Synthesis of an Efficient S/N-Based Flame Retardant and Its Application in Polycarbonate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ASN
2.3. Preparation of PC/ASN Specimens
2.4. Measurements and Characterization
3. Results and Discussion
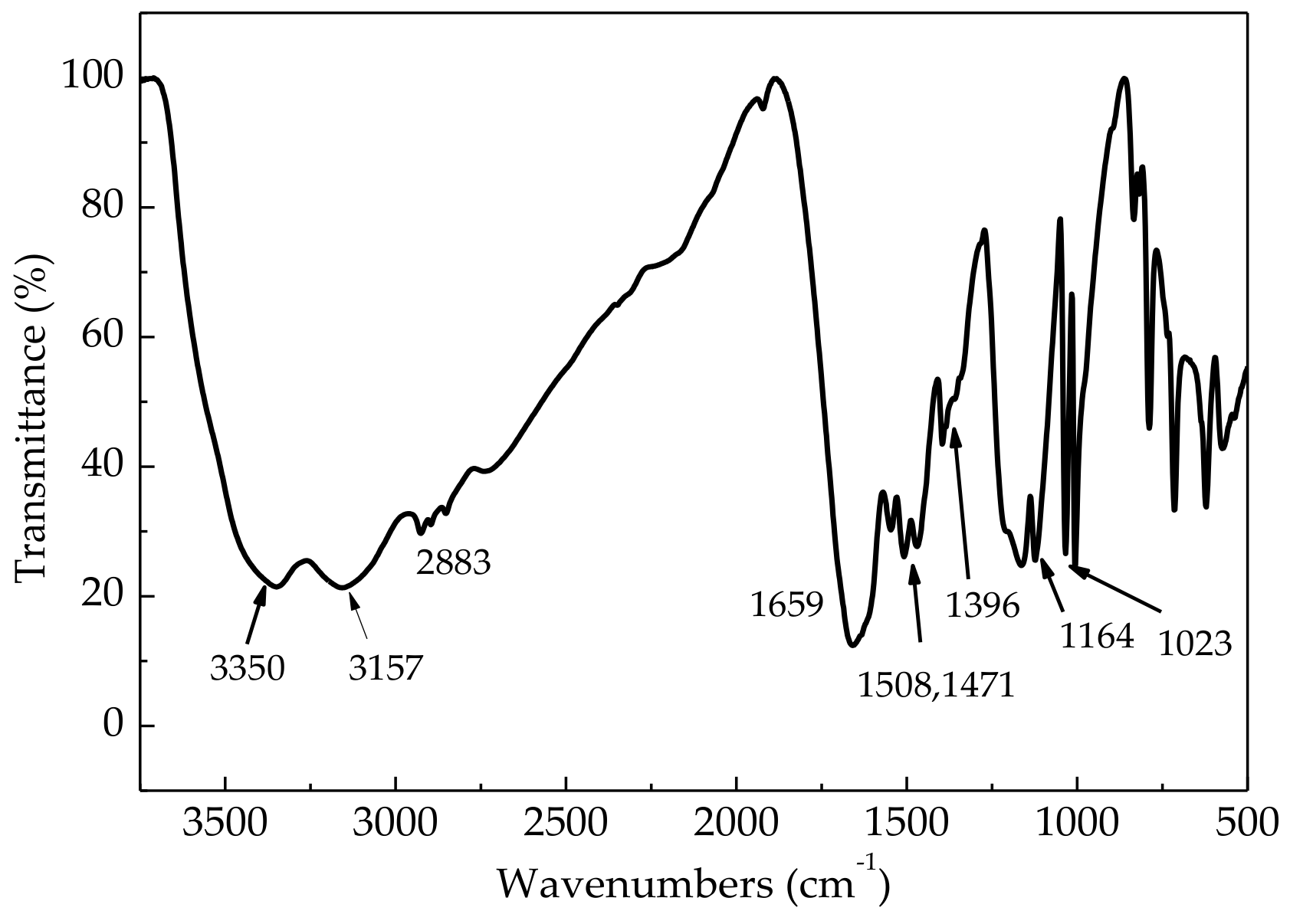
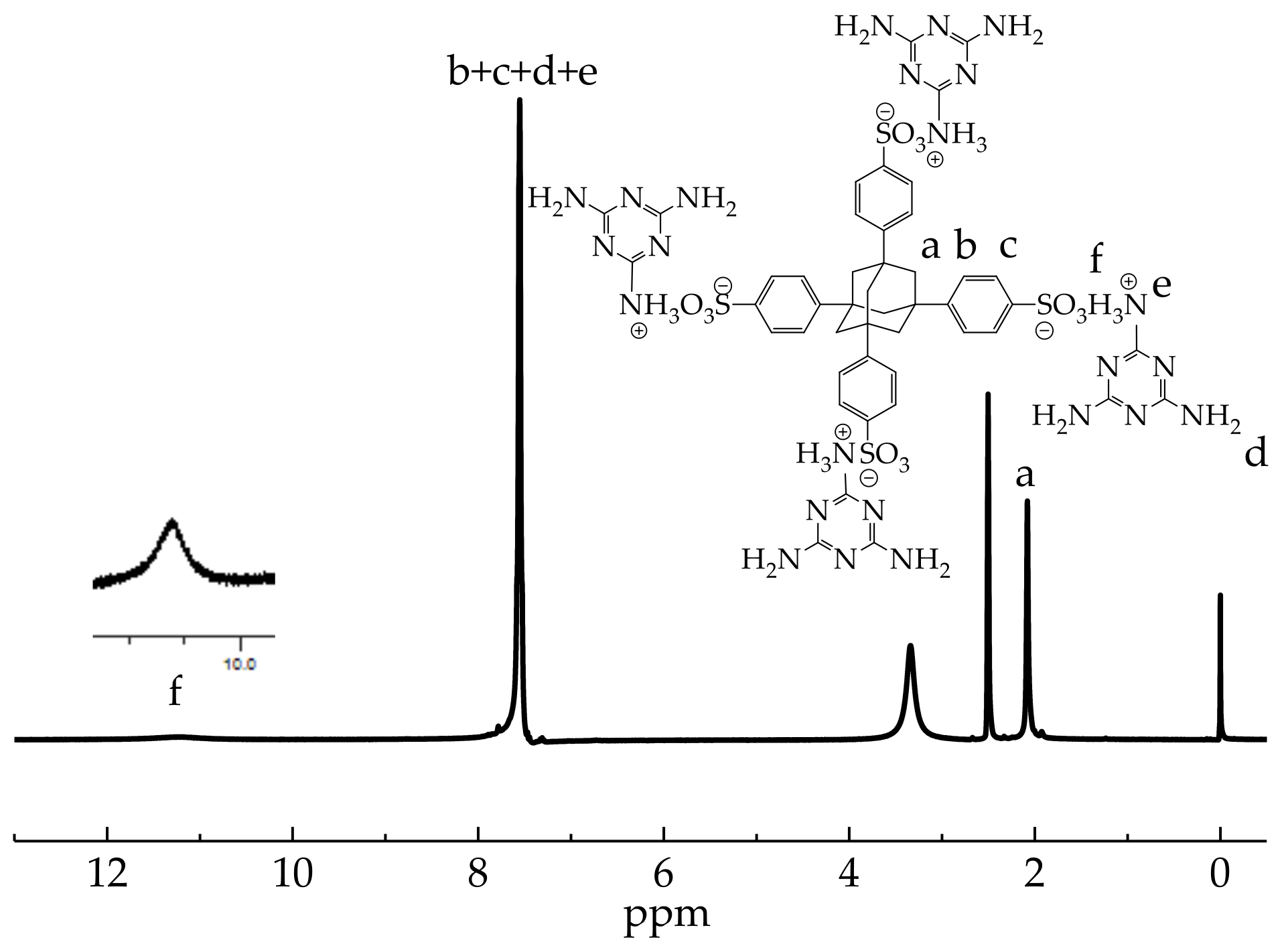
3.1. Characterization of ASN
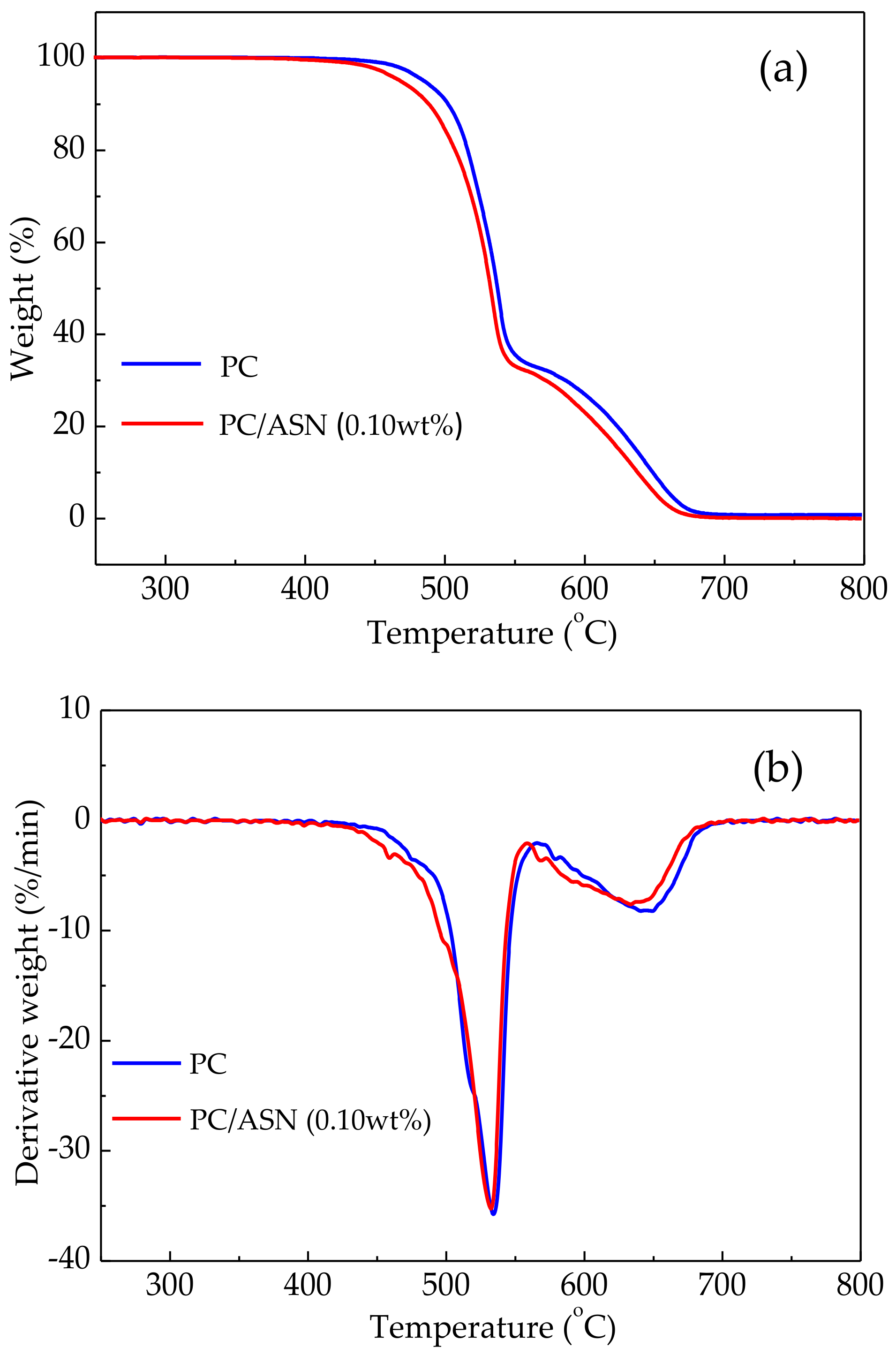
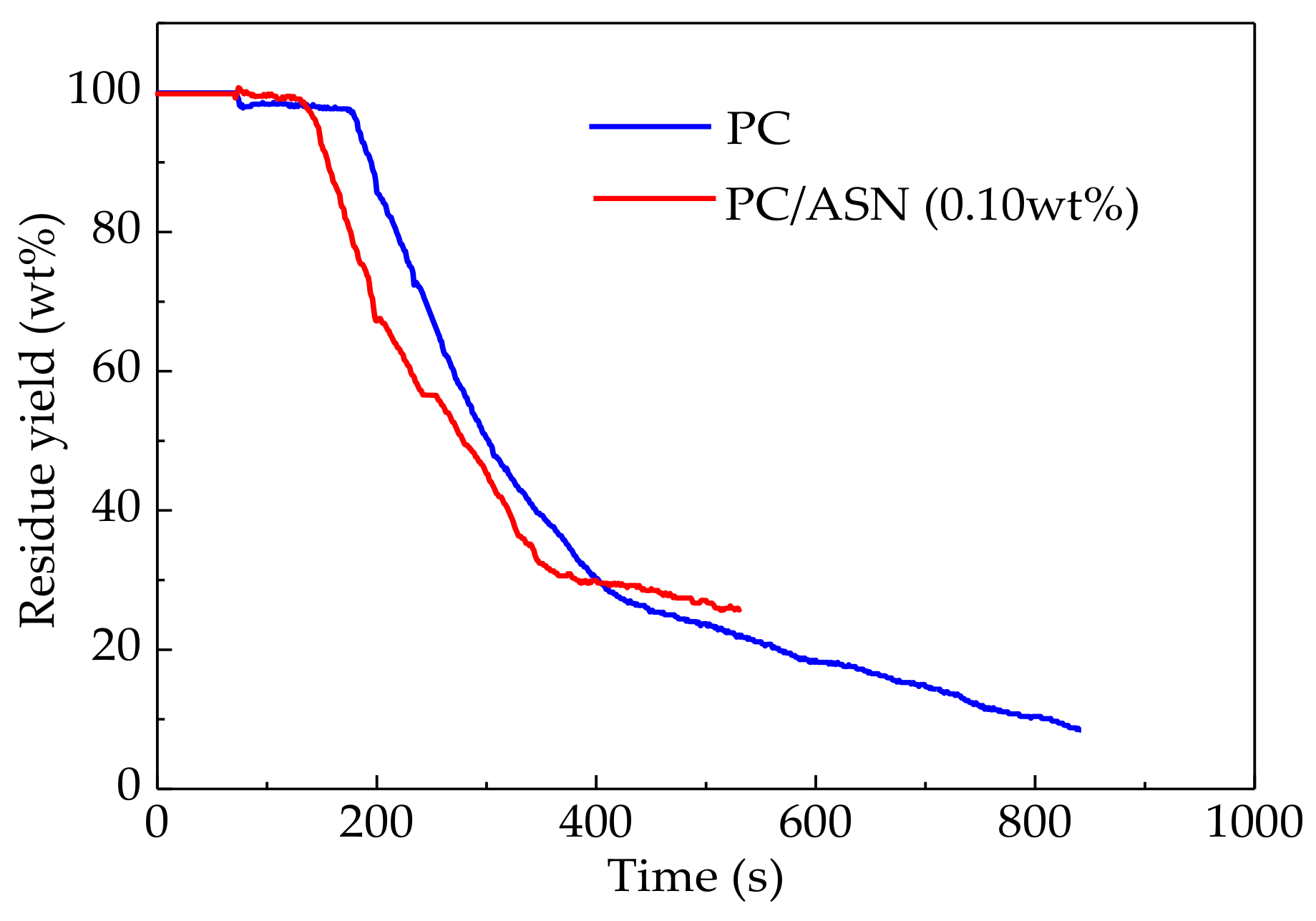
3.2. Thermal Degradation of PC Samples
3.3. Flammability Characteristics
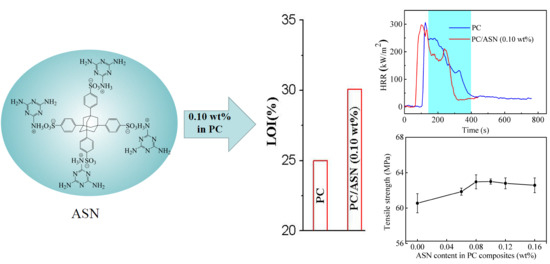
3.3.1. LOI and UL-94 Tests
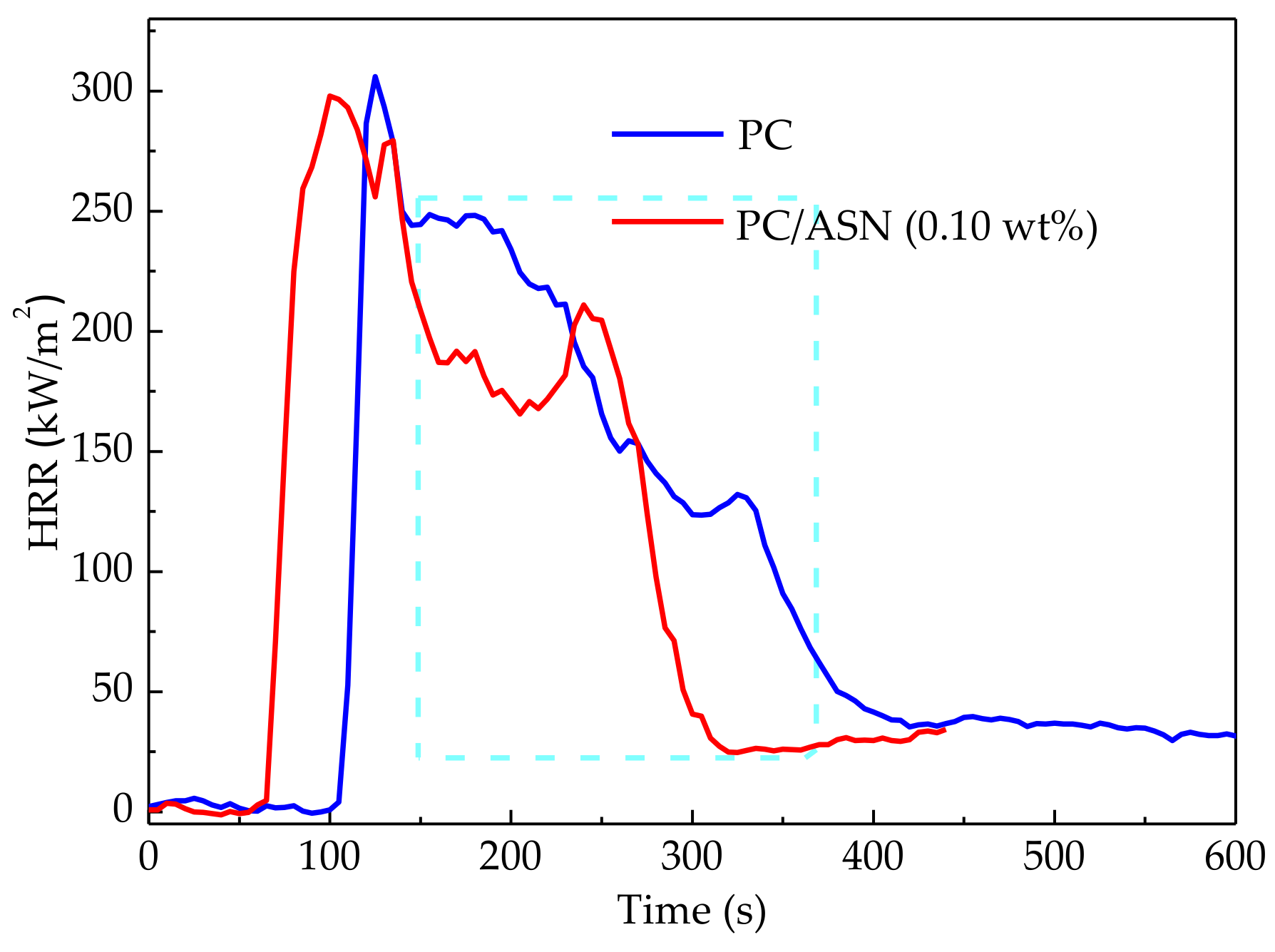
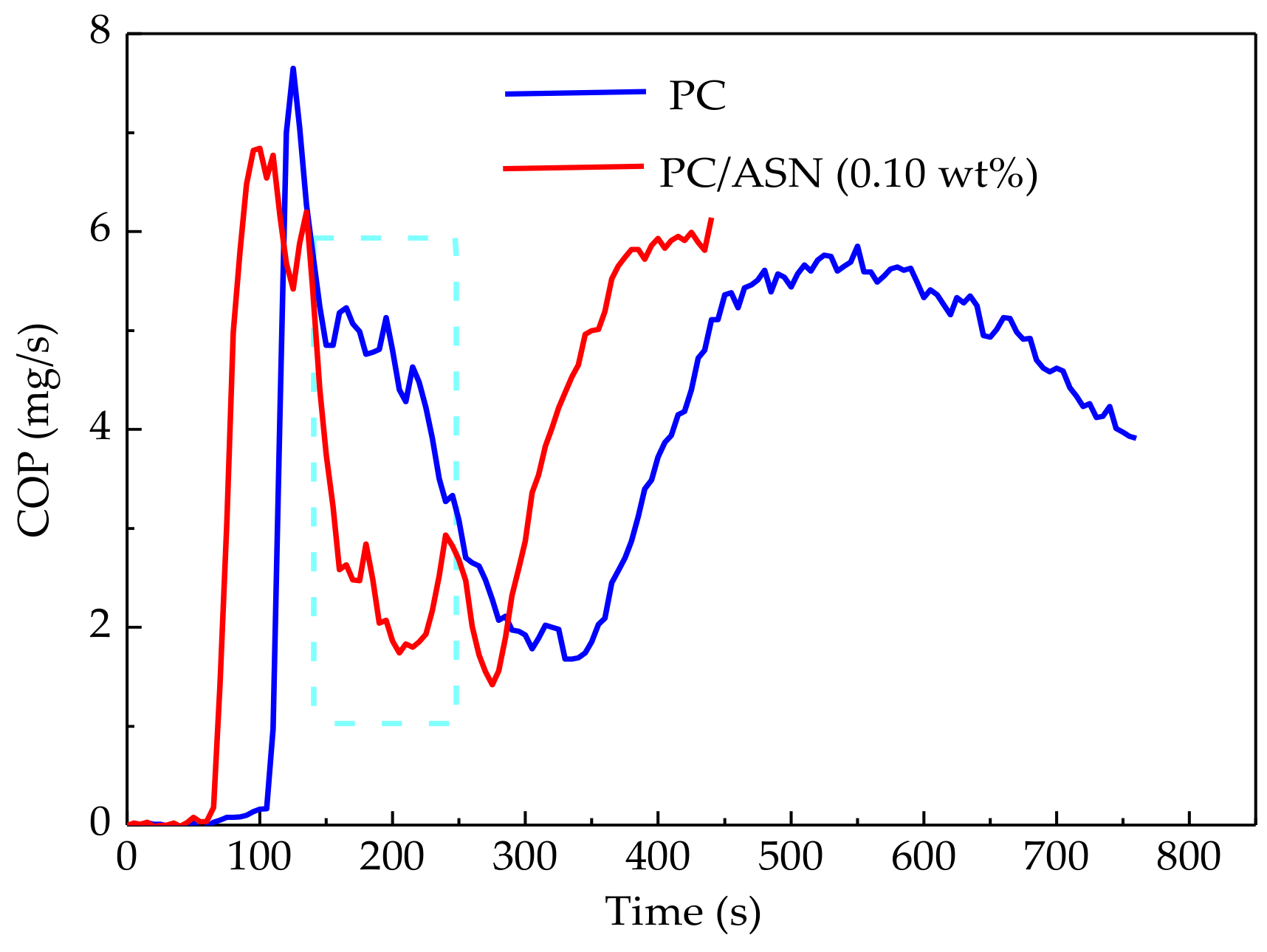
3.3.2. Cone Calorimeter Tests
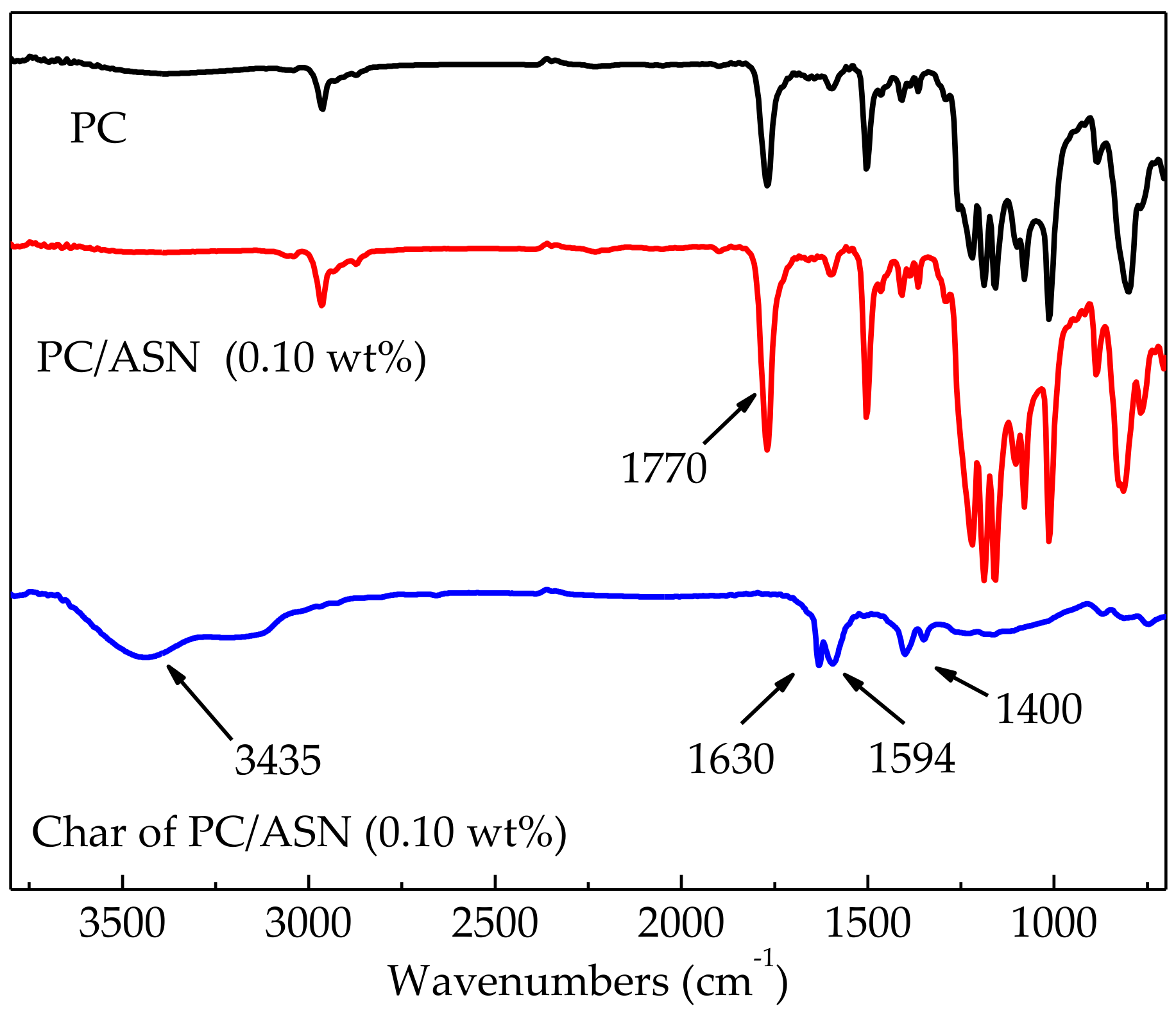
3.4. Analysis of Residual Char
3.5. Water Resistance
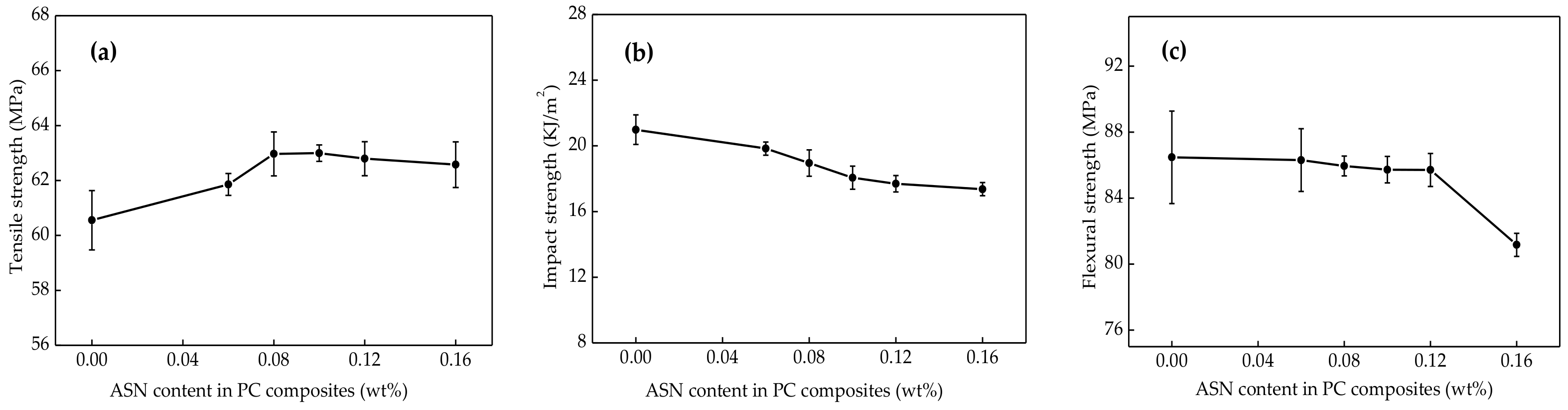
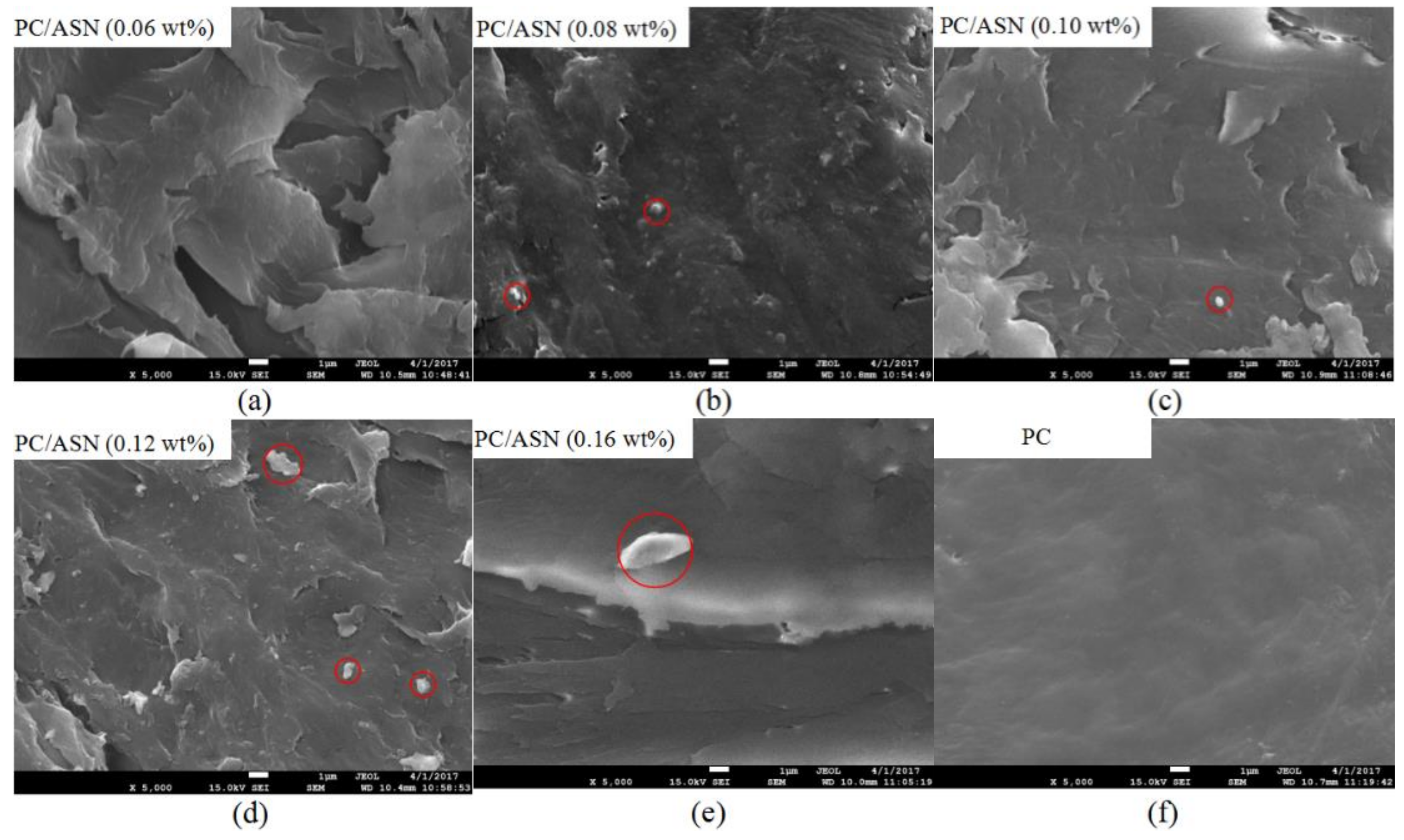
3.6. Mechanical Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xi, C.C.; Kang, G.Z.; Lu, F.C.; Zhang, J.W.; Jiang, H. An experimental study on uniaxial ratcheting of polycarbonate polymers with different molecular weights. Mater. Des. 2015, 67, 644–648. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Kong, W.B.; Cai, X.F. Two phosphorous-containing flame retardant form a novel intumescent flame-retardant system with polycarbonate. Polym. Degrad. Stab. 2016, 134, 136–143. [Google Scholar] [CrossRef]
- Feng, J.; Hao, J.W.; Du, J.X.; Yang, R.J. Flame retardancy and thermal properties of solid bisphenol A bis (diphenyl phosphate) combined with montmorillonite in polycarbonate. Polym. Degrad. Stab. 2010, 95, 2041–2048. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. Flame retardants in commercial use or in advanced development in polycarbonates and polycarbonate blends. J. Fire Sci. 2006, 24, 137–151. [Google Scholar] [CrossRef]
- Swoboda, B.; Buonomo, S.; Leroy, E.; Lopez-Cuesta, J.M. Fire retardant poly(ethylene terephthalate)/polycarbonate/triphenyl phosphite blends. Polym. Degrad. Stab. 2008, 93, 910–917. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P.H. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Wu, D.H.; Zhao, P.H.; Liu, Y.Q.; Liu, Y.X.; Wang, X.F. Halogen Free flame retardant rigid polyurethane foam with a novel phosphorus-nitrogen intumescent flame retardant. J. Appl. Polym. Sci. 2014, 131, 39581. [Google Scholar] [CrossRef]
- Zhao, X.L.; Chen, C.K.; Chen, X.L. Effects of carbon fibers on the flammability and smoke emission characteristics of halogen-free thermoplastic polyurethane/ammonium polyphosphate. J. Mater. Sci. 2016, 51, 3762–3771. [Google Scholar] [CrossRef]
- Blum, A. The fire retardant dilemma. Science 2007, 318, 194–195. [Google Scholar] [CrossRef] [PubMed]
- Mochane, M.J.; Luyt, A.S. Synergistic effect of expanded graphite, diammonium phosphate and Cloisite 15A on flame retardant properties of EVA and EVA/wax phase-change blends. J. Mater. Sci. 2015, 50, 3485–3494. [Google Scholar] [CrossRef]
- Liu, C.; Yao, Q. Design and Synthesis of Efficient Phosphorus Flame Retardant for Polycarbonate. Ind. Eng. Chem. Res. 2017, 56, 8789–8796. [Google Scholar] [CrossRef]
- Xiao, F.; Wu, K.; Luo, F.; Guo, Y.; Zhang, S.; Du, X.; Lu, M. An efficient phosphonate-based ionic liquid on flame retardancy and mechanical property of epoxy resin. J. Mater. Sci. 2017, 52, 13992–14003. [Google Scholar] [CrossRef]
- Pawlowski, K.H.; Schartel, B. Flame retardancy mechanisms of triphenyl phosphate, resorcinol bis(diphenyl phosphate) and bisphenol a bis(diphenyl phosphate) in polycarbonate/acrylonitrile–butadiene–styrene blends. Polym. Int. 2016, 56, 1404–1414. [Google Scholar] [CrossRef]
- Fu, S.Q.; Guo, J.W.; Zhu, D.Y.; Yang, Z.; Yang, C.F.; Xian, J.X.; Li, X. Novel halogen-free flame retardants based on adamantane for polycarbonate. RSC Adv. 2015, 5, 67054–67065. [Google Scholar] [CrossRef]
- Shen, M.Y.; Kuan, C.F.; Kuan, H.C.; Chen, C.H.; Wang, J.H.; Yip, M.C.; Chiang, C.L. Preparation, characterization, thermal, and flame-retardant properties of green silicon-containing epoxy/functionalized graphene nanosheets composites. J. Nanomater. 2013, 2013, 22. [Google Scholar] [CrossRef]
- Liu, S.H.; Kuan, C.F.; Kuan, H.C.; Shen, M.Y.; Yang, J.M.; Chiang, C.L. Preparation and Flame Retardance of Polyurethane Composites Containing Microencapsulated Melamine Polyphosphate. Polymers 2017, 9, 407. [Google Scholar] [CrossRef]
- Zhang, W.C.; Li, X.M.; Guo, X.Y.; Yang, R.J. Mechanical and thermal properties and flame retardancy of phosphorus-containing polyhedral oligomeric silsesquioxane (DOPO-POSS)/polycarbonate composites. Polym. Degrad. Stab. 2010, 95, 2541–2546. [Google Scholar] [CrossRef]
- Gao, M.; Yang, S. A novel intumescent flame-retardant epoxy resins system. J. Appl. Polym. Sci. 2010, 115, 2346–2351. [Google Scholar] [CrossRef]
- Martin, C.; Lligadas, G.; Ronda, J.C.; Galia, M.; Cadiz, V. Synthesis of novel boron-containing epoxy-novolac resins and properties of cured products. J. Polym. Sci. Part A 2006, 44, 6332–6344. [Google Scholar] [CrossRef]
- Yuan, D.D.; Yin, H.Q.; Cai, X.F. Synergistic effects between silicon-containing flame retardant and potassium-4-(phenylsulfonyl) benzenesulfonate (KSS) on flame retardancy and thermal degradation of PC. J. Therm. Anal. Calorim. 2013, 114, 19–25. [Google Scholar] [CrossRef]
- Dai, K.; Song, L.; Yuen, R.K.; Jiang, S.H.; Pan, H.F.; Hu, Y. Enhanced properties of the incorporation of a novel reactive phosphorus-and sulfur-containing flame-retardant monomer into unsaturated polyester resin. Ind. Eng. Chem. Res. 2012, 51, 15918–15926. [Google Scholar] [CrossRef]
- Li, Z.Q.; Yang, R.J. Flame retardancy, thermal and mechanical properties of sulfonate-containing polyhedral oligomeric silsesquioxane (S-POSS)/polycarbonate composites. Polym. Degrad. Stab. 2015, 116, 81–87. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Cai, X. Antagonistic flame retardancy between hexakis (4-nitrophenoxy) cyclotriphosphazene and potassium diphenylsulfone sulfonate in the PC system. J. Therm. Anal. Calorim. 2016, 126, 571–583. [Google Scholar] [CrossRef]
- Guo, J.W.; Wang, Y.Q.; Feng, L.J.; Zhong, X.; Yang, C.F.; Liu, S.; Cui, Y.D. Performance of a Novel Sulfonate Flame Retardant Based on Adamantane for Polycarbonate. Polymer (Korea) 2013, 37, 437–441. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Guo, J.W.; Xian, J.X.; Fu, S.Q. Novel sulfonate-containing halogen-free flame-retardants: Effect of ternary and quaternary sulfonates centered on adamantane on the properties of polycarbonate composites. RSC Adv. 2017, 7, 39270–39278. [Google Scholar] [CrossRef]
- Li, H.; Zhao, J.Q.; Liu, S.M.; Yuan, Y.C. Polycarbonate–acrylonitrile-butadiene-styrene blends with simultaneously improved compatibility and flame retardancy. RSC Adv. 2014, 4, 10395–10401. [Google Scholar] [CrossRef]
- Seo, J.; Jang, W.; Han, H. Thermal properties and water sorption behaviors of epoxy and bismaleimide composites. Macromol. Res. 2007, 15, 10–16. [Google Scholar] [CrossRef]
- Chiang, T.H.; Liu, C.Y.; Dai, C.Y. A study of the thermal, dielectric, and flame-retarding characteristics of various bismaleimide blended with halogen-free epoxy resin. J. Polym. Res. 2013, 20, 274. [Google Scholar] [CrossRef]
- Zhao, W.; Li, B.; Xu, M.J.; Yang, K.; Lin, L. Novel intumescent flame retardants: Synthesis and application in polycarbonate. Fire Mater. 2013, 37, 530–546. [Google Scholar] [CrossRef]
- Jian, R.K.; Wang, P.; Duan, W.S.; Wang, J.S.; Zheng, X.L.; Weng, J.B. Synthesis of a Novel P/N/S-Containing Flame Retardant and Its Application in Epoxy Resin: Thermal Property, Flame Retardance, and Pyrolysis. Ind. Eng. Chem. Res. 2016, 55, 11520–11527. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Peng, H.Q.; Li, J.H.; Xia, Z.X.; Tan, H. A novel flame retardant containing phosphorus, nitrogen, and sulfur. J. Therm. Anal. Calorim. 2014, 115, 1639–1649. [Google Scholar] [CrossRef]
- Verma, S.K.; Kaur, I. Gamma-induced polymerization and grafting of a novel phosphorous-, nitrogen-, and sulfur-containing monomer on cotton fabric to impart flame retardancy. J. Appl. Polym. Sci. 2012, 125, 1506–1512. [Google Scholar] [CrossRef]
- Lai, X.F.; Guo, J.W.; Fu, S.Q.; Zhu, D.Y. Synthesis of rigid cores based on 1, 1′-biadamantane. RSC Adv. 2016, 6, 8677–8680. [Google Scholar] [CrossRef]
- Guo, J.W.; Lai, X.F.; Fu, S.Q.; Yue, H.B.; Wang, J.W.; Topham, P.D. Microporous organic polymers based on hexaphenylbiadamantane: Synthesis, ultra-high stability and gas capture. Mater. Lett. 2017, 187, 76–79. [Google Scholar] [CrossRef]
- Li, X.; Guo, J.W.; Yue, H.B.; Wang, J.W.; Topham, P.D. Synthesis of thermochemically stable tetraphenyladamantane-based microporous polymers as gas storage materials. RSC Adv. 2017, 7, 16174–16180. [Google Scholar] [CrossRef]
- Zhan, J.; Song, L.; Nie, S.B.; Hu, Y. Combustion properties and thermal degradation behavior of polylactide with an effective intumescent flame retardant. Polym. Degrad. Stab. 2009, 94, 291–296. [Google Scholar] [CrossRef]
- Gao, S.; Liu, G.S. Synthesis of amino trimethylene phosphonic acid melamine salt and its application in flame-retarded polypropylene. J. Appl. Polym. Sci. 2018, 135, 46274. [Google Scholar] [CrossRef]
- Jiang, D.D.; Yao, Q.; McKinney, M.A.; Charles, A.W. TGA/FTIR studies on the thermal degradation of some polymeric sulfonic and phosphonic acids and their sodium salts. Polym. Degrad. Stab. 1999, 63, 423–434. [Google Scholar] [CrossRef]
- Lu, K.; Cao, X.J.; Liang, Q.S.; Wang, H.T.; Cui, X.W.; Li, Y.J. Formation of a compact protective layer by magnesium hydroxide incorporated with a small amount of intumescent flame retardant: New route to high performance nonhalogen flame retardant TPV. Ind. Eng. Chem. Res. 2014, 53, 8784–8792. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Huo, S.Q.; Wang, M.; Cheng, L.F. Synthesis of a phosphorus/nitrogen-containing additive with multifunction groups and its flame-retardant effect in epoxy resin. Ind. Eng. Chem. Res. 2015, 54, 7777–7786. [Google Scholar] [CrossRef]
- Schatel, B.; Hull, T.R. Development of fire-retarded materials-Interpretation of cone calorimeter date. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Purser, D.A. Toxic Combustion Product Yields as a Function of Equivalence Ratio and Flame Retardants in Under-Ventilated Fires: Bench-Large-Scale Comparisons. Polymers 2016, 8, 330. [Google Scholar] [CrossRef]
- Yu, H.Y.; Liu, J.; Wen, X.; Jiang, Y.J.; Wang, L.; Zheng, J.; Fu, S.Y.; Tang, T. Charing polymer wrapped carbon nanotubes for simultaneously improving the flame retardancy and mechanical properties of epoxy resin. Polymer 2011, 52, 4891–4898. [Google Scholar] [CrossRef]
- Manjunatha, N.L.; Bommulu, R.; Juikar, V.; Hatna, S. Investigation on the Influence of Different Compatibilizers on Polycarbonate and High Density Polyethylene Blends: Mechanical Properties, Thermal Properties, Morphology, and Chemical Resistance. Ind. Eng. Chem. Res. 2013, 52, 5672–5682. [Google Scholar] [CrossRef]
- Jang, K.S. Mineral filler effect on the mechanics and flame retardancy of polycarbonate composites: Talc and kaolin. E Polym. 2016, 16, 379–386. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Luo, H.; Cao, X.L.; Kong, W.B.; Cai, X.F. Preparation and characterization of a water resistance flame retardant and its enhancement on charring–forming for polycarbonate. J. Appl. Polym. Sci. 2017, 129, 809–820. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Kong, W.B.; Wang, Y.C.; Cai, X.F. Synthesis of tris (phenoxy) trifluorocyclotriphosphazenes and study of its effects on the flammable, thermal, optical, and mechanical properties of bisphenol-A polycarbonate. J. Appl. Polym. Sci. 2015, 122, 805–816. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Deng, C.L.; Chen, L.; Wang, D.Y.; Wang, Y.Z. Flame-retardant effect of sepiolite on an intumescent flame-retardant polypropylene system. Ind. Eng. Chem. Res. 2011, 50, 2047–2054. [Google Scholar] [CrossRef]



| Samples | PC/wt % | ASN/wt % | KSS/wt % | Dripping | Rating | LOI/% |
|---|---|---|---|---|---|---|
| PC | 100.00 | 0.00 | 0.00 | Yes | V-2 | 25.8 |
| PC/ASN (0.06 wt %) | 99.94 | 0.06 | 0.00 | No | V-1 | 28.5 |
| PC/ASN (0.08 wt %) | 99.92 | 0.08 | 0.00 | No | V-1 | 29.7 |
| PC/ASN (0.10 wt %) | 99.90 | 0.10 | 0.00 | No | V-0 | 30.1 |
| PC/ASN (0.12 wt %) | 99.88 | 0.12 | 0.00 | No | V-0 | 30.5 |
| PC/ASN (0.16 wt %) | 99.84 | 0.16 | 0.00 | No | V-0 | 30.2 |
| PC/KSS (0.10 wt %) | 99.90 | 0.00 | 0.10 | Yes | V-2 | 28.6 |
| Parameters | PC | PC/ASN (0.10 wt %) |
|---|---|---|
| TTI (s) | 102 | 60 |
| pk-HRR (kW/m2) | 305.9 | 297.8 |
| m-HRR (kW/m2) | 147.3 | 129.5 |
| THR (MJ/m2) | 61.0 | 49.3 |
| EHC (MJ/kg) | 19.7 | 21.0 |
| RY (wt %) | 8.5 | 25.7 |
| SEA (m3/kg) | 680.5 | 712.5 |
| pk-SPR (m3/s) | 0.133 | 0.113 |
| mean CO production (kg/kg) | 0.148 | 0.073 |
| Samples | PC/ASN (0.10 wt %) | PC/FR-A (0.10 wt %) | PC/KSS (0.10 wt %) |
|---|---|---|---|
| 0 h | V-0 | V-0 | V-1 |
| 24 h | V-0 | V-1 | V-2 |
| 48 h | V-0 | V-1 | V-2 |
| 72 h | V-0 | V-2 | V-2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, W.; Guo, J.; Zhao, X.; Li, X.; Yang, H.; Chen, J.-K. Synthesis of an Efficient S/N-Based Flame Retardant and Its Application in Polycarbonate. Polymers 2018, 10, 441. https://doi.org/10.3390/polym10040441
Wen W, Guo J, Zhao X, Li X, Yang H, Chen J-K. Synthesis of an Efficient S/N-Based Flame Retardant and Its Application in Polycarbonate. Polymers. 2018; 10(4):441. https://doi.org/10.3390/polym10040441
Chicago/Turabian StyleWen, Weiqiu, Jianwei Guo, Xi Zhao, Xiong Li, Hongmei Yang, and Jem-Kun Chen. 2018. "Synthesis of an Efficient S/N-Based Flame Retardant and Its Application in Polycarbonate" Polymers 10, no. 4: 441. https://doi.org/10.3390/polym10040441