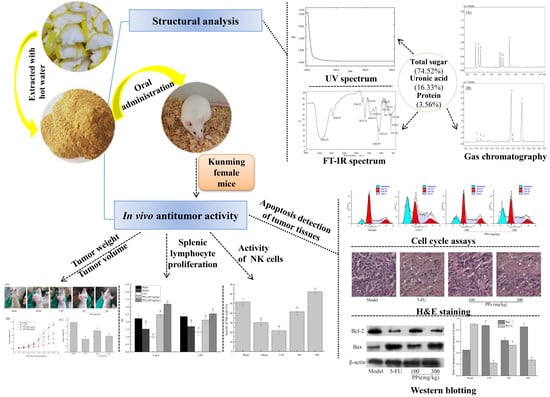
Preliminary Structural Characteristics of Polysaccharides from Pomelo Peels and Their Antitumor Mechanism on S180 Tumor-Bearing Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Standards and Reagents
2.3. Extraction Procedure of PPs
2.4. Physicochemical Properties Analysis
2.5. Antitumor Activity In Vivo
2.5.1. Design of Animal Model
2.5.2. Splenic Lymphocyte Proliferation Assay
2.5.3. NK Cells Activity Analysis
2.5.4. Evaluation of Serum Cytokines in Mice Serums
2.5.5. Assessment of Lymphocyte Subsets in Peripheral Blood
2.6. Apoptosis Detection of Tumor Tissues
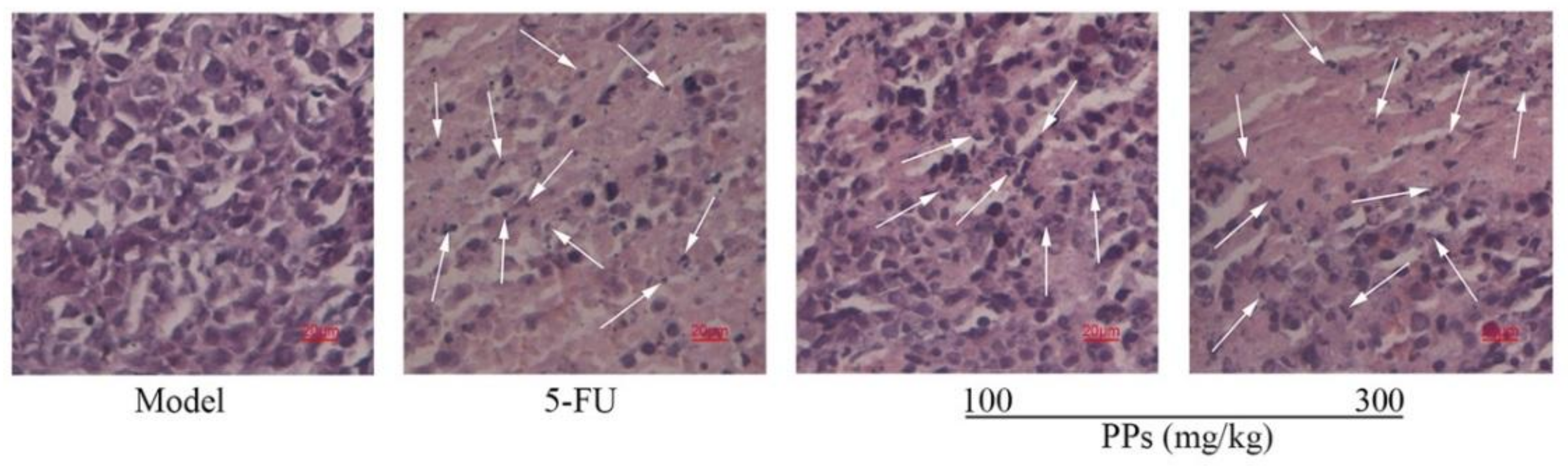
2.6.1. Histological Observations of Tumor Tissues
2.6.2. Cell Cycle Analysis Using Flow Cytometer
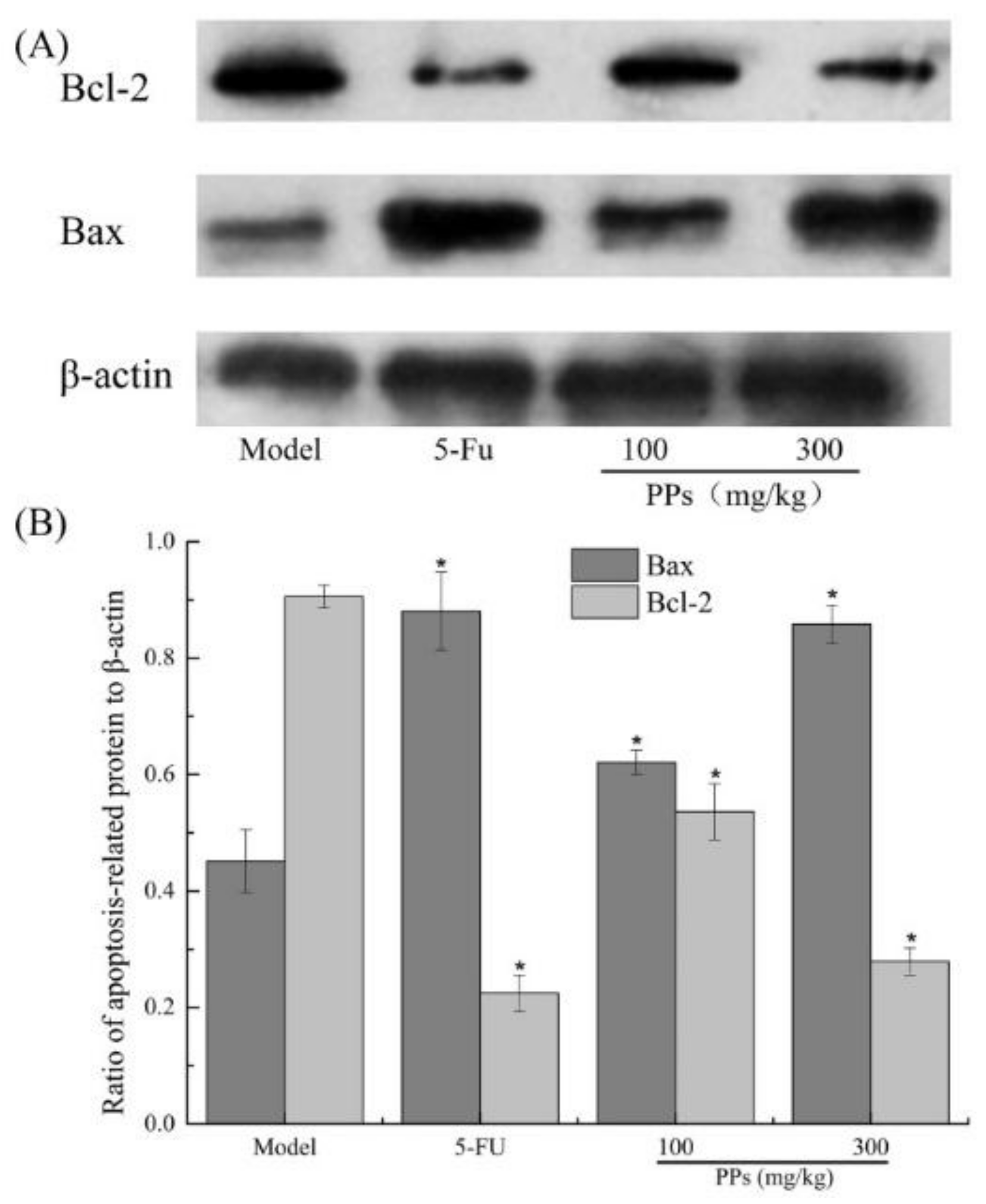
2.6.3. Western Blotting
2.7. Statistical Analysis
3. Results
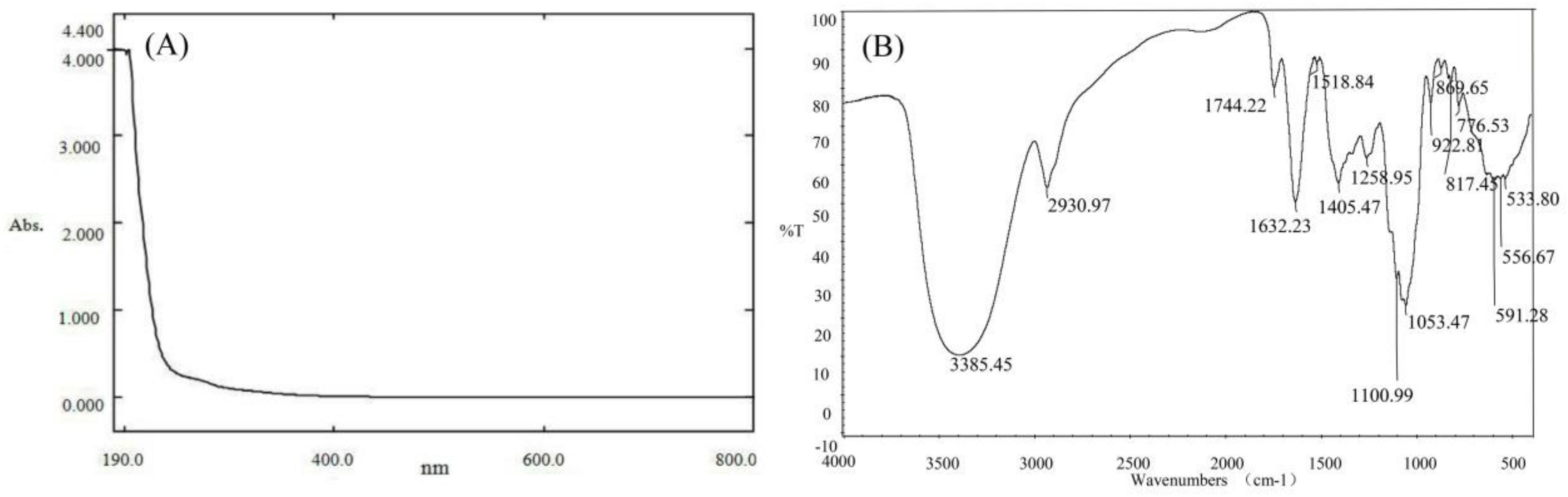
3.1. Physicochemical Characteristics of PPs
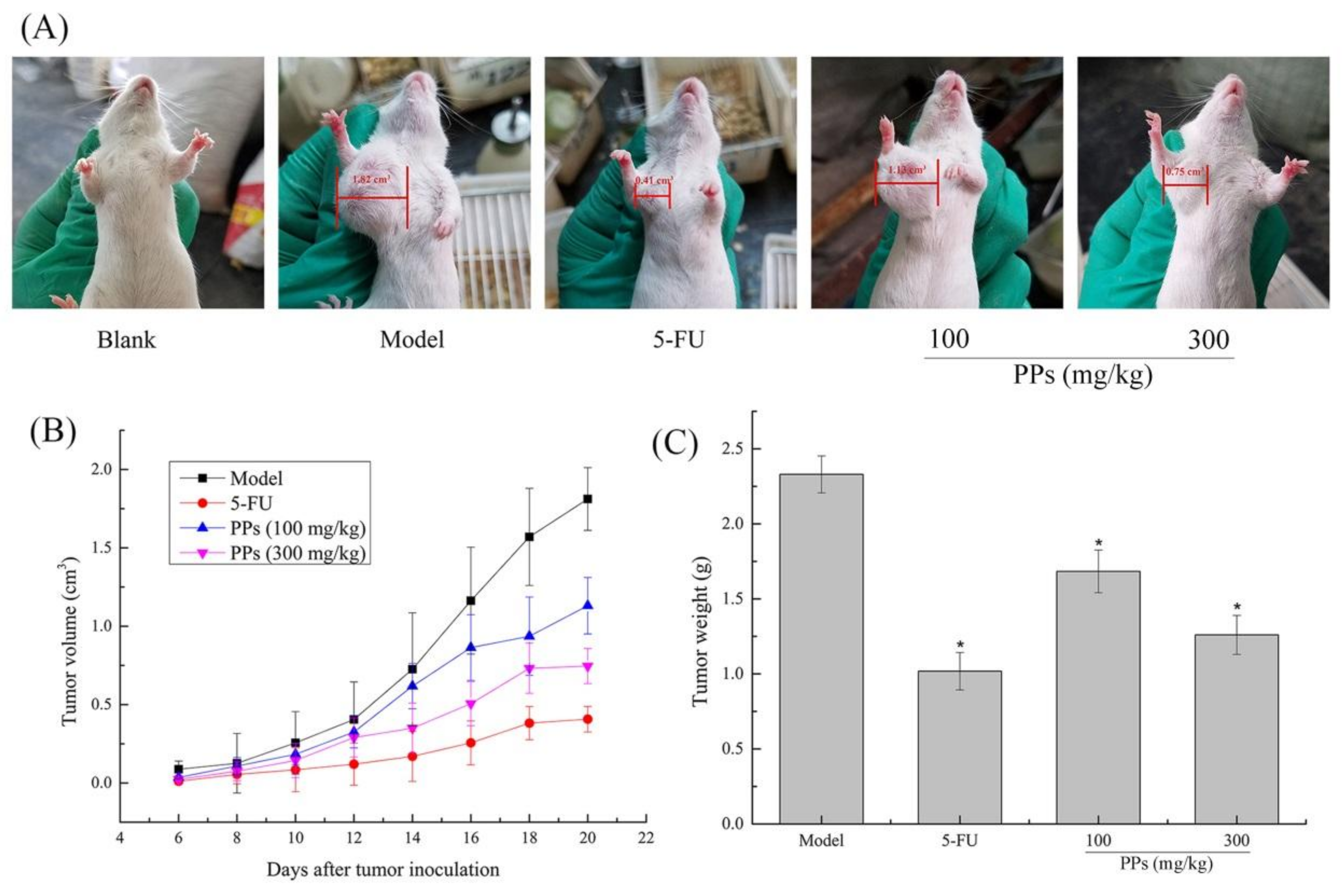
3.2. In Vivo Antitumor Activity of PPs on S180-Bearing Mice
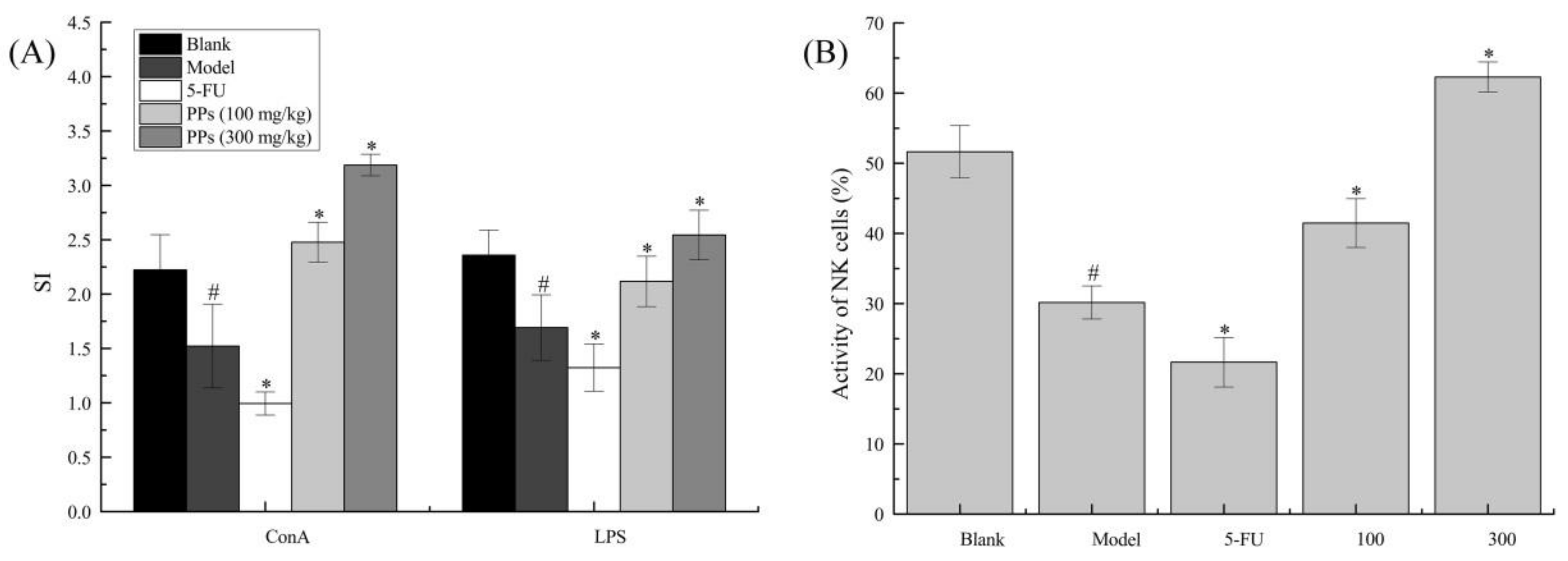
3.3. Splenocyte Proliferation and NK Cells Activities Analysis
3.4. Effects of PPs on Serum Cytokine Levels
3.5. Effects of PPs on T Lymphocyte Subsets in Peripheral Blood
3.6. Effects of PPs on Tumor Cells Apoptosis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PPs | Pomelo peels polysaccharides |
| MWCO | Molecular weight cut-off |
| FT-IR | Fourier transform infrared spectroscopy |
| GC | Gas chromatography |
| TFA | Trifluoroacetic acid |
| 5-FU | 5-fluorouraci |
| FBS | Fetal bovine serum |
| ConA | Concanavalin A |
| LPS | Lipopolysaccharide |
| NK | Natural killer |
| IL-2 | Interleukin-2 |
| IFN-γ | Interferon-γ |
| TNF-α | Tumor necrosis factor-α |
| BRM | Biological response modifier |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5diphenyltetrazolium bromide |
| DMSO | Dimethyl sulfoxide |
| FBS | Fetal bovine serum |
| H&E | Hematoxylin and eosin |
| PI | Propidium iodide |
| FCM | Flow cytometry |
| RIPA | Radio immunoprecipitation assay |
| BCA | Bicinchoninic acid |
| ECL | Enhanced chemiluminescence |
| WB | Western blotting |
References
- Sanjeewa, K.K.A.; Lee, J.-S.; Kim, W.-S.; Jeon, Y.-J. The potential of brown-algae polysaccharides for the development of anticancer agents: An update on anticancer effects reported for fucoidan and laminaran. Carbohydr. Polym. 2017, 177, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Chang, M.-C.; Cheng, W.-F. Metronomic chemotherapy and immunotherapy in cancer treatment. Cancer Lett. 2017, 400, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Asghari, M.H.; Ghobadi, E.; Moloudizargari, M.; Fallah, M.; Abdollahi, M. Does the use of melatonin overcome drug resistance in cancer chemotherapy? Life Sci. 2018, 196, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Tiwari, A.K.; Gandham, R.K.; Sahoo, A.P. Combined administration of the apoptin gene and poly (I:C) induces potent anti-tumor immune response and inhibits growth of mouse mammary tumors. Int. Immunopharmacol. 2016, 35, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, L.; Ren, Y.; Gao, Y.; Kang, L.; Qiao, Q. Anticancer and immunoregulatory activity of Gynostemma pentaphyllum polysaccharides in H22 tumor-bearing mice. Int. J. Biol. Macromol. 2014, 69, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Sun, H.; Li, J.; Ma, C.; Zhao, S.; Guo, Z.; Lin, Y.; Lin, Y.; Liu, L. A polysaccharide from Sanguisorbae radix induces caspase-dependent apoptosis in human leukemia HL-60 cells. Int. J. Biol. Macromol. 2014, 70, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xiao, B.; Sun, T. Antitumor and immunomodulatory activity of Astragalus membranaceus polysaccharides in H22 tumor-bearing mice. Int. J. Biol. Macromol. 2013, 62, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Chen, Y.; Li, W.; Guo, S.; Wang, X.; An, H.; Zhan, Y. Anti-tumor effects of (1→3)-β-d-glucan from Saccharomyces cerevisiae in S180 tumor-bearing mice. Int. J. Biol. Macromol. 2017, 95, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Zong, A.; Cao, H.; Wang, F. Anticancer polysaccharides from natural resources: A review of recent research. Carbohydr. Polym. 2012, 90, 1395–1410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chan, J.W.; Moretti, A.; Uhrich, K.E. Designing polymers with sugar-based advantages for bioactive delivery applications. J. Control. Release 2015, 219, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Ademosun, A.O.; Oboh, G.; Passamonti, S.; Tramer, F.; Ziberna, L.; Boligon, A.A. Modulation of HMG-CoA reductase and glutathione-linked enzymes and protection against pro-oxidant induced oxidative damage in colon (Caco-2) cells and rat colon homogenates by phenolic extracts from shaddock (Citrus maxima) peels. J. Appl. Biomed. 2017, 15, 1–8. [Google Scholar] [CrossRef]
- Methacanon, P.; Krongsin, J.; Gamonpilas, C. Pomelo (Citrus maxima) pectin: Effects of extraction parameters and its properties. Food Hydrocoll. 2014, 35, 383–391. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, Z.; Yao, F.Y.-D.; Liang, H. Study of two-stage microwave extraction of essential oil and pectin from pomelo peels. LWT Food Sci. Technol. 2016, 66, 538–545. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, W.; Liao, X.; Hu, X.; Wu, J.; Wang, X. Extraction of pectin from the peels of pomelo by high-speed shearing homogenization and its characteristics. LWT Food Sci. Technol. 2017, 79, 640–646. [Google Scholar] [CrossRef]
- Liu, Z.; Qiao, L.; Gu, H.; Yang, F.; Yang, L. Development of Brönsted acidic ionic liquid based microwave assisted method for simultaneous extraction of pectin and naringin from pomelo peels. Sep. Purif. Technol. 2017, 172, 326–337. [Google Scholar] [CrossRef]
- Safdar, M.N.; Kausar, T.; Jabbar, S.; Mumtaz, A.; Ahad, K.; Saddozai, A.A. Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. J. Food Drug Anal. 2016, 25, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Ademosun, A.O. Shaddock peels (Citrus maxima) phenolic extracts inhibit α-amylase, α-glucosidase and angiotensin I-converting enzyme activities: A nutraceutical approach to diabetes management. Diabetes Metab. Syndr. 2011, 5, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J. Antifungal activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. Food Control 2008, 19, 1130–1138. [Google Scholar] [CrossRef]
- Yang, X.B.; Zhao, Y.; Yang, Y.; Ruan, Y. Isolation and characterization of immunostimulatory polysaccharide from an herb tea, Gynostemma pentaphyllum makino. J. Agric. Food Chem. 2008, 56, 6905–6909. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhou, Y.; Wang, X.; Guo, J.; Li, J.; Fan, H.; Dou, J.; Shen, B.; Zhou, C. Enhanced antitumor activity of gemcitabine by polysaccharide-induced NK cell activation and immune cytotoxicity reduction in vitro/vivo. Carbohydr. Polym. 2017, 173, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Chen, J.; Li, Q.; Wang, T.; Li, H. Antitumor activity of polysaccharide from Laminaria japonica on mice bearing H22 liver cancer. Int. J. Biol. Macromol. 2016, 92, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, X.; Lu, X.; Wang, D.; Zhao, Y. Protective effects of Keemun black tea polysaccharides on acute carbon tetrachloride-caused oxidative hepatotoxicity in mice. Food Chem. Toxicol. 2013, 58, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Scoparo, C.T.; de Souza, L.M.; Rattmann, Y.D.; Dartora, N.; Paiva, S.M.M.; Sassaki, G.L.; Gorin, P.A.J.; Iacomini, M. Polysaccharides from green and black teas and their protective effect against murine sepsis. Food Res. Int. 2013, 53, 780–785. [Google Scholar] [CrossRef]
- Barbosa, H.; Slater, N.K.; Marcos, J.C. Protein quantification in the presence of poly(ethylene glycol) and dextran using the Bradford method. Anal. Biochem. 2009, 395, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Bitter, T.; Muir, H.M. A modified uronic acid carbazole reaction. Anal. Biochem. 1962, 4, 330–334. [Google Scholar] [CrossRef]
- Zhao, G.; Kan, J.; Li, Z.; Chen, Z. Structural features and immunological activity of a polysaccharide from Dioscorea opposita Thunb roots. Carbohydr. Polym. 2005, 61, 125–131. [Google Scholar] [CrossRef]
- Ren, Z.; He, C.; Fan, Y.; Si, H.; Wang, Y.; Shi, Z.; Zhao, X.; Zheng, Y.; Liu, Q.; Zhang, H. Immune-enhancing activity of polysaccharides from Cyrtomium macrophyllum. Int. J. Biol. Macromol. 2014, 70, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jia, J.; Zeng, G.; Zhao, Y.; Qi, X.; He, C.; Guo, W.; Fan, D.; Han, G.; Li, Z. Studies on immunoregulatory and anti-tumor activities of a polysaccharide from Salvia miltiorrhiza bunge. Carbohydr. Polym. 2013, 92, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-Z.; Tan, L.; Jin, C.-G.; Lu, J.; Tian, L.; Chang, Q.-Q.; Wang, K. Extraction, isolation, characterization and antioxidant activity of polysaccharides from Astragalus membranaceus. Ind. Crops Prod. 2015, 77, 434–443. [Google Scholar] [CrossRef]
- Li, C.; Fu, X.; Huang, Q.; Luo, F.; You, L. Ultrasonic extraction and structural identification of polysaccharides from Prunella vulgaris and its antioxidant and antiproliferative activities. Eur. Food Res. Technol. 2015, 240, 49–60. [Google Scholar] [CrossRef]
- Dou, J.; Meng, Y.; Liu, L.; Li, J.; Ren, D.; Guo, Y. Purification, characterization and antioxidant activities of polysaccharides from thinned-young apple. Int. J. Biol. Macromol. 2015, 72, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.J.; Tao, W.Y.; Xu, Z.H.; Guo, W.J.; Xu, H.Y.; Ao, Z.H.; Jin, J.; Wei, Y.Q. Structural analysis of anti-tumor heteropolysaccharide GFPS1b from the cultured mycelia of Grifola frondosa GF9801. Bioresour. Technol. 2007, 98, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.-H.; Zhu, L.; Jiang, J.-G. Immunoregulatory actions of polysaccharides from Chinese herbal medicine. Expert Opin. Ther. Targets 2010, 14, 1367–1402. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, W.; Song, W.; Chen, H.; Teng, A.; Liu, A. Partial characterization and anti-tumor activity of an acidic polysaccharide from Gracilaria lemaneiformis. Carbohydr. Polym. 2012, 88, 1313–1318. [Google Scholar] [CrossRef]
- Drake, C.G.; Antonarakis, E.S. Update: Immunological strategies for prostate cancer. Curr. Urol. Rep. 2010, 11, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, W.D.; Li, Y. Antitumor and immunomodulatory activity of polysaccharide isolated from Trametes orientalis. Carbohydr. Polym. 2015, 131, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Leiro, J.M.; Castro, R.; Arranz, J.A.; Lamas, J. Immunomodulating activities of acidic sulphated polysaccharides obtained from the seaweed Ulva rigida C. Agardh. Int. Immunopharmacol. 2007, 7, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-W.; Feng, M.; Zhai, X.; Hu, M.; You, L.; Luo, W.; Zhao, M. Optimization for the extraction of polysaccharides from Ganoderma lucidum and their antioxidant and antiproliferative activities. J. Taiwan Inst. Chem. Eng. 2013, 44, 886–894. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, C.; Pan, W.; Wang, J.; Wang, W. Carboxylate groups play a major role in antitumor activity of Ganoderma applanatum polysaccharide. Carbohydr. Polym. 2015, 123, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, F.; Yu, Z.; Lin, J.; Yang, L. Chemical characterization and in vitro antitumor activity of a single-component polysaccharide from Taxus chinensis var. Mairei. Carbohydr. Polym. 2015, 133, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.Z.; Lin, Z.B. Ganoderma lucidum polysaccharides peptide inhibits the growth of vascular endothelial cell and the induction of VEGF in human lung cancer cell. Life Sci. 2006, 78, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Ugolini, S.; Blaise, D.; Chabannon, C.; Brossay, L. Targeting natural killer cells and natural killer T cells in cancer. Nat. Rev. Immunol. 2012, 12, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yang, L.; Yao, D.; Wu, X.; Li, J.; Liu, X.; Deng, L.; Huang, C.; Wang, Y.; Li, D.; et al. Tumor antigen-specific CD8+ T cells are negatively regulated by PD-1 and TIM-3 in human gastric cancer. Cell. Immunol. 2017, 313, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Fesenkova, V.; Sultan, H.; Celis, E. CD4 T cells in antitumor immunity. Encycl. Immunobiol. 2016, 4, 441–450. [Google Scholar]
- Zan, X.; Cui, F.; Li, Y.; Yang, Y.; Wu, D.; Sun, W.; Ping, L. Hericium erinaceus polysaccharide-protein HEG-5 inhibits SGC-7901 cell growth via cell cycle arrest and apoptosis. Int. J. Biol. Macromol. 2015, 76, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Gelen, V.; Şengül, E.; Gedikli, S.; Atila, G.; Uslu, H.; Makav, M. The protective effect of rutin and quercetin on 5-FU-induced hepatotoxicity in rats. Asian Pac. J. Trop. Biomed. 2017, 7, 647–653. [Google Scholar] [CrossRef]


| Treatment | Dose (mg/kg) | Increased of Body Weight (g) | Thymus Index (mg/g) | Spleen Index (mg/g) | Numbers Start/End |
|---|---|---|---|---|---|
| Blank | - | 4.25 ± 1.28 | 2.64 ± 0.18 | 4.60 ± 0.29 | 10/10 |
| Model | - | 3.26 ± 0.91 # | 1.67 ± 0.35 # | 7.15 ± 0.25 # | 10/9 |
| 5-Fu | 20 | 1.25 ± 0.99 * | 1.27 ± 0.17 * | 3.87 ± 0.19 * | 10/8 |
| PPs | 100 | 3.74 ± 0.80 | 2.09 ± 0.28 * | 5.83 ± 0.22 * | 10/10 |
| 300 | 4.45 ± 1.39 * | 2.53 ± 0.28 * | 4.58 ± 0.16 * | 10/10 |
| Treatment | Dose (mg/kg) | IL-2 (pg/mL) | IFN-γ (pg/mL) | TNF-α (pg/mL) |
|---|---|---|---|---|
| Blank | - | 4.76 ± 0.13 | 20.54 ± 2.56 | 11.93 ± 2.13 |
| Model | - | 2.71 ± 0.16 # | 8.98 ± 3.21 # | 17.14 ± 2.24 # |
| 5-Fu | 20 | 1.28 ± 0.12 * | 10.68 ± 1.83 | 10.21 ± 1.94 * |
| PPs | 100 | 3.90 ± 0.15 * | 20.04 ± 2.31 * | 22.13 ± 2.82 * |
| 300 | 5.12 ± 0.25 * | 31.34 ± 4.09 * | 27.90 ± 2.92 * |
| Treatment | Dose (mg/kg) | CD3+ (%) | CD4+ (%) | CD8+ (%) |
|---|---|---|---|---|
| Blank group | - | 64.38 ± 5.13 | 43.43 ± 4.15 | 23.89 ± 3.33 |
| Model group | - | 46.12 ± 4.32 # | 27.68 ± 2.02 # | 21.60 ± 2.73 |
| 5-Fu | 20 | 40.11 ± 1.87 * | 21.51 ± 1.87 * | 15.60 ± 1.21 * |
| PPs | 100 | 50.09 ± 3.24 * | 32.35 ± 2.54 * | 21.98 ± 2.23 |
| 300 | 60.75 ± 5.18 * | 43.59 ± 3.76 * | 22.24 ± 3.11 |
| Treatment | Dose (mg/kg) | G0/G1 (%) | S (%) | G2/M (%) | Apoptosis (%) |
|---|---|---|---|---|---|
| Model | - | 53.19 ± 1.57 | 31.15 ± 1.85 | 15.66 ± 1.02 | 8.54 ± 1.23 |
| 5-Fu | 20 | 34.53 ± 2.12 * | 50.06 ± 2.32 * | 15.41 ± 0.87 | 31.38 ± 2.16 * |
| PPs | 100 | 46.01 ± 1.66 * | 38.23 ± 3.22 * | 15.76 ± 1.91 | 18.08 ± 1.24 * |
| 300 | 38.71 ± 2.77 * | 47.35 ± 2.73 * | 13.94 ± 0.94 * | 26.21 ± 1.17 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Ji, H.; Liu, A. Preliminary Structural Characteristics of Polysaccharides from Pomelo Peels and Their Antitumor Mechanism on S180 Tumor-Bearing Mice. Polymers 2018, 10, 419. https://doi.org/10.3390/polym10040419
Yu J, Ji H, Liu A. Preliminary Structural Characteristics of Polysaccharides from Pomelo Peels and Their Antitumor Mechanism on S180 Tumor-Bearing Mice. Polymers. 2018; 10(4):419. https://doi.org/10.3390/polym10040419
Chicago/Turabian StyleYu, Juan, Haiyu Ji, and Anjun Liu. 2018. "Preliminary Structural Characteristics of Polysaccharides from Pomelo Peels and Their Antitumor Mechanism on S180 Tumor-Bearing Mice" Polymers 10, no. 4: 419. https://doi.org/10.3390/polym10040419