1. Introduction
Nanoparticles grafted with thermoresponsive polymers have received much attention in recent years because of the multitude of applications for which they are of interest. Thermoresponsive core-shell nanoparticles are multifunctional materials, combining the nano-specific properties of the core with the thermoresponsive solubility of the shell. For example, Kakwere et al. report iron oxide cubes grafted with poly(
N-isopropylacrylamide) (PNIPAM), which can be used as drug delivery vehicles and for hyperthermia treatment [
1]. Such smart materials can be used not only for drug delivery [
2,
3,
4], but also for magnetic separation [
5], water desalination [
6], or catalysis [
7]. These applications require a detailed understanding of how thermoresponsive (reversible) aggregation of colloidally stable polymer-grafted nanoparticles can be controlled under relevant conditions. The impact of the grafting of thermoresponsive polymers on nanoparticles is still poorly investigated experimentally. This is mainly because of a lack of suitable model systems for which nanoparticle structure can be rationally designed and investigated, and which are colloidally stable under physiological conditions.
While nanoparticles grafted with poly(ethylene glycol) (PEG) dominate research in the practical use of non-responsive biomedical nanoparticles, PNIPAM-based systems account for the vast majority of publications in the field of thermoresponsive nanoparticles, despite the fact that PNIPAM has a thermal solubility transition in water that is below body temperature. However, for biomedical applications, poly(2-oxazolines) have emerged as a true alternative to PEG because of their non-toxic and biocompatible nature [
8] in the face of mounting antibody production to PEG [
9] and the stability of poly(2-oxazolines) to hydrolysis [
10]. In addition, poly(2-oxazolines) can be designed with a critical solution temperature (CST), above which they lose hydration. Through the monomer composition of poly(2-oxazolines), the CST can be designed to be close to or above body temperature [
11,
12,
13]; these combined properties make them potentially much more versatile for biotechnological and biomedical applications than PNIPAM or PEG.
Oxazolines are polymerized via cationic ring-opening polymerization (CROP), which allows for the synthesis of polymers with defined molecular weights (MW), composition, and end-group chemistry, as well as narrow molecular weight distribution [
14]. Poly(2-isopropyl-2-oxazoline) (PiPOx)—a structural isomer to PNIPAM—exhibits a lower critical solution temperature (LCST) in water at a few degrees below body temperature [
15,
16,
17]. During this thermally triggered solubility transition, the polymer undergoes a phase change from a soluble to a phase-separated state, driven mainly by entropy. The LCST describes the lowest temperature in the compositional phase diagram at which this transition occurs. The CST of a hybrid polymer system, such as nanoparticles grafted with polymers, therefore, can significantly deviate from the LCST, as the local concentration and imposed conformation of the polymer in such systems reflect other parts of the phase diagram [
18].
Several factors are known to shift the CST of PiPOx in addition to the concentration of the polymer [
19,
20], including the mixing of salt with the aqueous polymer solution [
21], the variation of end-groups [
22], and varying the polymer MW [
20,
23]. Short PiPOx chains (1.7 kg mol
−1) display higher transition temperatures (72.5 °C) than longer chains (5.7 kg mol
−1, 48.1 °C) [
23]. The enthalpy of the transition increases significantly with the MW [
23]. The concentration of free polymer has been shown to have smaller influence on the CST of PiPOx in water than that of the MW. Park et al. studied the cloud point of PiPOx with a MW of 9.7 kg mol
−1. Changing the concentration of the polymer from 0.1 to 1.0 wt % resulted in a decrease of the cloud point from 39.8 to 37.3 °C. Furthermore, the impact of the concentration on the CST decreases with increasing MW [
19]. The CST can be lowered by introducing a hydrophobic end-group to a polymer, whereas it increases with a hydrophilic end-group. For example, by changing a methyl end-group to a nonyl end-group, the CST of PiPOx was lowered by 19 °C [
22]. It also worth noting that the CST of PiPOx can be manipulated by copolymerization. The CST can be shifted downward by having more apolar moieties present [
24]. By incorporating shorter chains, such as ethyl or methyl moieties, the CST will increase to higher values when compared with PiPOx [
12].
The addition of salts to the aqueous polymer solution plays a crucial role in shifting the CST of thermoresponsive polymers. Many—in particular, medical, biotechnological, and environmental—applications require the function of responsive particles at both high ionic strength and in the presence of a large variety of different salts. The logic for the influence of salts is simple; salts are known to affect the internal hydrogen bonding of water, and thus strongly influence the solubility transition of polymers such as PiPOx, which is driven by the balance of water hydrogen bonding and entropy. Therefore, kosmotropes decrease the CST as the ionic strength is increased, while chaotropes will increase the CST. Previous studies on free PiPOx have found that kosmotropic anions decrease the transition temperature. With increasing salt concentration, a linear decrease in CST was observed. On the other hand, chaotropic anions increase the CST nonlinearly when the ion concentration is increased [
21,
25]. For poly(2-ethyl-2-oxazoline) (PEtOx)—which is structurally similar to PiPOx, but has higher LCST—mainly three mechanisms were found that cause a change in transition temperature. In the presence of chaotropic anions (NO
3−, I
−, ClO
−, SCN
−), a direct binding of the anions to the polymer is observed, leading to an increase in polymer solubility, tantamount to a shift of the CST to higher values. Divalent, kosmotropic anions (CO
32−, SO
42−, S
2O
32−) effectively desolvate the polymer chains and lower the CST. Monovalent, kosmotropic anions (H
2PO
4−, F
−, Cl
−, Br
−) cause desolvation and increase the surface tension of the solution. Therefore, the addition of monovalent, kosmotropic anions causes an increase in the free energy of the alkyl/water interface, leading to lower solubility and lower CST of the polymer [
25]. Much less attention has been paid to the influence of cations on poly(2-oxazoline) CST, but they have been claimed to have less impact on CST than anions [
21,
25].
Moreover, the polymer architecture has a significant influence on the CST. Star-shaped PiPOx display a lower aggregation temperature when compared with its linear analogs. Eight-arm, star-shaped PiPOx with a MW of 19.6 kg mol
−1 aggregate at a concentration of 2 g L
−1 at 37 °C, while the linear analog of one arm (MW 2.5 kg mol
−1) aggregates at 47 °C [
26]. It should, however, be noted that a more relevant comparison would require linear polymers of the same MW as the star-shaped polymer in order to investigate the influence of the branched topology. A strong effect of the architecture on the CST is also observed for comb copolymers [
27,
28,
29]. As expected, the CST can be modulated both by the MW of the side chain and by the length of the main chain [
27,
28,
29].
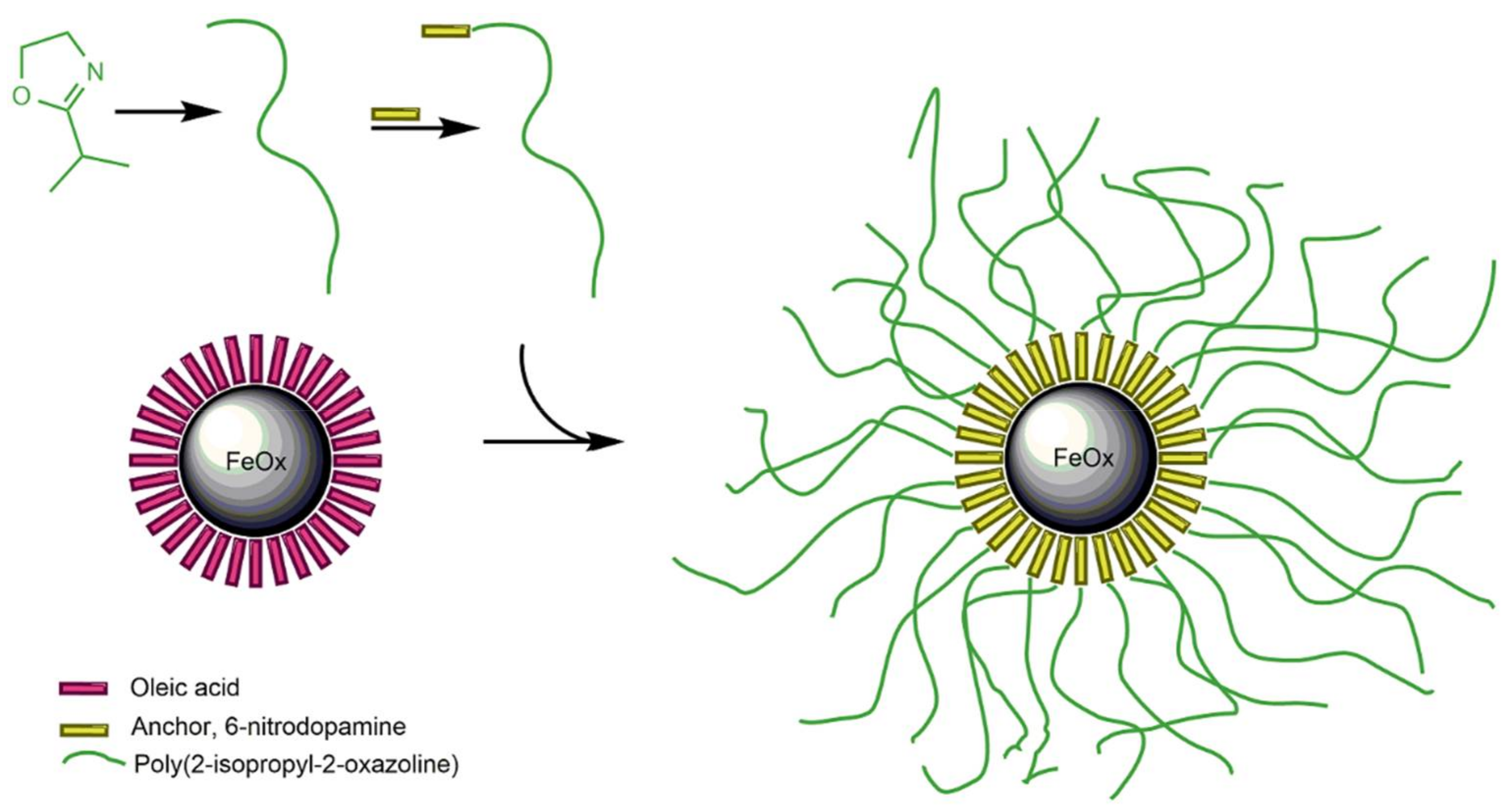
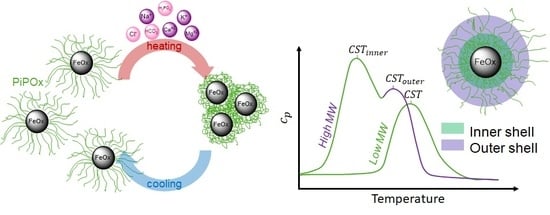
Core-shell nanoparticles with a spherical PiPOx shell are topologically and morphologically similar to star-shaped polymer architectures [
30]; thus a similar effect on the CST of the grafting of spherical brushes on nanoparticles as that of adding arms to a star-shaped polymer is expected. In a previous report, the thermal aggregation and deaggregation of poly(2-oxazoline)-grafted iron oxide nanoparticles were investigated with respect to shell composition and exposure to the physiological buffer and medium [
31]. Three different poly(2-oxazoline) shells—PiPOx, PEtOx, and a random copolymer PiPOx-
co-PEtOx in 87:13 ratio—were investigated. In all cases, the CST of the polymers shifted to lower values upon grafting. By adding the cell culture medium, which contains a high concentration of the kosmotrope NaCl, the critical solution temperature of the grafted PiPOx (16.5 kg mol
−1) brush dramatically shifted lower to 32.5 °C, compared to 38 °C in pure water. Analogue particles, with 14 kg mol
−1 grafted PEtOx at a concentration of 1 g L
−1, showed a decrease in CST from 74 °C in pure water to 47 °C in cell culture medium [
31]. However, an investigation into the influence of chain MW of PiPOx and ionic strength (type and concentration of ions) on the CST of the spherical brushes was not performed.
Given the potential applications of poly(2-oxazoline)-grafted nanoparticles, it is crucial to explore the impact of surface grafting, brush morphology, and the presence of ions on their thermal responsiveness. Therefore, we investigated monodisperse, superparamagnetic iron oxide nanoparticles regarding the influence of grafting, chain MW, and type of anions and cations from the Hofmeister series on the thermoresponsive properties of the PiPOx shell and on the thermally-induced aggregation of the nanoparticles. Differential scanning calorimetry (DSC) and dynamic light scattering (DLS) were used to elucidate the influence of the unique internal structure, provided by grafting of the linear PiPOx into a nanoscale spherical brush, on the thermal colloidal properties.
4. Conclusions
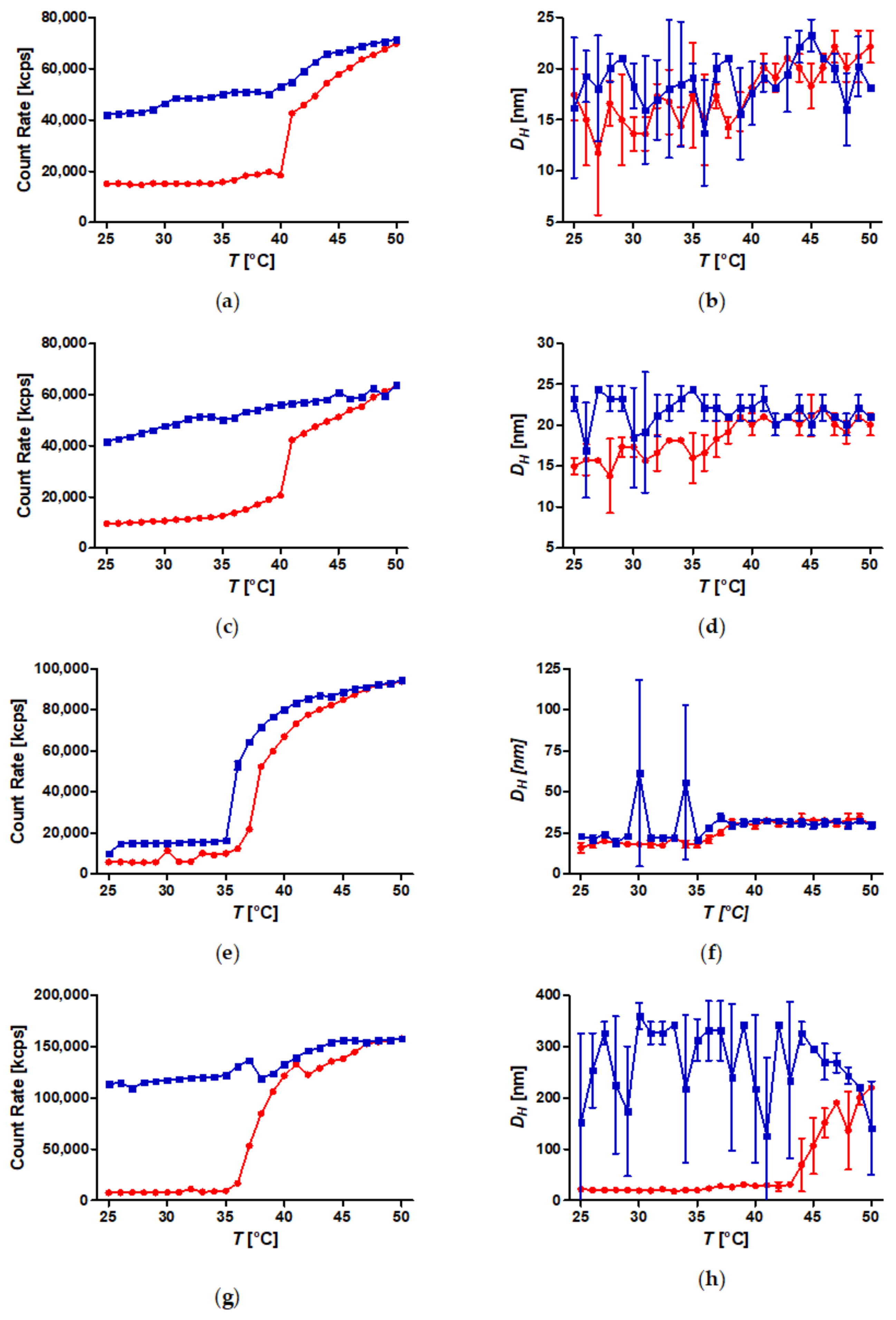
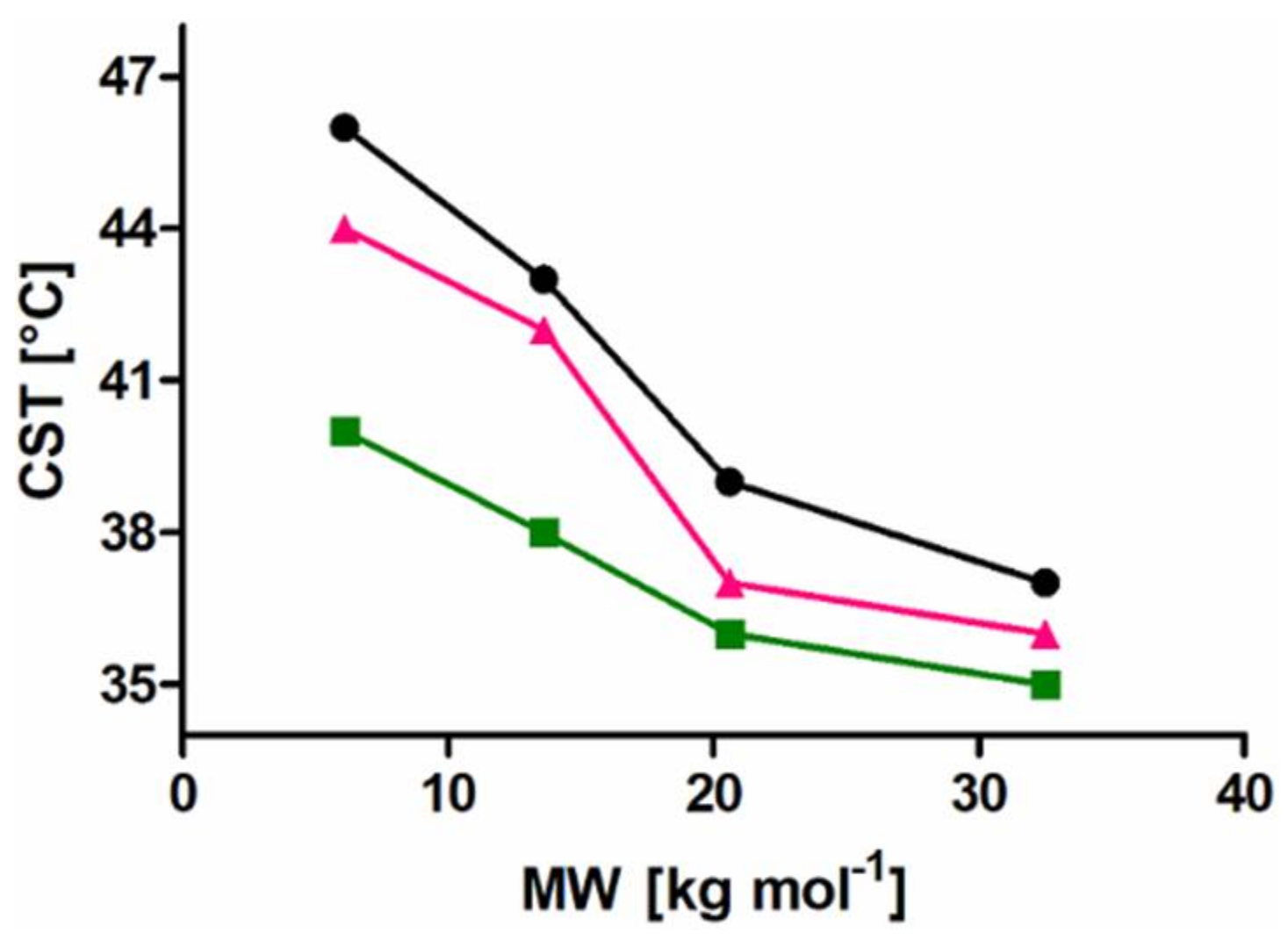
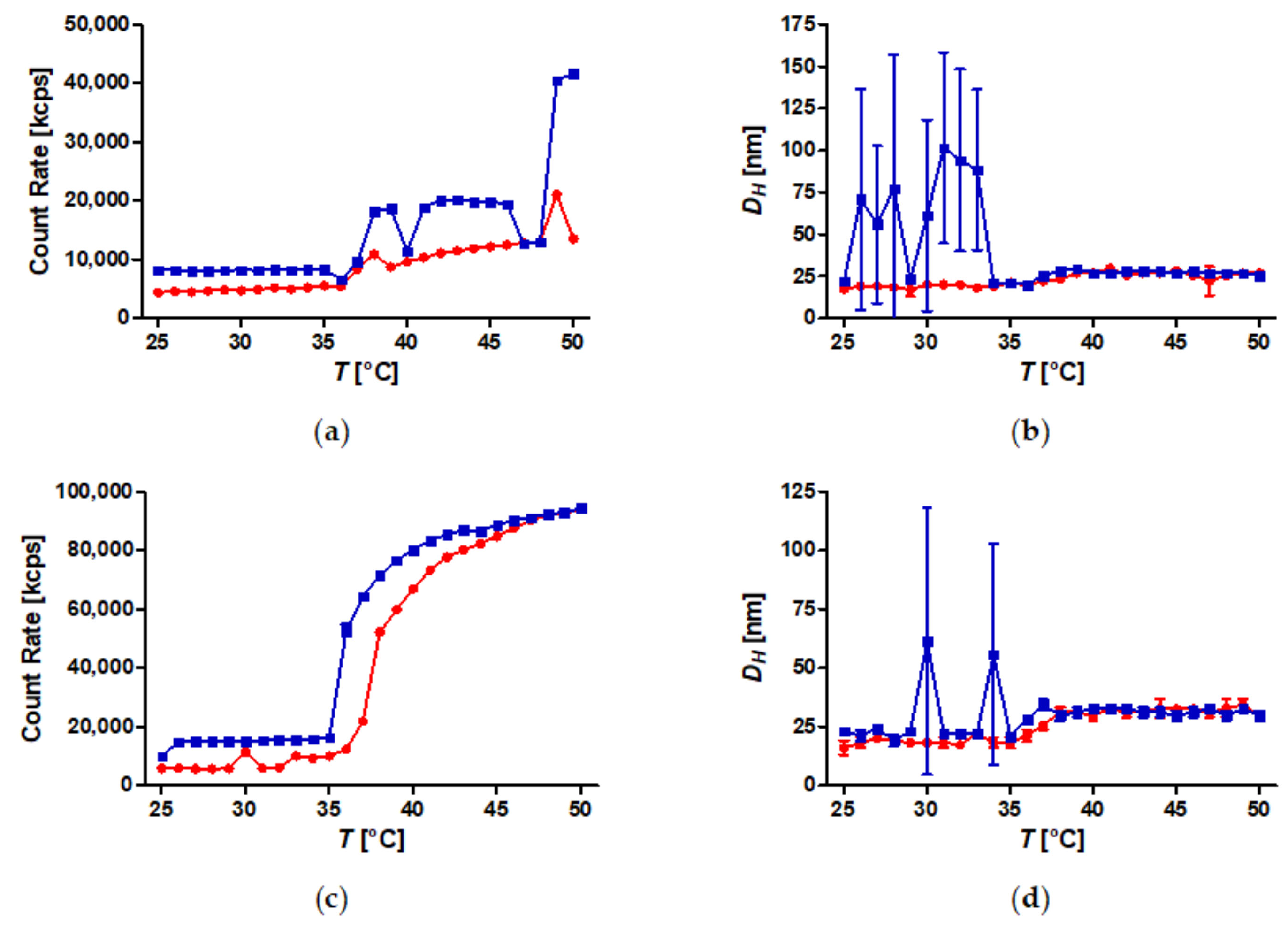
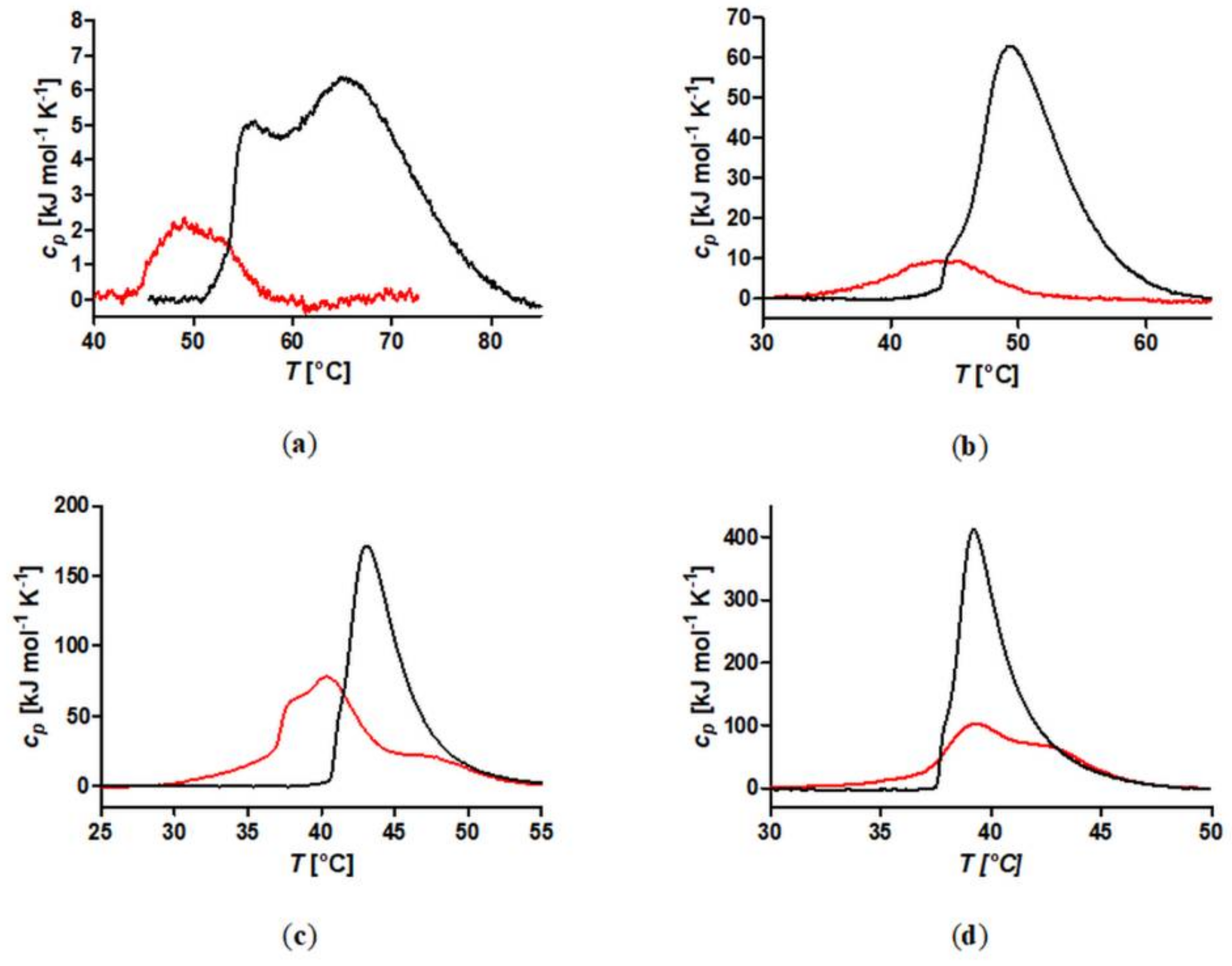
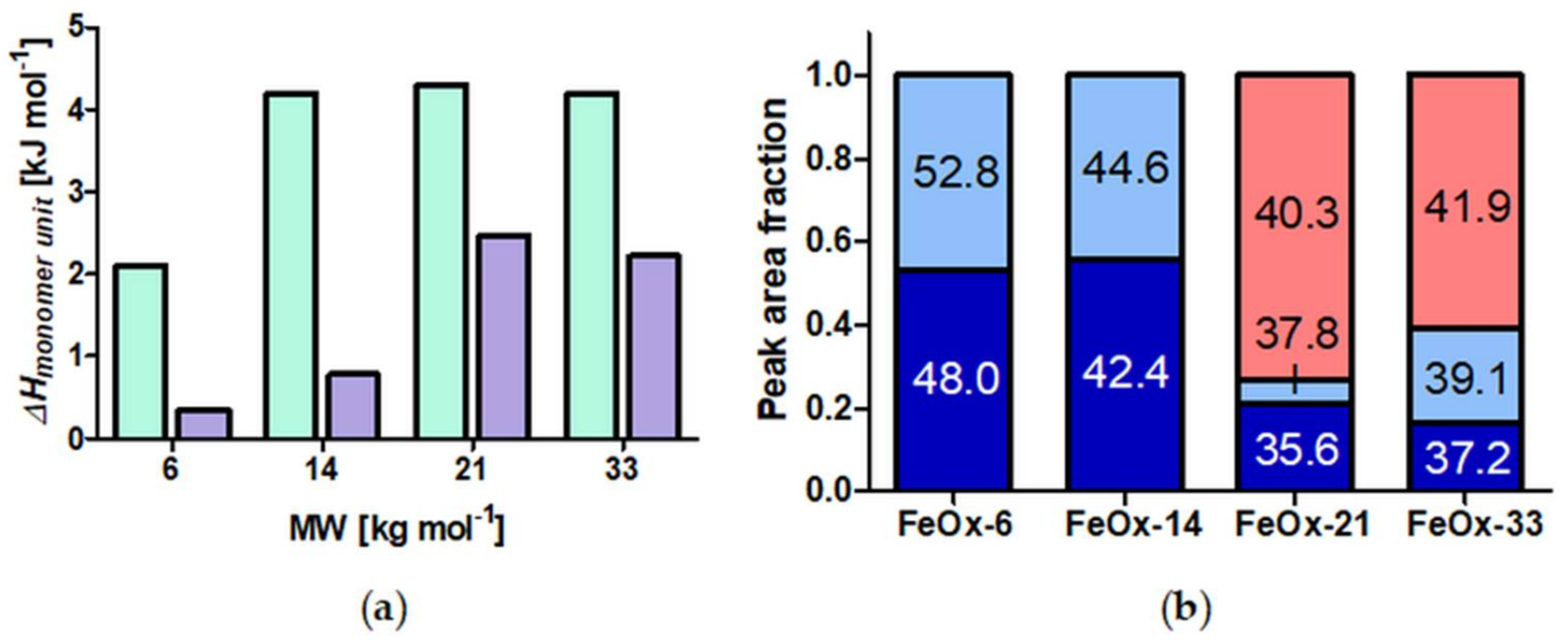
A set of well-defined SPION with spherical PiPOx brushes was prepared using state-of-the-art techniques. By keeping the core diameter and the grafting density constant, the influence of the PiPOx molecular weight on the thermoresponsiveness of the core-shell nanoparticle dispersions could be studied in detail by DLS and DSC. All of the particles displayed reversible aggregation/deaggregation in water. Partial rehydration of the shell, investigated by DSC, proceeded on the experimental (minute) time scale, while spontaneous colloidal redispersion, investigated by DLS, was generally reversible only on a much longer (hour) time scale. For both free polymers and spherical brushes, the CSTs decreased and transition enthalpies increased with molecular weight. A reduction in the CST and transition enthalpy was observed after grafting to SPION, confirming the strong impact of polymer topology on the thermoresponsive properties. The reduction in CST observed for PiPOx-grafted SPION was more pronounced than for analogous systems based on PNIPAM. The PiPOx-grafted brushes displayed broadened transitions in DSC, which could be attributed to a main transition at a lower temperature for the part of the brush closest to the core that is identical for all molecular weights and a second distinct transition at a higher temperature, observed only for high molecular weight grafted PiPOx corresponding to a mushroom-like state in the outer shell. Importantly, the thermally-induced aggregation of thermoresponsive SPION in terms of cluster size appeared to be controlled by the outer shell transition.
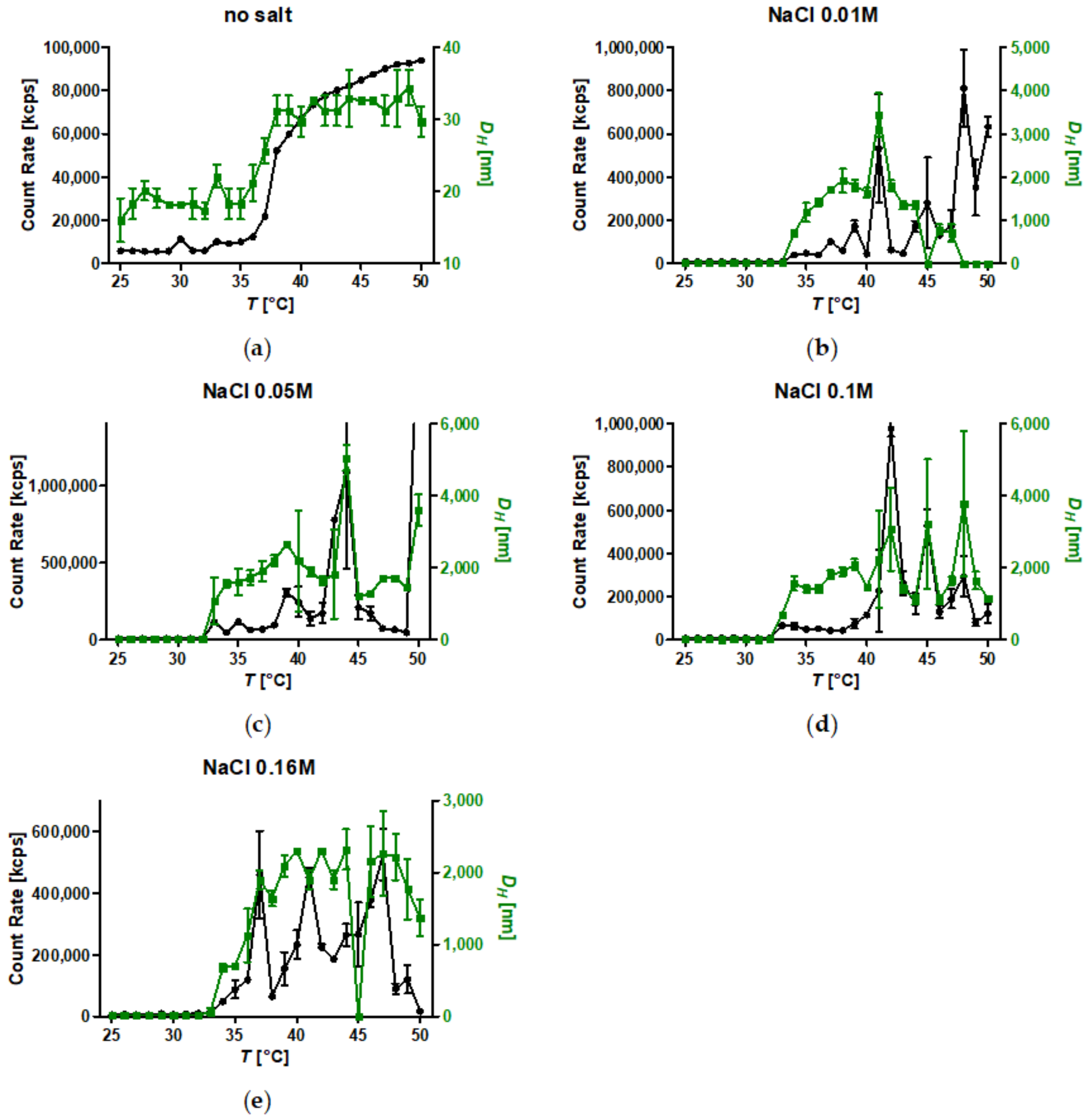
The addition of kosmotropic salts resulted in a reduction of CSTs for PiPOx-grafted SPION with anions having a stronger impact than cations. Aggregation in pure water led to the formation of small clusters consisting of only a few SPION. By contrast, the addition of kosmotropes enabled sedimentation of PiPOx-grafted SPION upon heating, which would facilitate easy magnetic removal of the material, a crucial feature for potential applications in the areas of catalysis, cell separation, and molecular fishing. Consequently, designing thermoresponsive core-shell nanoparticles for a specific application requires taking the dispersion medium into account, as the CSTs of free polymers and spherical brushes in water are misguiding. Poly(2-oxazolines) possess the advantage that the highlighted effects can be compensated by the choice of side chains and monomer composition of the grafted polymer to design both the CST and tune colloidal stability as desired for applications.