Effect of the Pleurotus eryngii (King Oyster Mushroom) Polysaccharide from Solid-State Fermentation on Both Macrophage-Derived Foam Cells and Zebrafish Models" Polymers 10, no. 5: 492. https://doi.org/10.3390/polym10050492